 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
An increased and prolonged duration of pain relief after morphine administration has been found in elderly patients. Whether this is due to alterations in pharmacokinetics, receptor binding profile or other factors remains unsolved. The aims were to elucidate the pharmacokinetics after intravenous administration of morphine and oxycodone in elderly patients older than 70 years.
Methods
A randomized non-blinded study with 16 patients aged older than 70 years scheduled for elective hip replacement receiving morphine or oxycodone 0.05 mg/kg as an IV infusion over 15 minutes.
Results
A 2-compartment pharmacokinetic model best described the disposition of morphine and oxycodone. The estimated elimination half-lives for morphine and oxycodone were (mean ± SD) 2.7 ± 3.6 (range 0.8–11.6) and 3.1 ± 1.3 (range 1.1–4.8) hr, respectively. Volume of distribution at steady state was estimated to be 243 ± 256 and 277 ± 187 L, and clearance to be 1748 ± 623 and 1206 ± 546 ml/min for morphine and oxycodone, respectively.
Conclusion
The increased and prolonged duration of pain relief after morphine administration seen in some elderly patients cannot, based on these findings, be ascribed to changes in the pharmacokinetic parameters between elderly and younger patients. Similar for oxycodone, no changes in the pharmacokinetic could be found when comparing the parameters found in elderly patients with those from younger healthy volunteers. A great variability within the individual pharmacokinetic parameters was seen for both drugs. Therefore, we recommend that treatment with morphine and oxycodone in elderly patients is initiated very conservatively and is titrated slowly to effect.
Introduction
In developed countries the percentage of the population over 80 years will more than triple by 2050. Older people have the highest rates of surgical procedures (CitationGibson and Weiner 2005). Standard postoperative pain treatment is often intravenous administration of morphine during the first 24 hours after operation followed by oral sustained release morphine. An increased and prolonged duration of pain relief after morphine administration has been found in the elderly patients (CitationBellville et al 1971; CitationKaiko 1980). However, whether this is due to alterations in pharmacokinetics, in receptor binding profiles or to other factors still remains unsolved.
Oxycodone is another opioid, which is widely used in postoperative settings. Its main benefit over morphine is the lack of active metabolites contributing to the analgesic effect. Morphine and oxycodone are believed to act on different classes of opioid receptors and with different affinities and different kinetics. The pharmacokinetic of morphine in elderly people (age over 60 years) have been investigated in a few studies (CitationBerkowitz et al 1975; CitationStanski et al 1978; CitationOwen et al 1983; CitationBaillie et al 1989; CitationSear et al 1989), whereas no studies on the pharmacokinetics of oxycodone has so far been conducted in this patient population.
The aims of this study were to elucidate the pharmacokinetics after intravenous administration of morphine and oxycodone in elderly patients older than 70 years and compare this to the literature data on pharmacokinetics of morphine and oxycodone of younger people.
Materials and methods
Study design, subjects and blood sampling
The study was conducted as a randomized non-blinded study. The Regional Committee on Biomedical Research Ethics for Copenhagen County and the Danish Medicines Agency approved the study protocol, the informed consent form and the subsequent amendments for the study. Verbal and written information concerning the study was given to the patients and written informed consent was obtained according to the ethical principles stated in the Declaration of Helsinki II. Study subjects were 16 patients older than 70 years scheduled for elective hip replacement. Exclusion criteria included mental illness assessed subjectively by a physician, dialysis, deviant liver and/or kidney function assessed by deviant laboratory values, regular treatment with opioids for pain due to osteoarthritis, treatment with morphine, oxycodone or codeine from three days prior to the study day, treatment with MAO-inhibitors or fluoxetine (SSRI-inhibitor) from 2 weeks before the study day. Known hypersensitivity to morphine or oxycodone was also an exclusion criterion.
The study subjects were asked to be fasting from midnight prior to the study day, which was the day before the surgery. In the morning, the study subjects received either morphine or oxycodone 0.05 mg/kg as an IV infusion over 15 minutes. The drugs, morphine hydrochloride 10 mg/ml (SAD, County Medical Regulatory Office I/S, Denmark) and oxycodone hydrochloride 10 mg/ml (Oxynorm®, norpharma a/s, Denmark) in ampoules of 1 ml were supplied by the hospital pharmacy at Herlev University Hospital. A peripheral vein contra lateral to the vein used for drug administration was used for blood sampling. Blood samples were collected before infusion, at 5, 10, and 15 minutes after the beginning of the infusion, and at 5, 10, 15, 30, 60, 90 minutes and 2, 4, 6, 8, 11, and 24 hours after the end of the infusion period. The last blood sample was sampled as close as possible to the scheduled time, but before the patient went into surgery. The actual sampling times were noted in the individual case report form and subsequently used in the pharmacokinetic analysis. Blood samples were drawn into dry glass tubes, which were placed on ice until centrifugation at 3000 rpm for 10 minutes. Serum was separated into tubes, which were stored below −20 °C until analysis.
Routine vital signs including blood pressure, pulse rate, respiratory rate, and oxygen saturation were obtained at the same times as the blood samples were taken until 30 min after drug administration. These parameters were also recorded in the individual case report form. The study subjects were allowed breakfast 60 min after infusion.
LC-MS analyses
Serum samples obtained after morphine administration were analyzed for morphine and the metabolites morphine-3-glucuroninde (M3G) and morphine-6-glucuronide (M6G). Serum samples obtained after oxycodone administration were analyzed for oxycodone and the metabolites oxymorphone and noroxycodone.
Quantification was done by high-performance liquid chromatography (HPLC) using mass spectrometry as the detection principle. The LC-MS system used consisted of a Hewlett Packard 1100 series chromatograph (Palo Alto, California, USA) equipped with a Quatpump, a degasser, column oven (colcomp), an autosampler (ALS), a DAD UV detector operated at 280 nm (bandwidth 16) and a MSD detector. Data were collected using the HP ChemStation software, version 6.03 (Palo Alto, California, USA). The analytical column was a reversed phase Synergi 4 μ Polar RP 80A (Phenomenex, Torrance, CA, USA) column (4.6 mm I.D. × 150 mm, 4 μm particles). A linear gradient system was applied. Eluent A consisted of 1% formic acid in MeCN:water (3:97 v/v), eluent B of 1% formic acid in MeCN:water (27:73 v/v) and eluent C of 1% formic acid in MeCN:water (80:20, v/v). The linear gradient was applied from 100% A to 100% B over 4 min. One hundred percent B was maintained for 3 min and then to 100% C over 1 min and maintained at 100% C for 3 min. Finally the gradient returned to 100% A in 1 min. The flow-rate was 0.5 mL/min. and the total run time was 20 min.
The MSD detector was equipped with an electrospray interface and used in positive mode for SIM detection of the masses: 286.1 (morphine, and the internal standard hydromorphone, which are well separated in the HPLC system), 462.2 (M-6-G and M-3-G which are well separated in the HPLC system), 316.1 (oxycodon), and 302.1 (oxymorphone and noroxycodone being well separated in the HPLC system).
The fragmentor voltage was set to 100 V for all masses except for mass 462.2 where it was set at 120 V. The voltage over the capillary was kept at 3000 V. The temperature of the nitrogen drying gas was set to 350 °C with a flow-rate of 13 L/min. The nebulizer gas pressure was 30 psig.
After addition of the internal standard, hydromorphone, serum samples and calibration standards in serum were subject to solid phase extraction using 1.0 ml of serum on Oasis HLB SPE cartridges (30 mg). The final eluent was evaporated to dryness under nitrogen at ambient temperature and the residue was dissolved in 500 μL of eluent A. 25 μL was injected onto the HPLC column.
Limit of quantification (LOQ) for morphine and M3G/M6G was 3.5 nmol/L and 5.0 nmol/L, respectively. Linearity of the calibration curve was proven from 3.5 to 10.000 nmol/L. This was used for the detection of M3G. Narrow ranges were used for the detection of morphine and M6G, 3.5 to 50 nmol/L and 5.0 to 1000 nmol/L, respectively. The LOQ for oxycodone was 3.5 nmol/L. The CV of the method determined at three concentration levels were in all cases below 15% for the inter-day variation. Intra-day variations were a little less than the inter-day CV but at the same order of magnitude.
Data analysis
Pharmacokinetic analysis
Individual serum concentrations for morphine and oxycodone were analyzed using WinNonLin, Version 3.3 (Pharsight Corporation, Mountain View, CA, USA). The data was fitted to 1-, 2- and 3-compartment pharmacokinetic models. Selection criteria for the final model was a low Akaike Criteria (AIC), a statistical significant (p < 0.05) improvement of the fit as determined by the F-test based upon the residual sum of squares, and an even distribution of residuals.
The following pharmacokinetic parameter estimates were determined using compartmental analysis: systemic clearance (CL), volume of distribution at steady state (Vss), central compartment volume (V2), peripheral compartment volume (V1), and elimination half-life (t½). Area under the serum concentration-time curve (AUC) was calculated by using the software GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, California, USA). Intercompartmental rate constants (k12 and k21) and elimination rate constant (ke) were calculated using standard pharmacokinetic equations. Peripheral compartment morphine concentrations were calculated from mean data as described by Rowland and Tozer (CitationRowland and Tozer 1995).
Kidney function was evaluated by estimation of the creatinine clearance:
F equals 1.23 for men and 1.04 for women.
Statistical analysis
GraphPad Prism version 4.00 for Windows was used for all statistical analyses. Normally distributed data was tested for differences between the two treatment means with a t-test with two-tail p-value. A p-value of 0.05 was considered statistically significant.
Results
All results are presented as mean values with standard deviation (± S.D.). All patients completed the study. Mean age, height and weight of the study subjects was 76.1 ± 4.5 years, 167.4 ± 8.3 cm, and 77.8 ± 16.9 kg, respectively. Kidney function estimated by creatinine clearance was found to be normal except for two patients, where the kidney function was mildly reduced.
Pharmacokinetic analysis
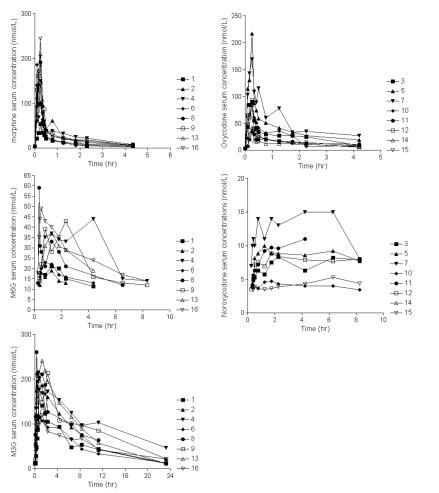
The individual serum concentrations versus time profiles after IV administration of morphine, M3G and M6G, and oxycodone and noroxycodone are shown in . Mean dose of morphine and oxycodone was 10744 ± 3017 and 9393 ± 1015 nmol, respectively. Peak drug serum concentrations (Cmax) occurred at 15 min (end of infusion) and were 150 ± 50 nmol/L and 98 ± 62 nmol/L for morphine and oxycodone, respectively. The metabolites M3G and M6G were measurable in the samples after 10 and 20 min, respectively. Maximum concentration was reached at 75 min for both metabolites, for M3G 166 ± 46 nmol/L and for M6G 30 ± 8 nmol/L. Oxymorphone was not quantifiable in any of the samples, but noroxycodone was measurable in 4 patients after 20 min. Maximum concentration was after 6 hours, 8 ± 4 nmol/L. In a single patient (no. 14) noroxycodone was not quantifiable in any of the samples.
Figure 1 The individual serum concentrations versus time profiles of morphine, M3G and M6G after IV administration of morphine and of oxycodone and noroxycodone after IV administration of oxycodone. Please notice the different axes.
A 2-compartment pharmacokinetic model best described the disposition of morphine and oxycodone. The best fits of morphine and oxycodone serum concentrations after applying the 2-compartment model are shown in . The AUCs for morphine and oxycodone were 159 ± 38 and 230 ± 196 nmol/L*hr, respectively. After approximately 4.3 hr the morphine serum concentrations were below the limit of quantification. For oxycodone serum concentrations were still quantifiable at 11 hours. The results of the pharmacokinetic modeling are summarized in . The estimated elimination half-lives for morphine and oxycodone were 2.7 ± 3.6 (range 0.8–11.6) and 3.1 ± 1.3 (range 1.1–4.8) hr, respectively. Volume of distribution at steady state (Vss) was estimated to be 243 ± 256 and 277 ± 187 L, and clearance (CL) to be 1748 ± 623 and 1206 ± 546 ml/min for morphine and oxycodone, respectively. The intercompartmental rate constants k12 and k21 were for morphine 29.0 ± 36.9 and 1.9 ± 1.2 hr−1, and for oxycodone 11.44 ± 15.0 and 2.2 ± 0.9 hr−1. Maximum morphine peripheral compartment concentration was 20.8 nmol/L at 1.26 hr (tmax) and the elimination rate constant was 0.32 hr−1.
Figure 2 The best fit of morphine (A) and oxycodone (B) serum concentrations after applying a 2-compartment pharmacokinetic model. Open circles are mean observed concentrations, while the solid lines are the mean predicted concentrations using the best model. The dotted lines are 95% confidence interval on the mean observed values.
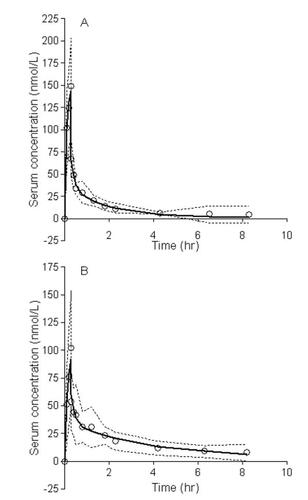
Table 1 Pharmacokinetic characteristics of morphine and oxycodone in elderly patients
Discussion
Drug disposition of both morphine and oxycodone followed a biexpontential decline with an initial rapid distribution phase followed by a slower elimination phase. The corresponding elimination half-life t½ was 2.7 ± 3.6 (range 0.8–11.6) and 3.1 ± 1.3 (range 1.1–4.8) hr for morphine and oxycodone, respectively. For morphine, this is in agreement with what have been reported by others in healthy elderly volunteers (age 60–90 years) (CitationStanski et al 1978; CitationOwen et al 1983; CitationBaillie et al 1989; CitationSear et al 1989) and elderly patients (age 61–83 years) (CitationStanski et al 1978; CitationOwen et al 1983; CitationBaillie et al 1989; CitationSear et al 1989). To the best of our knowledge, the pharmacokinetics of oxycodone has not earlier been investigated in an old population. The parameters found for oxycodone in this study are in agreement with those found in younger patients (t½: 2.3-5.9 hr, Vss: 140–279 L, and CL: 49–1130 L) (CitationPöyhiä et al 1991, Citation2004; CitationLeow, Smith, Watt, et al 1992; CitationLeow, Smith, Williams, et al 1992; CitationLeow et al 1995; CitationKirvela et al 1996; CitationTallgren et al 1997).
The Vss of morphine has been found to considerably smaller in elderly patients than younger patients (CitationOwen, Sitar, Berger, Brownell, Duke, and Mitenko 1983). However, the Vss found in this study is in range with what others have reported in both healthy volunteers (CitationStanski et al 1978; CitationPatwardhan et al 1981; CitationOwen et al 1983; CitationAitkenhead et al 1984; CitationMazoit et al 1987; CitationBaillie et al 1989; CitationHasselström and Säwe 1993; CitationWesterling et al 1993, Citation1994, Citation1995; CitationStuart-Harris et al 2000) and patients (CitationStanski et al 1976, Citation1978; CitationMurphy and Hug 1981; CitationSäwe et al 1981; CitationMoore et al 1984; CitationChauvin et al 1987; CitationSäwe and Odar-Cederlöf 1987; CitationSear et al 1989, Citation1989a, Citation1989b) regardless of age. Also clearance of morphine has been found to be significantly reduced in elderly people (CitationOwen et al 1983; CitationBaillie et al 1989; CitationSear et al 1989) compared to middle-aged as well as young people. In this study the CL of morphine was estimated to be 1748 ± 623 ml/min, which in agreement with the estimates found by others. For all studies, a great variability exists. However, there are some indications that the older the patients or study population are, the greater variability. In this study great inter-patient variability is seen in parent drugs as well as in the metabolites. Owen et al compared the disposition of morphine in elderly (age 60-69) with younger (age 23–28) healthy volunteers. The coefficient of variance (% CV) was approximately twice as big in the elderly group compared to the younger group (CitationOwen et al 1983). In this study the patients are older (age 70–84), and the % CV were even greater. Similar for oxycodone, great variability in the parameters exists. This great variability could be the explanation for the increased sensitivity observed in some elderly patients and complicates the treatment with morphine and oxycodone in this patient population.
Owen et al have also suggested that the increased analgesic potency of morphine seen in elderly subjects might be related to increased peripheral compartment concentration in elderly subjects (CitationOwen et al 1983). In the present study the patients were older (age 70–84), the %CV greater and the calculated peripheral compartment morphine concentrations higher (dose difference taken into consideration). The analgesic effect of morphine may partly be related to altered disposition, however great variability exists and no easy correlation can be made. Animal and human studies indicate that also polymorphism may contribute to the variability of morphine efficacy. However, the role of polymorphism for morphine is controversial. A recent report suggested that cancer patients homozygous for the 118G allele caused by the single nucleotide polymorphism at nucleotide position 118 in the μ-opioid receptor gene, require higher doses of morphine to relieve pain (CitationKlepstad et al 2004). Sawyer et al saw an effect on M3G and M6G-concentrations in patients with variations in the uridine diphosphate-glucuronosyltransferase (by UGT2B7*2), which indicates that inter-individual differences in morphine glucuronidation may be the result of genetic variation (CitationSaywer et al 2003). The pharmacokinetics and pharmacodynamics of M6G also seems to be related to A118G mutation of the human μ-opioid receptor gene resulting in reduced analgesic responses to M6G (CitationRomberg et al 2004, Citation2005). CitationKlepstad et al (2005) concludes in a short review that opioid efficacy is partly related to inborn properties caused by genetic variability related to metabolism, receptors and transporters, and that variation in other non-opioid biological systems may indirectly influence the pharmacology.
The role of polymorphism for oxycodone seems to be a bit clearer. The metabolic pathway for oxycodone is by the cytochrome P450 2D6 iso-enzyme. Approximately 10% of Caucasians are poor metabolizers, eg, they do not express this enzyme, and hence only form small amount of oxymorphone. As oxymorphone is not considered an important contributor to the analgesia seen after oxycodone administration, the component of polymorphism does not seem so important. However, as for morphine many factors influence the variation and the efficacy of the drugs, and further studies investigating this is necessary.
As both the peripheral compartment concentrations and the variability increases with increasing age, treatment with morphine and probably oxycodone becomes more complicated the older the patient is, and treatment should be conservative. Furthermore, it is important to acknowledge the risk of the potential life-threatening side effects, such as respiratory depression, with opioid during the acute phase of post-operative pain relief, which further supports a conservative treatment.
Conclusion
The increased and prolonged duration of pain relief after morphine administration, which has been seen in elderly patients, cannot, based on these findings, be ascribed to changes in the pharmacokinetic parameters between elderly and younger patients. Similar for oxycodone, no changes in the pharmacokinetic could be found when comparing the parameters found in elderly patients with those from younger healthy volunteers. A great variability within the individual pharmacokinetic parameters was seen for both drugs.
As age-related drug effect could not solely be translated to alterations in pharmacokinetics, in order to fully understand the pharmacological basis for these changes in the elderly people, pharmacodynamic parameters must also be considered when conducting such studies. Therefore, we recommend that treatment with morphine and oxycodone in elderly patients is initiated very conservatively and is titrated slowly to effect.
Acknowledgements
The Authors would like to thank Orthopedic Surgery Unit at Gentofte and Herlev University hospital for help during the study. Oxycodone was kindly provided by norpharma a/s, Denmark.
References
- AitkenheadARVaterMAcholaKPharmacokinetics of a single-dose i.v. morphine in normal volunteers and patients with end-stage renal failureBritish Journal of Anaesthesia19845681396743445
- BaillieSPBatemanDNCoatesPEAge and the pharmacokinetics of morphineAge and Ageing198918258622816559
- BellvilleJWForrestWHMillerEInfluence of age on pain relief from analgesicsJAMA19712171835415109724
- BerkowitzBANgaiSHYangJCThe disposition of morphine in surgical patientsClinical Pharmacology and Therapeutics197517629351139854
- ChauvinMSandoukPScherrmannJ-MMorphine pharmacokinetics in renal failureAnesthesiology198766327313826690
- GibsonSJWeinerDKPain in older persons2005Seattle, USAIASP Press
- HasselströmJSäweJMorphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrationsClinical Pharmacokinetics199324344548491060
- KaikoRFAge and morphine analgesia in cancer patients with postoperative painClinical Pharmacology and Therapeutics19802882367438698
- KirvelaMLindgrenLSeppalaTThe pharmacokinetics of oxycodone in uremic patients undergoing renal transplantationJournal of Clinical Anesthesia199681388695073
- KlepstadPDaleOSkorpenFGenetic variability and clinical efficacy of morphineActa Anaesthesiol Scand200549902816045647
- KlepstadPRakvagTTKaasaSThe 118 A > G polymorphism in the human micro-opioid receptor gene may increase morphine requirements in patients with pain caused by malignant diseaseActa Anaesthesiol Scand2004481232915504181
- LeowKPCramondTSmithMTPharmacokinetics and pharmacodynamics of oxycodone when given intravenously and rectally to adult patients with cancer painAnesthesia and Analgesia1995802963027818116
- LeowKPSmithMTWattJAComparative oxycodone pharmacokinetics in humans after intravenous, oral, and rectal administrationTherapeutic Drug Monitoring199214479841485370
- LeowKPSmithMTWilliamsBSingle-dose and steady-state pharmacokinetics and pharmacodynamics of oxycodone in patients with cancerClinical Pharmacology and Therapeutics199252487951424423
- MazoitJ-XSandoukPZetlaouiPPharmacokinetics of unchanged morphine in normal and cirrhotic subjectsAnesthesia Analgesia19876629383565791
- MooreASearJBaldwinDMorphine kinetics during and after renal transplantationClinical Pharmacology and Therapeutics19843564156370555
- MurphyMRHugCCJrPharmacokinetics of intravenous morphine in patients anesthetized with enflurane-nitrous oxideAnesthesiology198154187927469101
- OwenJASitarDSBergerLAge-related morphine kineticsClinical Pharmacology and Therapeutics19833436486883911
- PatwardhanRVJohnsonRFHoyumpaANormal metabolism of morphine in cirrhosisGastroenterology1981811006117286578
- PöyhiäRHynynenMSeppalaTPharmacodynamics and pharmacokinetics of high-dose oxycodone infusion during and after coronary artery bypass graftingJournal of Cardiothoracic and Vascular Anesthesia2004187485415650985
- PöyhiäROlkkolaKTSeppalaTThe pharmacokinetics of oxycodone after intravenous injection in adultsBritish Journal of Clinical Pharmacology19913251681958450
- RombergROlofsenESartonEPharmacokinetic-pharmacodynamic modeling of morphine-6-glucuronide-induced analgesia in healthy volunteersAnesthesiology20041001203314695733
- RombergROlofsenETaschnerPEMPolymorphism of mu-opioid receptor gene (OPRM1:c. 118A > G) does not protect against opioid-induced respiratory depression despite reduced analgesic responseAnesthesiology20051025223015731588
- RowlandMTozerTNClinical pharmacokinetics. concepts and applications19953PhilidelphiaLippincott Williams & Wilkins
- SäweJDahlstromBPaalzowLMorphine kinetics in cancer patientsClinical Pharmacology and Therapeutics198130629357297022
- SäweJOdar-CederlöfIKinetics of morphine in patients with renal failureEuropean Journal of Pharmacology19873237782
- SaywerMBInnocentiFDasSA pharmacogentic study of uridine diphosphate-glucuronosyltranferase 2B7 in patients receiving morphineClinical Pharmacology and Therapeutics2003735667412811366
- SearJWHandCWMooreRAStudies on morphine disposition: plasma concentrations of morphine and its metabolites in anesthetized middle-aged and elderly surgical patientsJournal of Clinical Anesthesia1989116492627383
- SearJWHandCWMooreRAStudies on morphine disposition: Influence of general anaesthesia on plasma concentrations of morphine and its metabolitesBritish Journal of Anaesthesia1989a622272917110
- SearJWHandCWMooreRAStudies on morphine disposition: Influence of renal failure on the kinetics of morphine and its metabolitesBritish Journal of Anaesthesia1989b6228322644963
- StanskiDRGreenblattDJLappasDGKinetics of high-dose ontraveneous morphine in cardiac surgery patientsClinical Pharmacology and Therapeutics19761975261269215
- StanskiDRGreenblattDJLowensteinEKinetics of intravenous and intramuscular morphineClinical Pharmacology and Therapeutics197824529657720
- Stuart-HarrisRJoelSPMeDonaldPThe pharmacokinetics of morphine and morphine glucuronide metabolites after subcutaneous injection and subcutaneous infusion of morphineBritish Journal of Clinical Pharmacology2000492071410718775
- TallgrenMOlkkolaKTSeppalaTPharmacokinetics and ventilatory effects of oxycodone before and after liver transplantationClinical Pharmacology and Therapeutics199761655619209248
- WesterlingDFrigrenLHöglundPMorphine Pharmacokinetics and Effects on Salivation and Continuous Reaction Times in Healthy VolunteersTherapeutic Drug Monitoring199315364748249042
- WesterlingDHöglundPLundinSTransdermal administration of morphine to healthy subjectsBritish Journal of Clinical Pharmacology19943757167917776
- WesterlingDPerssonCHöglundPPlasma Concentrations of Morphine, Morphine-3-Glucuronide, and Morphine-6-Glucuronide After Intravenous and Oral Administration to healthy Volunteers: Relationship to nonanalgesic ActionTherapeutic Drug Monitoring1995172873017624926