Abstract
Lenalidomide (also known as Revlimid®, CC-5013) is an immunomodulatory derivative of thalidomide and has more potent anti-tumor and anti-inflammatory effects than thalidomide. The molecular mechanisms of anti-tumor activity of lenalidomide have been extensively studied in multiple myeloma (MM) both preclinical models and in clinical trials. Lenalidomide: directly triggers growth arrest and/or apoptosis of drug resistant MM cells; inhibits binding of MM cells to bone marrow (BM) extracellular matrix proteins and stromal cells; modulates cytokine secretion and inhibits angiogenesis in the BM milieu; and augments host anti-tumor immunity. Lenalidomide achieved responses in patients with relapsed refractory MM. Moreover, lenalidomide with dexamethasone (Dex) demonstrates more potent anti-MM activities than Dex both in vitro and in randomized phase III clinical trials. Specifically, the combination improved overall and extent of response, as well as prolonged time to progression and overall survival, resulting in FDA approval of lenalidomide with Dex for therapy MM relapsing after prior therapy.
Introduction
Multiple myeloma (MM) is a B cell malignancy characterized by excess monotypic plasma cells in the BM in association with monoclonal protein in serum and/or urine, decreased normal immunoglobulin (Ig) levels, and lytic bone disease. The 2006 estimate of multiple myeloma incidence in the United States is 16,570 cases, with an estimated number of 11,300 deaths. Conventional therapies with alkylating agents, anthracyclines, and corticosteroids can extend patient survival to a median of 3–4 years (CitationGregory et al 1992; CitationGroup 1998), and high dose therapy followed by autologous transplantation can modestly prolong median survival to 4–5 years (CitationFermand et al 1998; CitationLenhoff et al 2000). Attempts to improve autografting include repeated use of high dose therapies (CitationDesikan et al 2000; CitationAttal et al 2003), as well as immune strategies to treat minimal residual disease post-transplant (CitationMassaia et al 1999) can improve outcome in some studies, few, if any, patients are cured. MM remains incurable due to the development of tumor cell resistance to all therapies, highlighting the urgent need for novel treatment strategies.
Thalidomide (Thal) has shown to be useful in various diseases including MM; however, it is a potent teratogen and causes side effects including peripheral neuropathy (CitationTseng et al 1996). Attempts were therefore made to develop Thal analogs which are more potent and have less adverse effects: lenalidomide (C13H13N3O3, MW = 259.26) is one such analog belonging to the class ofimmunomodulatory drugs (IMiDs) developed by the drug discovery program.
Preclinical studies of lenalidomide
Overview ()
The interaction of multiple myeloma (MM) cells with bone marrow (BM) extracellular matrix (ECM) proteins and BM accessory cells, BM stromal cells (BMSCs), osteoblasts, osteoclasts, endothelial cells, as well as other factors in the BM milieu (ie, cytokines, angiogenesis) plays a crucial role in MM pathogenesis and drug resistance (CitationDamiano et al 1999; CitationAkiyama et al 2002; CitationHideshima and Anderson 2002; CitationHideshima et al 2003, Citation2004, Citation2006; CitationChauhan et al 2004). These accessory cells not only physically interact with MM cells, but also secrete growth and/or anti-apoptotic factors such as interleukin (IL)-6, insulin-like growth factor (IGF)-1, vascular endothelial growth factor (VEGF), and tumor necrosis factor (TNF)-α (CitationAkiyama et al 2002; CitationChauhan et al 1996, Citation2004, Citation2005; CitationCatley et al 2004; CitationHideshima et al 2006). Delineation of the mechanisms of BM stromal cell (SC)-mediated MM cell proliferation, survival, drug resistance, and migration therefore provides the framework for identification and validation of novel therapeutic targets.
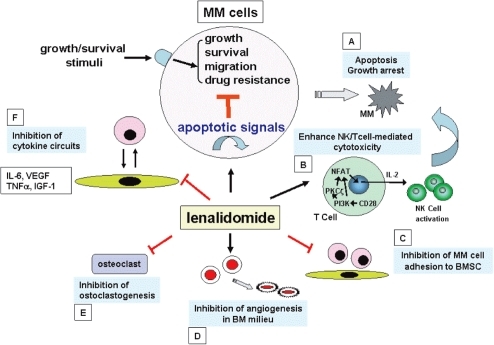
Figure 1 Potential mechanisms of action of anti-MM activity of lenalidomide. Lenalidomide: directly induces tumor cell apoptosis and/or growth arrest (A); enhances NK and/or NK cell activity via activation of CD28/NF-AT2 pathway (B); inhibits MM cell adhesion to host microenvironment (C); inhibits angiogenesis (D); inhibits osteoclastogenesis (E); as well as inhibits cytokine secretion (F).
Within the BM microenvironment, several proliferative/antiapoptotic signaling cascades are activated in MM cells: phosphatidylinositol-3 kinase (PI3K)/Akt (also known as protein kinase B); I κ B kinase (IKK)/nuclear factor κ-B (NFκB); Ras/Raf/mitogen-activated protein kinase (MAPK) kinase (MEK)/extracellular signal-related kinase (ERK); and Janus kinase (JAK) 2/signal transducers and activators of transcription (STAT)-3 (, ). These signaling cascades mediate: cytoplasmic sequestration of many transcription factors; upregulation of cyclin D and anti-apoptotic Bcl-2 family members; as well as augmentation of telomerase activity (CitationHideshima et al 2001a; CitationAkiyama et al 2002). Importantly, these molecular events are triggered by both MM cell adherence to BMSCs and by cytokines secreted from BMSCs (CitationDankbar et al 2000; CitationHideshima et al 2004; CitationMitsiades et al 2004). Cytokines secreted from MM cells and BMSCs and other cells may in turn further augment cytokine secretion.
Table 1 Selected ongoing clinical trials of lenalidomide based combination treatment in multiple myeloma
Novel biologically based agents target not only the MM cell, but also MM cell–host interactions, cytokines, and their sequelae in the BM milieu. Thalidomide and its immunomodulatory derivative (IMiD) lenalidomide (Revlimid®; Celgene Corp., Summit, NJ, USA) are examples of such agents targeting the tumor cell in its BM milieu which can achieve responses even in refractory relapsed MM. Lenalidomide may inhibit MM cell growth by several different mechanisms (). First, lenalidomide has a direct effect on MM cells to induce G1 growth arrest or apoptosis even of drug resistant cells (CitationHideshima et al 2000; CitationMitsiades et al 2002). Second, lenalidomide inhibits adhesion of MM cells to BMSCs, and thereby can overcome cell adhesion mediated drug resistance (CAM-DR); third, lenalidomide inhibits bioactivity and/or secretion in MM cells and/or BM stromal cells of cytokines [eg, interleukin (IL)-6, IL-1β, IL-10, and tumor necrosis factor (TNF)α] which augment MM cell growth, survival, drug resistance, migration, and expression of adhesion molecules. Importantly, lenalidomide is several thousand fold more potent than Thal at inhibiting TNFα/IL-1β secretion from mononuclear cells stimulated with lipopolysaccharide (LPS) in vitro (CitationCorral et al 1999; CitationMuller et al 1999). Fourth, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are secreted by MM cells and/or BMSCs, and lenalidomide may inhibit activity of VEGF, bFGF, and angiogenesis in MM. Lenalidomide also acts against MM through immunomodulatory effects such as augmentation of activity of cytotoxic T-cells and natural killer (NK) cells, associated with secretion of IL-2 and interferon-γ (CitationDavies et al 2001; CitationLeBlanc et al 2004; CitationHayashi et al 2005).
Bone destruction is a hallmark of MM, with 70%–80% of patients manifesting bone involvement. Recently, Anderson et al demonstrated that an IMiD CC-4047 (Actimid®; Celgene Corp., Summit, NJ, USA) inhibits osteoclastgenesis via downregulation of transcription factor PU.1 (CitationAnderson et al 2006). Lenalidomide also has inhibitory effect on osteoclastogenesis (CitationTerpos et al 2007).
Direct anti-tumor activities of lenalidomide
Although the targets of whereby lelalidomide mediates antitumor activity of lenalidomide have not been fully delineated, several studies have examined the molecular mechanisms mediating sequelae of lenalidomide. Our previous studies demonstrated that lenalidomide induces G0/G1 growth arrest associated with p21Cip1 upregulation and/or apoptosis which is mediated via caspase-8 activation (CitationHideshima et al 2000; CitationMitsiades et al 2002). Lenalidomide inhibits LPS-mediated induction of Cox-2 and prostaglandin E2 (PGE2) production by a post-transcriptional mechanism in RAW 364.7 cells (CitationFujita et al 2001), suggesting that the anti-tumor activity induced by lenalidomide may also be due to inhibition of Cox-2 and PGE2. Lenalidomide inhibits nuclear factor (NF)-κB subunit activity in MM cell lines (CitationMitsiades et al 2002), which is consistent with reports that Thal inhibits DNA binding activity of the p50/p65 NF-κB triggered by TNFα and IL-1β in Jurkat cell line (CitationKeifer et al 2001) and in PBMCs (CitationRowland et al 2001). Since NF-κB plays an essential role in cell cycle regulation, cell survival, anti-apoptosis, and cytokine production in MM cells (CitationHideshima et al 2001b, Citation2002), inhibition of NF-κB activity by lenalidomide may also enhance or restore sensitivity to other chemotherapeutic agents. Specifically, we have demonstrated that MM cell adhesion-mediated upregulation of IL-6 is mediated via NF-κB activation (CitationChauhan et al 1996; CitationHideshima et al 2002). Recently, CitationStewart et al (2004) reported pharmacogenomic studies suggesting that hyperactivation of the Wnt signaling antagonist DKK-1 is associated with response to the immunomodulators Thal and lenalidomide. Furthermore, β-catenin expression is downregulated by lenalidomide in MM cell lines.
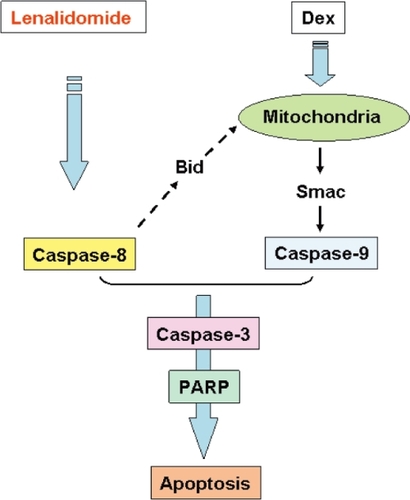
Lenalidomide in combination with Dex is one of the most promissing MM novel treatment options. It induces at least additive direct cytotoxicity in MM cells (CitationHideshima et al 2000), associated with activation of dual apoptotic signaling cascades: Dex induces caspase-9 (CitationChauhan et al 2001; CitationHideshima et al 2001a) and lenalidomide triggers caspase-8 activation (CitationMitsiades et al 2002) (). Most recently, enhanced anti-MM activity of rapamycin, a specific mTOR inhibitor, in combination with lenalidomide has been reported (CitationRaje et al 2004). In this study, the combination of rapamycin plus lenalidomide overcomes drug resistance in MM cell lines resistant to conventional chemotherapy. Interestingly, differential signaling cascades, including the ERK and PI3-K/Akt pathways, are targeted by these drugs individually and in combination, suggesting the molecular mechanism by which they inhibits MM growth and survival.
Anti-angiogenic activity
Previous studies have shown that oral administration of lenalidomide attenuates growth factor-induced angiogenesis in vivo. This effect is correlated with the inhibitory effect of lenalidomide on growth factor-induced Akt phosphorylation, thereby providing a potential mechanism for its anti-migratory and subsequent anti-angiogenic effects (CitationDredge et al 2005). In MM, an anti-angiogenic effect of Thal in vitro has been demonstrated (D’Amato et al 1004; CitationSinghal et al 1999; CitationLentzsch et al 2002; CitationFujita et al 2004); however, to date no strong evidence of an anti-angiogenic effect of lenalidomide in vivo has been demonstrated. Moreover, CitationSinghal et al (1999) reported no correlation of BM angiogenesis with response to Thal in patients with relapsed refractory MM, suggesting that lenalidomide may mediate its anti-MM activity via mechanisms other than anti-angiogenesis.
Immunomodulatory activities
A unique feature of the anti-tumor effect of Thal and lenalidomide is their ability to modulate and potentiate host immune responses against MM. Several studies have demonstrated the effects of lenalidomide on peripheral blood lymphocytes (CitationDavies et al 2001; CitationHaslett et al 2003; CitationLeBlanc et al 2004; CitationHayashi et al 2005). Co-culture of naive splenocytes with anti-CD3 monoclonal antibody and IMiD1 (Actimid®) directly costimulates T cells and increases Th-1-type cytokines. Most excitingly, IMiDs augment CTL and NK cell activity against MM cell lines and autologous MM cells, associated with increased IL-2 levels in serum (CitationDavies et al 2001). Although Thal/IMiDs induce IL-2 secretion from T cells (CitationCorral et al 1999; CitationShannon et al 2000), the mechanisms whereby these compounds induce IL-2 production from T cells has not totally been defined. Importantly, our recent studies demonstrated that lenalidomide significantly costimulates proliferation of CD3+ T cells induced by CD3 ligation, immature dendritic cells (DCs; SI, 2.1), or mature DCs (SI, 2.6). T-cell proliferation triggered by DCs is abrogated by cytotoxic T lymphocyte antigen 4-immunoglobulin (CTLA-4-Ig). Lenalidomide also overcomes the inhibitory effects of CTLA-4-Ig on Epstein-Barr virus and influenza-specific CD4 and CD8 T-cell responses, as measured by cytokine capture and enzyme-linked immunosorbent spot (ELISPOT) assays. Importantly, lenalidomide triggers tyrosine phosphorylation of CD28 on T cells, followed by activation of NF-κB (CitationLeBlanc et al 2004). Furthermore, we have demonstrated that IMiDs facilitate the nuclear translocation of nuclear factor of activated T cells (NF-AT)-2 and activator protein-1 via activation of PI3-K/Akt signaling, with resultant IL-2 secretion. IMiDs enhance both NK cell cytotoxicity and ADCC induced by triggering IL-2 production from T cells (CitationHayashi et al 2005). These studies therefore define the molecular mechanisms whereby lenalidomide triggers NK cell-mediated cytotoxicity against MM cells, further supporting their therapeutic use in MM. More recently, we have shown that lenalidomide enhances ADCC induced by SGN-40, a humanized IgG1 anti-CD40 monoclonal antibody (CitationTai et al 2005).
Clinical studies of lenalidomide
Pharmacokinetics
Pharmacokinetics (PK) of lenalidomide in MM patients has been reported by CitationWu and Scheffler (2004) at American Society of Clinical Oncology in 2004. In this single-center, open-label, non-randomized, phase I dose escalation study in relapsed and refractory MM, the doses of lenalidomide used were 5, 10, 25 or 50 mg/day orally for 28 days. Blood samples were collected before and at 15 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 4 h, 6 h, 8 h, 10 h, 12 h, 18 h, 24 h, 48 h, and 72 h after administration on both days 1 and 28. No lenalidomide dose-limiting toxicity was observed at any dose level within the first 28 days. Absorption of lenalidomide was rapid on both day 1 and 28, with tmax ranging from 0.7 to 2.0 h at all dose levels. Plasma levels of lenalidomide declined in a monophasic manner, with elimination half-life ranging from 2.8 to 6.1 h on both days 1 and 28 at all four doses. No plasma accumulation was observed upon multiple dosing. Importantly, daily oral doses of lenalidomide up to 50 mg produced no dose-limiting toxicity within the first 28 days.
The other PK study has been reported by CitationRichardson et al (2006). In this study, plasma concentration of lenalidomide was determined in 39 patients during the first and second cycles in both 15 mg and 30 mg dose groups, and when Dex was added due to progressive disease (PD) or stable disease (SD) on lenalidomide alone. The mean minimum (Cmin) plasma lenalidomide concentrations on days 1, 2, 3, 4, and 21 during the first and second 21-day cycles of lenalidomide alone and with the addition of Dex are shown for the 30 mg once-daily and 15 mg twice-daily cohorts. The average Cmin plasma levels were less in the twice-daily compared with daily dosing regimens. No obvious effect on lenalidomide plasma concentrations was seen with addition of Dex in either once- or twice-daily treatment.
Clinical trials of lenalidomide
Only a limited number of reports are available for clinical studies of lenalidomide (CitationBartlett et al 2004). A phase I clinical study of lenalidomide was completed at Dana-Farber Cancer Institute (CitationRichardson et al 2002a). In this study, dose-escalation (5 mg/day, 10 mg/day, 25 mg/day, and 50 mg/day) of lenalidomide was evaluated in 27 patients (median age 57 years; range, 40–71 years) with relapsed and refractory relapsed MM (CitationRichardson et al 2002b). These patients received a median of 3 (range, 2–6) prior regimens, including autologous stem cell transplantation and Thal in 15 and 16 patients, respectively. In 24 evaluable patients, no dose-limiting toxicity (DLT) was observed in patients treated at any dose level within the first 28 days; however, grade 3 myelosuppression developed after day 28 in all 13 patients treated with 50 mg/day lenalidomide. Dose reduction to 25 mg/day was well tolerated in 12 patients and therefore considered to be the maximal tolerated dose (MTD). Most importantly, no significant somnolence, constipation, or neuropathy, the most common toxicities of Thal, have been seen in any cohort. Best responses of at least 25% reduction in paraprotein occurred in 17 of 24 (71%) patients (90% confidence interval [CI], 52%–85%), including 11 (46%) patients who had received prior Thal; stable disease (less than 25% reduction in paraprotein) was observed in an additional 2 (8%) patients. This study therefore demonstrates that lenalidomide can overcome conventional drug resistance, even resistance to Thal. Given that lenalidomide is an oral agent, it is currently being evaluated in a randomized trial post autografting in an attempt to prolong progression free and overall survival.
A multicenter, open-label, randomized phase II study to evaluate 2 dose regimens of lenalidomide for relapsed, refractory MM has been performed. In this study, 70 patients were randomized to receive either 30 mg once-daily or 15 mg twice-daily oral lenalidomide for 21 days of every 28-day cycle. An additional 32 patients received 30 mg once daily. Patients with progressive or stable disease after 2 cycles received additional Dex. Responses were evaluated according to European Group for Blood and Marrow Transplantation (EBMT) criteria. Overall response rate (CR+PR+MR) to lenalidomide alone was 25%; 24% for 30 mg once-daily and 29% for 15 mg twice-daily cohort. Median overall survival in 30-mg once-daily and 15 mg twice-daily groups was 28 and 27 months, respectively. However, median progression-free survival was 7.7 months on 30 mg once-daily versus 3.9 months on 15 mg twice-daily lenalidomide. Dex was added in 68 patients and 29% responded. Importantly, time to first occurrence of clinically significant grade 3/4 myelosuppression was shorter in the 15 mg twice-daily group (1.8 months) than 30 mg once-daily (5.5 months, p = 0.05) group. Moreover, analysis of the first 70 patients showed increased grade 3/4 myelosuppression in patients receiving 15 mg twice-daily (41% vs 13%, p = 0.03). This study indicate that lenalidomide is active and well tolerated in relapsed, refractory myeloma, with the 30-mg once-daily regimen providing the basis for future studies as monotherapy and with Dex (CitationRichardson et al 2006).
Clinical studies of lenalidomide in combination with Dex
As described above, preclinical studies have demonstrated the efficacy of combination treatment of lenalidomide with Dex in MM and several clinical trials of this combination treatment have been completed.
In two double blind, multicenter, international phase III clinical trials (MM-009, North American, 353 patients; MM-010, Europe, Australia, and Israel, 351 patients), patients with relapsed or refractory MM not resistant to Dex were treated with Dex 40 mg daily on days 1–4, 9–12, and 17–20 every 28 days and were randomized to receive either lenalidomide 25 mg daily orally on days 1–21 every 28 days or placebo. At a median follow-up from randomization of 17.1 months (MM-009) and 16.5 months (MM-010), both studies show significant improvement with lenalidomide plus Dex compared to Dex in overall response (OR) (MM-009: 1% vs 20.5%, p < 0.001; MM-010: 59.1% vs 24%, p < 0.001, respectively), time to progression (TTP) (MM-009: 11.1 months vs 4.7 months, p < 0.001; MM-010: 11.3 months vs 4.7 months, p < 0.001, respectively), and overall survival (OS) (MM-009: 29.6 months vs 20.5 months, p < 0.001; MM-010: not estimable vs 20.6 months, p < 0.001, respectively). In a subgroup analysis on patients with impaired creatinine clearance, no significant difference in response rate, TTP, or OS was observed in patients with creatinine clearance above or below 50 mL/min who were treated with lenalidomide plus Dex; however, for 16 patients with creatinine clearance <30 mL/min, median TTP and OS was shorter than for those with creatinine clearance >30 mL/min, but still significantly longer than for patients treated with Dex (CitationWeber et al 2006).
Treatment with lenalidomide plus Dex in newly diagnosed MM patients has also reported by CitationRajkumar et al (2005). In this study, lenalidomide was given orally 25 mg daily on days 1–21 of a 28-day cycle. Dex was given orally 40 mg daily on days 1–4, 9–12, and 17–20 of each cycle. Thirty-one of 34 patients achieved an objective response, including 2 (6%) achieving complete response (CR) and 11 (32%) meeting criteria for both very good partial response and near complete response, resulting in an overall objective response rate of 91%. This study indicated that lenalidomide plus Dex is a highly active regimen with manageable side effects in the treatment of newly diagnosed MM.
A number of studies demonstrated that MM is characterized by cytogenetic abnormalities causing dysregulation of the genes at the breakpoints, and by point mutations (CitationKuehl et al 2002; CitationFonseca et al 2004; CitationCarrasco et al 2006). Specifically, chromosome 13 deletions are present in over 50% of MM patients and considered to be associated with poor prognosis. In addition, t(4; 14) in MM also predicts poor response to conventional and high dose treatment and shortened survival. A recent study has shown that lenalidomide overcomes the poor prognosis conferred by chromosome 13 deletion and t(4; 14) in MM patients, evidenced by event free survival and response rate (CitationBahlis et al 2006).
Recently, a phase I/II 3 combination treatment of lenalidomide, Dex and adriamycin (RAD therapy) for relapsed MM patient has been reported. In this study, 31 patients were evaluated for response and toxicity: 26 patients achieved reduction of paraprotein levels of at least 50% for a response rate of 84%, including one confirmed CR and 14 PRs according to the EBMT criteria. Importantly, 8 of 10 patients who displayed del (13) on cytogenetic analysis responded, including 6 confirmed PRs. One patient each experienced acute renal failure due to emesis and hypovolemia, pneumocystis pneumonitis, and catheter related infection. Somnolence, constipation, thromboembolism, or neuropathy was not observed. This study showed that RAD induces substantial responses with an acceptable toxicity profile, and thus significantly contributes to the therapeutic armamentarium even in heavily pretreated MM patients (CitationKnop et al 2006). Most recently, a phase I study of lenalidomide and dexamethasone in combination with Akt inhibitor perifosine for patients with relapsed or refractory MM, and a phase I/II study of lenalidomide, dexamethasone and bortezomib combination therapy for newly diagnosed MM patients are ongoing.
The common side effects of lenalidomide treatment in phase 2 clinical trials of relapsed refractory MM are summarized in . The most common toxicities associated with Thal (eg, constipation, neuropathy, tremors) were not observed. Toxicities associated with lenalidomide were primarily hematologic and reversible. The most common grade 3 or higher adverse events during lenalidomide therapy were neutropenia and thrombocytopenia. Grade 4 neutropenia occurred in 2 of 34 (5.8%) patients treated at 15 mg twice daily vs 4 of 68 (5.9%) patients treated with 30 mg daily. Grade 4 thrombocytopenia occurred in 2 (5.8%) of 34 patients on 15 mg twice daily vs 2 of 68 patients (2.9%) treated with 30 mg daily. Deep vein thrombosis (DVT) was reported in 1 patient on the 30 mg daily and 2 patients (5.8%) on the 15 mg twice-daily treated regimen. Sedation or neurologic toxicities were not observed in most of these studies (CitationRichardson et al 2006). The differences in the side effect profile between Thal and lenalidomide may reflect distinct patterns of antiangiogenic, cytokine-related, microenvironmental, and immunomodulatory activity, rather than distinct separate mechanisms of action.
In phase III trials of lenalidomide plus Dex for newly diagnosed MM patients, 47% of patients experienced grade III or higher nonhematologic toxicity. The most common adverse effects were fatigue (15%), muscle weakness (6%), anxiety (6%), pneumonitis (6%), and rash (6%) (CitationRajkumar et al 2006a). Recently, a randomized phase III trial of lenalidomide plus high-dose Dex versus lenalidomide plus low-dose Dex in newly diagnosed MM has also reported by CitationRajkumar et al (2006b). In this study, patients in both arms received lenalidomide 25 mg/day orally on days 1–21 every 28 days. In addition, patients in the high-dose Dex arm received Dex 40 mg on days 1–4, 9–12, and 17–20 orally every 28 days, while patients in the low-dose Dex arm received Dex 40 mg on days 1, 8, 15, and 22 orally every 28 days. Although response rate (RR) has not yet been reported, toxicity rates are higher in the high-dose Dex arm than low-dose Dex arm. For example, Grade 3 and above toxicities in cardiac ischemia (2.7% vs 0.5%), hypercalcemia (5.8% vs 1.8%), infection (18.8% vs 9%), thromboembolism (18.4% vs 5.4%), and non-hematologic toxicities (22% vs 12.6%) are higher in high-dose Dex arm than low-dose Dex arm. If RR is similar in both arms, dosage of Dex can be reduced to 25 mg.
Conclusion
Lenalidomide plus Dex treatment is highly effective in both preclinical and clinical studies. It is one of the promising treatment options against both relapsed/refractory and newly diagnosed MM patients. Adverse effects of this combination can be markedly reduced by lowering the Dex dosage.
Future directions
Lenalidomide plus Dex treatment can be further combined with other novel or conventional agents to improve patient outcome in MM. Indeed, potent Akt inhibitor Perifosine, proteasome inhibitor bortezomib (Velcade®; Millennium Pharmaceuticals Inc.), and anti-angiogenic agent bevacizumab (Avastin®; Genentech) are already under evaluation in combination clinical trials.
Acknowledgements
Supported by National Institutes of Health Grant PO-1 78378 and RO-1 CA 50947; the Doris Duke Distinguished Clinical Research Scientist Award (KCA); the Multiple Myeloma Research Foundation (TH, NR, KCA); and the Myeloma Research Fund (KCA).
References
- AkiyamaMHideshimaTHayashiTCytokines modulate telomerase activity in a human multiple myeloma cell lineCancer Res20026238768212097303
- AndersonGGriesMKuriharaNThalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1Blood2006107309810516373662
- AttalMHarousseauJLFaconTSingle versus double autologous stem-cell transplantation for multiple myelomaN Engl J Med2003349249550214695409
- BahlisNJMansoorALateganJCLenalidomide overcomes poor prognosis conferred by deletion of chromosome 13 and t(4; 14) in multiple myeloma: MM016 TrialBlood20061081016a
- BartlettJBDredgeKDalgleishAGThe evolution of thalidomide and its IMiD derivatives as anticancer agentsNat Rev Cancer200443142215057291
- CarrascoDRTononGHuangYHigh-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patientsCancer Cell200693132516616336
- CatleyLTaiYTShringarpureRProteasomal degradation of topoisomerase I is preceded by c-Jun NH2-terminal kinase activation, Fas up-regulation, and poly(ADP-ribose) polymerase cleavage in SN38-mediated cytotoxicity against multiple myelomaCancer Res20046487465315574786
- ChauhanDCatleyLLiGA novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from BortezomibCancer Cell200584071916286248
- ChauhanDHideshimaTRosenSApaf-1/cytochrome c independent and Smac dependent induction of apoptosis in multiple myeloma cellsJ Biol Chem200127624453611356822
- ChauhanDLiGHideshimaTBlockade of ubiquitin-conjugating enzyme CDC34 enhances anti-myeloma activity of Bortezomib/Proteasome inhibitor PS-341Oncogene200423359760215094775
- ChauhanDUchiyamaHAkbaraliYMultiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kBBlood1996871104128562936
- CorralLGHaslettPAJMullerGWDifferential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-αJ Immunol1999163380610384139
- D’AmatoRJLoughmanMSFlynnEThalidomide is an inhibitor of angiogenesisProc Natl Acad Sci USA199491408257513432
- DamianoJSCressAEHazlehurstLACell adhesion mediated drug resistance (CAM-DR): Role of integrins and resistance to apoptosis in human myeloma cell linesBlood19999316586710029595
- DankbarBPadroTLeoRVascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myelomaBlood2000952630610753844
- DaviesFERajeNHideshimaTThalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myelomaBlood2001982101611418482
- DesikanRBarlogieBSawyerJResults of high-dose therapy for 1000 patients with multiple myeloma: durable complete remissions and superior survival in the absence of chromosome 13 abnormalitiesBlood20009540081010845942
- DredgeKHorsfallRRobinsonSPOrally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitroMicrovasc Res200569566315797261
- FermandJ-PRavaudPChevretSHigh-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: Up-front or rescue treatment? Results of a multicenter sequential randomized clinical trialBlood199892313169787148
- FonsecaRBarlogieBBatailleRGenetics and cytogenetics of multiple myeloma: a workshop reportCancer Res20046415465814989251
- FujitaJMestreJRZeldisJBThalidomide and its analogues inhibit lipopolysaccharide-mediated Iinduction of cyclooxygenase-2Clin Cancer Res2001733495511705847
- FujitaKAsamiYTanakaKAnti-angiogenic effects of thalidomide: expression of apoptosis-inducible active-caspase-3 in a three-dimensional collagen gel culture of aortaHistochem Cell Biol2004122273315221409
- GregoryWMRichardsMAMalpasJSCombination chemotherapy versus melphalan and prednisolone in the treatment of multiple myeloma: an overview of published trialsJ Clin Oncol199210334421531068
- Group MTsCCombination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trialsJ Clin Oncol1998163832429850028
- HaslettPAHanekomWAMullerGThalidomide and a thalidomide analogue drug costimulate virus-specific CD8+ T cells in vitroJ Infect Dis20031879465512660941
- HayashiTHideshimaTAkiyamaMMolecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical applicationBr J Haematol200512819220315638853
- HideshimaTAndersonKCMolecular mechanisms of novel therapeutic approaches for multiple myelomaNat Rev Cancer200229273712459731
- HideshimaTBergsagelPLKuehlWMAdvances in biology of multiple myeloma: clinical applicationsBlood20041046071815090448
- HideshimaTCatleyLYasuiHPerifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cellsBlood200610740536216418332
- HideshimaTChauhanDRichardsonPNF-κB as a therapeutic target in multiple myelomaJ Biol Chem2002277166394711872748
- HideshimaTChauhanDShimaYThalidomide and its analogues overcome drug resistance of human multiple myeloma cells to conventional therapyBlood20009629435011049970
- HideshimaTNakamuraNChauhanDBiologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myelomaOncogene2001205991600011593406
- HideshimaTRichardsonPAndersonKCNovel therapeutic approaches for multiple myelomaImmunol Rev20031941647612846814
- HideshimaTRichardsonPChauhanDThe proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cellsCancer Res2001613071611306489
- KeiferJAGuttridgeDCAshburnerBPInhibition of NF-κB activity by thalidomide through suppression of IkappaB kinase activityJ Biol Chem200127622382711297551
- KnopSGereckeCToppMSLenalidomide (Revlimid), adriamycin and dexamethasone chemotherapy (RAD) is safe and effective in treatment of relapsed multiple myeloma -first results of a German multicenter phase I/II trialBlood2006108125a
- KuehlWMBergsagelPLMultiple myeloma: evolving genetic events and host interactionsNat Rev Cancer200221758711990854
- LeBlancRHideshimaTCatleyLPImmunomodulatory drug costimulates T cells via the B7-CD28 pathwayBlood200410317879014512311
- LenhoffSHjorthMHolmbergEImpact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based studyBlood20009571110607678
- LentzschSRogersMSLeBlancRS-3-Amino-phthalimido-glutarimide inhibits angiogenesis and growth of B-cell neoplasias in miceCancer Res2002622300511956087
- MassaiaMBorrionePBattaglioSIdiotype vaccination in human myeloma: generation of tumor-specific immune responses after high-dose chemotherapyBlood1999946738310397734
- MitsiadesCSMitsiadesNMunshiNCFocus on multiple myelomaCancer Cell200464394415542427
- MitsiadesNMitsiadesCSPoulakiVApoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implicationsBlood20029945253012036884
- MullerGWChenRHuangSYAmino-substituted thalidomide analogs: potent inhibitors of TNF-α productionBioorg Med Chem Lett1999916253010386948
- RajeNKumarSHideshimaTCombination of the mTOR inhibitor Rapamycin and Revlimid (CC-5013) has synergistic activity in multiple myelomaBlood200410441889315319277
- RajkumarSVJacobusSCallanderNA randomized phase III trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed multiple myeloma (E4A03): a trial coordinated by the Eastern Cooperative Oncology GroupBlood2006b108239a
- RajkumarSVBloodEVesoleDPhase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology GroupJ Clin Oncol2006a24431616365178
- RajkumarSVHaymanSRLacyMQCombination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myelomaBlood20051064050316118317
- RichardsonPJagannathSSchlossmanRA multi-center, randomized, phase II study to evaluate the efficacy and safety of two CC-5013 dose regimens when used alone or in combination with dexameyhasone (Dex) for the treatment of relapsed or refractory multiple myeloma (MM)Blood2002100104a
- RichardsonPGBloodEMitsiadesCSA randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myelomaBlood200610834586416840727
- RichardsonPGSchlossmanRLWellerEImmunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myelomaBlood20021003063712384400
- RowlandTLMcHughSMDeightonJDifferential effect of thalidomide and dexamethasone on the transcription factor NF-κBInt Immunopharmacol20011496111367517
- ShannonEAseffaAPankeyGThalidomide’s ability to augment the synthesis of IL-2 in vitro in HIV-infected patients is associated with the percentage of CD4+ cells in their bloodImmunopharmacology200046175910647875
- SinghalSMehtaJDesikanRAntitumor activity of thalidomide in refractory multiple myelomaN Engl J Med199934115657110564685
- StewartAKScangaSEZhuYXInhibition of Wnt pathway signaling by thalidomide and revlimid: studies in a drosophila model systemBlood2004104916a
- TaiYTLiXFCatleyLImmunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implicationsCancer Res200565117122016357183
- TerposEDimopoulosMASezerOThe effect of novel anti-myeloma agents on bone metabolism of patients with multiple myelomaLeukemia20072118758417611556
- TsengSPakGWashenikKRediscovering thalidomide: a review of its mechanism of action, side effects, and potential usesJ Am Acad Dermatol199635969798959957
- WeberDWangMChenCLenalidomide plus high-dose dexamethasone provides improved overall survival compared to high-dose dexamethasone alone for relapsed or refractory multiple myeloma (MM): results of 2 phase III studies (MM-009, MM-010) and subgroup analysis of patients with impaired renal functionBlood20061081012a
- WuASchefflerMRMulti-dose phamacokinetics and safety of CC-5013 in 15 multiple myeloma patientsJ Clin Oncol200422141s