Abstract
Allergic rhinitis (AR) is a prevalent disease with great morbidity and significant societal and economic burden. Intranasal corticosteroids are recommended as first-line therapy for patients with moderate-to-severe disease, especially when nasal congestion is a major component of symptoms. To compare the efficacy and safety profile of different available intranasal corticosteroids for the treatment of AR, it is important to understand their different structures and pharmacokinetic and pharmacodynamic properties. Knowledge of these drugs has increased tremendously over the last decade. Studies have elucidated mechanisms of action, pharmacologic properties, and the clinical impact of these drugs in allergic respiratory diseases. Although the existing intranasal corticosteroids are already highly efficient, the introduction of further improved formulations with a better efficacy/safety profile is always desired. Fluticasone furoate nasal spray is a new topical corticosteroid, with enhanced-affinity and a unique side-actuated delivery device. As it has high topical potency and low potential for systemic effects, it is a good candidate for rhinitis treatment.
Allergic rhinitis
Allergic rhinitis (AR) is an inflammatory disease of nasal mucosa induced by an IgE-mediated immune response. It is clinically defined as a symptomatic condition with four major symptoms: rhinorrhea, sneezing, nasal itching and obstruction (CitationInternational Rhinitis Management Working Group 1994; CitationBousquet et al 2001).
Patients with AR can also experience fatigue, sleep disturbance, social function impairment, depressed mood, anxiety, learning and attention impairment, increased work or school absenteeism, and decreased work or school performance and productivity. The impact is made worse because of co-morbidities such as sinusitis, otitis media with effusion, allergic conjunctivitis, bronchial asthma, and dental disorders. Therefore, AR has a high morbidity with significant societal and economic burden, due to direct and indirect costs (CitationInternational Rhinitis Management Working Group 1994; CitationYawn et al 1999; CitationCrystal-Peters et al 2000; CitationLeynaert et al 2000a; CitationBousquet et al 2001; CitationO’Connell 2004; CitationSchoenwetter et al 2004).
AR has an estimated prevalence of 30% of the general population, which has been increasing, particularly in Western countries (CitationThe International Study of Asthma and Allergies I Childhood – ISAAC – Steering committee 1998; CitationUpton et al 2000; CitationBousquet et al 2001). It is the most common chronic disorder in children and can be considered a major public health problem.
Allergic rhinitis and its impact on asthma
The ARIA (Allergic Rhinitis and its Impact on Asthma) guideline was published in 2001, bringing some conceptual changes for rhinitis, such as the modification of its classification, and emphasizing the relationships between upper and lower airways (; CitationBousquet et al 2001).
AR can be classified as perennial or seasonal (hay fever), depending on the timing and type of allergen involved in triggering the allergy. Patients with seasonal AR experience symptomatic exacerbations primarily during pollen seasons. However, more recently, AR has also been classified as intermittent or persistent, according to symptoms duration and frequency. This classification also divides AR into mild or moderate/severe. Severity is measured as a short assessment of the impairment in the day-to-day life of the patient and not as a nasal symptom score (CitationBousquet et al 2001).
Nowadays, rhinitis and asthma are recognized as manifestations of one syndrome, the chronic allergic respiratory syndrome, also known as united airway disease. There is epidemiologic, immunopathologic, and clinical evidences that support an integrated view of these diseases and permit an understanding of their interactions (CitationLeynaert et al 2000b; CitationBousquet et al 2001; CitationLinneberg et al 2002; CitationTogias 2003). Almost all patients with asthma have rhinitis and the presence of severe rhinitis in patients with asthma is associated with worse asthma outcomes. AR is a risk factor for asthma development. Besides, beneficial effects of nasal treatment on the lower airways have been reported, with fewer emergency service visits, fewer hospitalizations, and declining bronchial responsiveness (CitationCrystal-Peters et al 2002; CitationTaramarcaz 2003).
Rhinitis treatment
Rhinitis treatment includes allergen avoidance, pharmacotherapy, and immunotherapy. Intranasal corticosteroids (INS) are recommended as first-line therapy for patients with moderate-to-severe AR, especially when nasal congestion is a major component of symptoms (CitationInternational Rhinitis Management Working Group 1994; CitationBousquet et al 2001; Citationvan Cauwenberge et al 2005; CitationAntonicelli et al 2007). INSs improve nasal congestion more effectively and are more cost-effective than nonsedating antihistamines, the most commonly prescribed AR medications (CitationCraig et al 1998; CitationSchoenwetter et al 2004; CitationPrice et al 2006). Oral antihistamines may be used concomitantly with INS in more severe cases, in rhinitis exacerbations, and in patients with ocular and skin symptoms that can occur, since atopic diseases are components of a systemic syndrome.
The major advantage of INS administration is that high concentrations of the drug, with rapid onset of action, can be delivered directly into the target organ, so that systemic effects are avoided or minimized. INS exert their anti-inflammatory effect through the inhibition of the production of many different cytokines, chemokines, enzymes, and cell adhesion molecules, after their interaction with intracellular glucocorticoid receptors.
To compare the efficacy and safety profile of different available INS for the treatment of AR, it is important to understand the different structures and their pharmacokinetic and pharmacodynamic properties (CitationCorren 1999; CitationHübner et al 2005). Pharmacokinetics are related to the concentration of a drug at the site of action over time, whereas pharmacodynamics relate to drug’s concentration to its clinical effect. To determine the overall effect of a drug over time, a combination of pharmacokinetics/pharmacodynamics parameters has to be accomplished (CitationHübner et al 2005).
Receptor potency is a pharmacodynamic parameter and represents the binding ability of INS that is expressed by its receptor affinity compared with dexamethasone. Topical potencies of glucocorticoids have been most often compared with use of the Mckenzie assay, which assesses skin-blanching responses as a measure of cutaneous vasoconstriction (CitationMcKenzie 1962). Another recent method for comparing the biologic effects of topical corticosteroids has been to evaluate the inhibitory effects of various compounds on the production of T lymphocyte-derived cytokines (CitationEnglish et al 1994; CitationUmland et al 1997).
Some important pharmacokinetic parameters are: prodrug design, organ deposition, onset of action, lipophilicity, bioavailability, systemic clearance, protein binding, volume of distribution, device of administration, and nasal residence time.
A Cochrane Systematic Review compared the efficacy and safety of fluticasone propionate (FP) with beclomethasone dipropionate and budesonide in the treatment of chronic asthma. FP-treated participants had slightly better lung function, but with increased hoarseness and, probably, with a higher risk of sore throat (CitationAdams et al 2007).
It is important to emphasize that decisions on the use of INS, especially in children, should be guided by the physician’s clinical experience and patients’ individual circumstances and preferences (CitationAl Sayyad et al 2007).
Fluticasone furoate
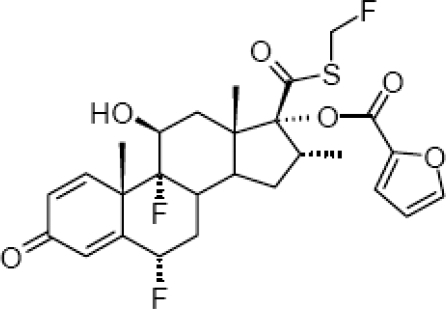
Fluticasone furoate (FF) is a new, topical, intranasal, enhanced-affinity trifluorinated glucocorticoid, with potent anti-inflamatory activity and low systemic exposure. FF is a synthetic fluorinated corticosteroid having the chemical name (6α,11β,16α,17α)-6,9-difluoro-17-{[(fluoro-methyl)thio]carbonyl}-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl 2-furancarboxylate (). The drug (GW685698X; Veramyst™; Avamys™) comes in a nasal spray, as an aqueous suspension of micronized fluticasone furoate for topical administration to the nasal mucosa by means of a metering, atomizing spray pump. Each actuation delivers 27.5 μg of FF in a volume of 50 μL of suspension that also contains 0.015% w/w benzalkonium chloride, dextrose anhydrous, edetate disodium, microcrystalline cellulose, carboxymethylcellulose sodium, polysorbate 80, and purified water.
It has been developed for the treatment of AR in patients 2 years of age and older and is administered via a unique, side-actuated device. FF is administered once daily and its recommended starting dose is 55 μg for children and 110 μg for adults and adolescents (CitationFDA 2007; CitationGlaxoSmithKline 2007; CitationMcCormack and Scott 2007; CitationRxList 2007).
Pharmacodynamic profile
Fluticasone furonate has high receptor affinity, with low equilibrium dissociation constant (kd = 0.3 nmol/L) and with greater relative receptor affinity (2989) than mometasone furoate (2244), fluticasone propionate (1775), beclomethasone-17-monopropionate (1345), ciclesonide active principle (1212), and budesonide (855) (CitationBiggadike et al 2007).
Some in vitro studies showed that FF displayed greater potency than other corticosteroids in inhibiting tumor necrosis factor synthesis and action. It was also more potent in preventing damage to cultured human lung epithelial cells by different stimulus. Experimental studies demonstrated more potent and faster anti-inflammatory activity of FF than fluticasone propionate (CitationSalter et al 2006, Citation2007).
FF displayed high selectivity for the glucocorticoid receptor in vitro and had no effect on the hypothalamic-pituitary-adrenal (HPA) axis in children or adults during clinical trials (CitationPastel et al 2007; CitationSalter et al 2007; CitationTripathy et al 2007). Laboratory tests that assess basal and dynamic function of HPA axis are frequently used to determine the systemic effects of INS.
Pharmakokinetic profile
After single- and multiple-dose intranasal administration, plasma fluticasone furoate concentrations are below the lower limit of quantification in most patients (CitationAllen et al 2007; CitationHughes et al 2007; CitationMartin 2007). One study showed that only 2% of samples from patients receiving 110 μg of FF had quantifiable plasma drug concentrations (CitationMartin 2007).
Systemic bioavailability is determined by the sum of 2 components, including the portion of the drug that is absorbed via the nasal mucosa plus the portion that is swallowed. The last one is the major route for circulation, what makes the first-pass hepatic metabolism after drug absorption in the gastrointestinal tract very important.
Intranasal FF 880 μg was administered every 8 hours for 10 doses in healthy adult volunteers and the average absolute bioavailability was 0.5%. Oral bioavailability after 2 mg single oral dose is 1.26% and elimination half-life after single intravenous dose is 15.1 hours (CitationFDA 2007; CitationAllen et al 2007).
FF was 99.4% bound to plasma protein in vitro and other research indicated extensive first-pass metabolism of the absorbed drug (CitationSalter et al 2006; CitationAllen et al 2007). Protein binding is highly relevant because only the unbound free drug can exert an effect at the receptor site. As long as the corticosteroid is bound to a protein, it is unable to bind to its receptor. Clearance of FF is primarily by hydrolysis in the liver by the cytochrome P450 isozyme (CYP) 3A4 that converts the drug to the 17[beta]-carboxylic acid metabolite (M10), which displays low glucocorticoid receptor agonist potency. The drug is excreted mainly in the feces, with only minor amounts in the urine (Hughes et al 2005; CitationFDA 2007).
FF is a synthetic, lipophilic, corticosteroid (Biggadike et al 2006). Agents highly lipophilic will demonstrate a higher and faster rate of uptake by the nasal mucous membrane, a higher level of retention within the nasal tissue, and an enhanced ability to reach the glucocorticoid receptor.
It has become widely recognized that many patients use INS on an as-need basis only, stopping medication when symptoms substantially abate. In support of this approach are recent studies demonstrating that intermittent use of INS is moderately effective in many patients (CitationJuniper et al 1993). Therefore, onset of action can become an important feature of these drugs. In a perennial AR clinical trial, a statistically significant difference between FF and placebo was first noted at 24 hours after the first dose for instantaneous total nasal symptom score and after 2 days for reflective total nasal symptom score (CitationVasar et al 2007).
Drug formulation and delivery device
Drug’s formulation and delivery device may affect the efficacy, tolerability, drug retention and deposition in nasal tissue, safety and patient preference and adherence to treatment (CitationHübner et al 2005; CitationMeltzer 2007). Optimization of formulation is one way to improve rhinitis treatment.
Additives and preservatives are included in INS formulations to prevent bacterial growth, confer both taste and smell, absorb extra water, and maintain appropriate moisture levels. Some of these agents may irritate or dry nasal tissue and/or, rarely, lead to hypersensitivity. There is benzalkonium chloride, polysorbate, and carboxymethylcellulose in the FF formulation.
Benzalkonium chloride is a cationic surfactant used as a preservative in nasal solutions. Studies have showed that it can induce nasal mucociliary dysfunction, nasal irritation and hypersecretion, burning sensation, degenerative changes in supportive and olfactory cells, and squamous cell metaplasia (CitationSteinsvag et al 1996; CitationMcMahon et al 1997; CitationHofmann et al 2004; CitationMeltzer 2007). However, the clinical impact of these effects on the nasal mucosa is unclear (CitationBraat et al 1995; CitationBernstein 2000; CitationMarple et al 2004; CitationVerret and Marple 2005). Perhaps the nasal toxicity of benzalkonium chloride could be neutralized by nasal secretions and corticosteroids actions (CitationRiechelmann et al 2004).
The polysorbates are nonionic surfactants and emulsifying agents used as additives in drugs, food, shampoo, and lotions. Polysorbate 80 reversibly inhibited ciliary beat frequency in cultured human nasal epithelial cells and has been associated with allergy or sensitivity (CitationShelley et al 1995; CitationDimova et al 2003).
Carboxymethylcellulose is a thixotropic agent that increases nasal drug concentration, but also confers viscosity to INS solution, which is one of the reasons why the suspension must be shaken before use (CitationMeltzer 2007). It exerts a drying effect on the nasal mucosa that may contribute to the incidence of epistaxis and it also has been involved in rare cases of allergic anaphylactic reactions (CitationPatterson et al 1995; CitationOppliger et al 2004).
Sensory attributes are an important factor in patient preference and adherence to INS treatment. Patients consider several sensory attributes during INS therapy: aftertaste, taste, smell, run out of nose, throat rundown (drip down), irritation, and urge to sneeze (CitationMahadevia et al 2004; CitationMeltzer et al 2005; CitationMeltzer 2007); sensation of moisture and soothing have been reported as good attributes. A study showed that benzalkonium chloride has a bitter taste that can be unpleasant (CitationMahadevia et al 2004).
The FF delivery device is an easy-to-use aqueous pump spray that presents low risk for nasal tissue damage and with a new trigger mechanism that minimizes potential variation in the dose delivered (CitationFDA 2007; CitationBerger et al 2007). The device delivers a low spray volume, which minimizes the amount of drug available to run down the back of the throat or leak out the nose. It is suitable for use in young children aged 2 years and in the elderly.
Clinical trials
Therapeutic efficacy of fluticasone furonate in AR has been proven by double-blind, placebo-controlled, clinical trials that can be differentiated according to drug dosage, duration of treatment, age of patients, type of rhinitis, and end-points ().
Table 1 Double-blind, placebo-controlled, clinical trials of fluticasone furonate in the treatment of allergic rhinitis
A dose-ranging study in adolescents and adults with seasonal allergy to mountain cedar pollen established that the 110 μg dose provided the optimal benefit-risk ratio. The significant reduction in morning, predose, instantaneous total nasal symptom score (iTNSS) indicated, at least, 24-hour duration of efficacy (CitationMartin et al 2007).
FF was also superior to placebo for reductions in ocular symptoms of adults and adolescents suffering from seasonal and perennial AR (CitationStuebner 2006; CitationFokkens et al 2007a; CitationHampel et al 2007; CitationKaiser et al 2007; CitationRatner et al 2007; CitationVasar et al 2007). The mechanism by which it alleviates allergic conjunctivitis has yet to be fully elucidated. Possible mechanisms include: reduced nasal inflammation resulting in reduced release of inflammatory mediators and, hence, less activation of inflammatory cells in the neighbouring tissues; improved drainage away from the eye down the nasolacrimal duct; and modulation of a naso-ocular neurogenic reflex. It is unlikely that the observed effect results from systemic action of FF, since it has a low absolute bioavailability.
Oral antihistamines may be used concomitantly with INSs in patients for whom ocular symptoms are troublesome. However, in a meta-analysis of studies comparing INS with antihistamines, INS treatment was shown to reduce ocular symptoms as effectively as oral antihistamines (CitationWeiner et al 1998).
Safety and tolerability
The severe adverse effects of chronic therapy with systemic corticosteroids are well documented. INS, at recommended doses, are generally not associated with long-term, clinically significant, or irreversible adverse effects. However, many physicians and patients are still concerned about the potential adverse effects of these drugs and these feelings can reduce medication adherence, which is one of the biggest challenges that physicians tackle on a daily basis. If the health care provider can effectively communicate and convince the patient of the benefit/risk ratio of steroids, patient outcomes can be improved (CitationRao and Apter 2005).
In a pooled analysis of clinical trials, the overall incidence of adverse events with intranasal fluticasone furoate was similar to that with placebo, as was rate of withdrawal from therapy. The most common adverse events (incidence >1% in adolescents/adults or >3% in children, and with a higher frequency than placebo) were: headache, epistaxis, nasopharyngitis, pyrexia, pharyngolaryngeal pain, nasal ulceration, cough, and back pain (CitationFDA 2007).
Treatment of adults and adolescents with FF for the long term (12 months) was likewise well tolerated, with no unusual or unexpected events. Epistaxis was the only adverse event occurring more frequently and with more severity among FF recipients (CitationFDA 2007; CitationRosenblut et al 2007). There was no evidence during long-term therapy of adverse events suggestive of clinically relevant systemic corticosteroid exposure.
There is consistent evidence that INS therapy in children can reduce short-term growth and growth velocity, especially during the first year of treatment. However, studies suggest that usual doses of these drugs do not cause clinically relevant growth suppression or reduced final height in the overall majority of patients (CitationBrand 2001; CitationGulliver and Eid 2005). INS can reduce growth only after they become available systemically. FF systemic bioavailability is low and it had no effect on lower-leg growth rate assessed by knemometry in children (CitationGradman et al 2007).
Caution is necessary if co-administered with potent CYP3A4 inhibitors, such as ketoconazole and ritonavir, since the increased exposure to FF may increase the risk of systemic adverse effects (FDA).
There is low potential risk for systemic effects at recommended doses of INS. When higher doses are administered, the physician should weight the benefits against the risks and consider the morbidity of uncontrolled rhinitis. To reduce any potential risk for systemic effects, the lowest effective dose of INS should be used.
Conclusion
The role of INS in the treatment of AR is well established. They are proven to be efficacious and are recommended as first-line therapy for individuals with persistent moderate/severe rhinitis.
Knowledge about INS has increased tremendously over the last decade. Studies have elucidated mechanisms of action, pharmacologic properties, and the clinical impact of these drugs in allergic respiratory diseases. Although the existing ICS are already highly efficient, the introduction of further improved formulations with a better efficacy/safety profile is always desired.
FF nasal spray is a new topical corticosteroid, with enhanced-affinity and a unique side-actuated delivery device, which is effective in improving nasal symptoms of AR. Significant improvement in ocular symptoms and in quality of life was also demonstrated. Its low oral bioavailability and high plasma protein binding minimize systemic adverse effects. A potentially prolonged nasal retention time may further enhance the efficacy of FF, which may allow for a once-daily dosing regimen in adults, adolescents, and children.
FF with high topical potency and low potential for systemic effects is a good candidate for rhinitis treatment. As expected for all new drugs, long-term safety and efficacy studies are required, which can establish the potential modification of AR course.
References
- AdamsNLassersonTJCatesCJFluticasone versus beclomethasone or budesonide for chronic asthma in adults and childrenCochrane Database Syst Rev20074CD00231017943772
- Al SayyadJJFedorowiczZAlhashimiDTopical nasal steroids for intermittent and persistent alergic rhinitis in childrenCochrane Database Syst Rev20071CD00316317253485
- AllenADownGNewlandsATolerability, safety, pharmacokinetics and bioavailability of the novel intranasal corticosteroid fluticasone furoate in healthy subjects [abstract no P210]Ann Allergy Asthma Immunol200798A89
- AntonicelliLMicucciCVoltoliniSRelationship between ARIA classification and drug treatment in allergic rhinitis and asthmaAllergy20076210647017686109
- BergerWGodfreyJWGrantACFluticasone furoate (FF) nasal spray: development of a next-generation delivery system for allergic rhinitis [abstract no 907]J Allergy Clin Immunol2007119S231
- BernsteinILIs the use of benzalkonium chloride as a preservative for nasal formulations a safety concern? A cautinary note based on compromised mucociliary transportJ Allergy Clin Immunol2000105394410629450
- BiggadikeKBledsoeRHassellAFluticasone furoate (FF): interactions with the glucocorticoid receptor [abstract no P219]Ann Allergy Asthma Immunol200798A912
- BousquetJVan CauwenbergePKhaltaevNARIA Workshop Group. Allergic rhinitis and its impact on asthmaJ Allergy Clin Immunol2001108S133311449199
- BraatJAingeGBowlesJAThe lack of effect of benzalkonium chloride on the cilia of the nasal mucosa in patients with perennial allergic rhinitis: a combined functional, light, scanning and transmission electron microscopy studyClin Exp Allergy199525957658556567
- BrandPLPInhaled corticosteroids reduce growth. Or do they?Eur Respir J2001172879411334133
- CorrenJIntranasal corticosteroids for allergic rhinitis: How do different agents compare?J Allergy Clin Immunol1999104S144910518811
- CraigTJTeetsSLehmanEBNasal congestion secondary to allergic rhinitis as a cause of sleep disturbance and daytime fatigue and the response to topical nasal corticosteroidsJ Allergy Clin Immunol199810163379600500
- Crystal-PetersJCrownWHGoetzelRZThe cost of productivity losses associated with allergic rhinitisAm J Manag Care20006373810977437
- Crystal-PetersJNeslusanCCrownWHTreating allergic rhinitis in patients with comorbid asthma: The risk of asthma-related hospitalizations and emergency department visitsJ Allergy Clin Immunol2002109576211799366
- DimovaSMugabowindekweRWillemsTSafety-assessment of 3-methoxyquercetin as an anthirhinoviral compund for nasal application: effect on ciliary beat frequencyInt J Pharm20032639510312954184
- EnglishAFNeateMSQuintDJBiological activities of some corticosteroids used in asthmaAm J Respir Crit Care Med1994149A212
- FDAVeramyst™ (fluticasone furoate) nasal spray: US prescribing information [online] Accessed 2007 Dec 17. URL: http://www.fda.gov/cder/
- FokkensWJJogiRReinartzSOnce daily fluticasone furoate nasal spray is effective in seasonal allergic rhinitis caused by grass pollenAllergy2007a6210788417686111
- FokkensWJoggiRSidorenkoIFluticasone furoate nasal spray (FFNS) 110 mcg once-daily is effective in seasonal allergic rhinitis (SAR) caused by grass pollen [abstract no. 352]2007bXXVI Congress of the European Academy of Allergology and Clinical ImmunologyJun 9-13, 2007Göteborg
- GivenJTolerTEllsworthAOnce-daily fluticasone furoate nasal spray (FFNS) 110 mcg improves quality of life (QoL) in subjects with seasonal allergic rhinitis (SAR) during the ragweed season [abstract no 1188]J Allergy Clin Immunol2007119S304
- GlaxoSmithKline.Veramyst™ Accessed 2007 Dec 17. URL: http://www.gsk.com/
- GradmanJCaldwellMWolthersOKnemometric assessment of short-term lower-leg growth in children with allergic rhinitis (AR) treated with fluticasone furoate (FF) nasal spray [abstract no 1187]J Allergy Clin Immunol2007119S304
- GulliverTEidNEffects of glucocorticoids on the hypothalamic-pituitary-adrenal axis in children and adultsImmunol Allergy Clin N Am20052554155
- HampelFCJrJacobsRMartinBOnce-daily fluticasone furoate nasal spray (FF) provides 24-hour symptom relief in subjects with seasonal allergic rhinitis (SAR) caused by mountain cedar pollen [abstract no 1190]J Allergy Clin Immunol2007119S3045
- HofmannTGugatschgaMKoidlBInfluence of preservatives and topical steroids on ciliary beat frequency in vitroArch Otolaryngol Head Neck Surg2004130440515096427
- HübnerMHochhausGDerendorfHComparative pharmacology, bioavailability, pharmacokinetics, and pharmacodynamics of inhaled glucocorticosteroidsImmunol Allergy Clin N Am20052546988
- HughesSShardlowPRousellVDisposition and metabolism of a novel enhanced affinity glucocorticoid, [14C]-fluticasone furoate (FF) after oral and intravenous (IV) administration in healthy male subjects [abstract no P216]Ann Allergy Asthma Immunol200798A901
- International Rhinitis Management Working GroupInternational Consensus Report on the diagnosis and management of rhinitisAllergy199449134
- JuniperEFGuyattGHArcherBAqueous beclomethasone dipropionate in the treatment of ragweed pollen-induced rhinitis: further exploration of “as needed” useJ Allergy Clin Immunol19939266728335858
- KaiserHBNaclerioRMGivenJFluticasone furoate nasal spray: a single treatment option for the symptoms of seasonal allergic rhinitisJ Allergy Clin Immunol20071191430717418384
- LeynaertBNeukirchCLiardRQuality of life in allergic rhinitis and asthma: a population based study of young adultsAm J Respir Crit Care Med2000a1621391611029350
- LeynaertBNeukirchFDemolyPEpidemiological evidence for asthma and rhinitis comorbidityJ Allergy Clin Immunol2000b106S201511080732
- LinnebergAHenrik NielsenNFrolundLCopenhagen Allergy Study. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy StudyAllergy20025710485212359002
- MahadeviaPJShahSLeibmanCPatient preferences for sensory attributes of intranasal corticosteroids and willingness to adhere to prescribed therapy for allergic rhinitis: a conjoint analysisAnn Allergy Asthma Immunol2004933455015521370
- MarpleBRolanfPBenningerMSafety review of benzalkonium chloride used as a preservative in intranasal solutions: an overview of conflicting data and opinionsOtolaryngol Head Neck Surg20041301314114726922
- MartinBGRatnerPHHampelFCOptimal dose selection of fluticasone furoate nasal spray for the treatment of seasonal allergic rhinitis in adults and adolescentsAllergy Asthma Proc2007282162517479608
- MasperoJFRosenblutAFinnAJrOnce-daily fluticasone furoate nasal spray (FF) is safe and effective in the long-term treatment of perennial allergic rhinitis (PAR) in children ages 2 to 11 years [abstract no 1189]J Allergy Clin Immunol2007119S304
- McCormackPLScottLJFluticasone furoate: intranasal use in allergic rhinitisDrugs20076719051517722960
- McKenzieAWPercutaneous absorption of steroidsArch Dermatol1962866114
- McMahonCDarbyYRyanRImmediate and short-term effects of benzalkonium chloride on the human nasal mucosa in vivoClin Otolaryngol Allied Sci199722318229298605
- MeltzerEOBardelasJGoldsobelAA preference evaluation study comparing the sensory attributes of mometasone furoate and fluticasone propionate nasal sprays by patients with allergic rhinitisTreat Respir Med200542899616086602
- MeltzerEOFormulation considerations of intranasal corticosteroids for the treatment of allergic rhinitisAnn Allergy Asthma Immunol200798122117225715
- MeltzerEOTripathyILeeJOnce-daily fluticasone furoate nasal spray (FF) provides 24-hour relief of the nasal symptoms of seasonal allergic rhinitis (SAR) in children ages 2-11 years [abstract no 1193]J Allergy Clin Immunol2007a119S305
- MeltzerEOLeeJTripathyIStudy to assess the efficacy and safety of two doses of fluticasone furoate nasal spray in children with seasonal allergic rhinitis [abstract no 343]2007bXXVI Congress of the European Academy of Allergology and Clinical Immunology2007 Jun 9-13Göteborg
- NathanRBergerWYangWOnce daily fluticasone furoate nasal spray (FFNS), a novel enhanced affinity steroid, provides 24-hour relief for the nasal symptoms of perennial allergic rhinitis (PAR) [abstract no 254]J Allergy Clin Immunol2007119S65
- O’ConnellEJThe burden of atopy and asthma in childrenAllergy20045971115245350
- OppligerRHauserCAnaphylaxis after injection of corticosteroid preparations-carboxymethylcellulose as a hidden allergen [in German]J Dtsch Dermatol Ges200429283016281611
- PastelDRatnerPClementsDLack of efect on hypothalamic-pituitary-adrenal (HPA) axis function by once-daily fluticasone furoate nasal spray (FFNS) 110 mcg in adolescents and adults with perennial allergic rhinitis [abstract no 909]J Allergy Clin Immunol2007119S305
- PattersonDLYungingerJWDunnWFAnaohykaxis induced by carboxymethylcellulose component of injectable triamcinolone acetonide suspension (Kenalog)Ann Allergy Asthma Immunol19957416367697477
- PriceDBondCBouchardJInternational Primary Care Respiratory Group (IPCRG) Guidelines: management of allergic rhinitisPrim Care Respir J200615587016701759
- RaoVUApterAJSteroid phobia and adherence-problems, solutions, impact on benefit/risk profileImmunol Allergy Clin N Am20052558195
- RatnerPAndrewsCvan BavelJOnce-daily fluticasone furoate nasal spray (FF) effectively treats ocular symptoms of seasonal allergic rhinitis (SAR) caused by mountain cedar pollen [abstract no 908]J Allergy Clin Immunol2007119S231
- RiechelmannHDeutschleTStuhlmillerANasal toxicity of benzalkonium chlorideAm J Rhinol200418291915586800
- RosenblutABardinPGMullerBLong-term safety of fluticasone furoate nasal spray in adults and adolescents with perennial allergic rhinitisAllergy2007621071717686110
- RxListThe internet drug index Veramyst™ Accessed 2007 Dec 17. URL: http://www.rxlist.com/cgi/generic/veramyst/
- SalterMBiggadikeKClackersMGW685698X – enhanced affinity for the glucocorticoid receptor: cellular and in nvivo pharmacology [abstract no 781]200625th Congress of the European Academy of Allergology and Clinical Immunology2006, Jun 10-14Vienna
- SalterMBiggadikeKClackersMFluticasone furoate (FF): enhanced cellular and tissue protection with a new selective glucocorticoid agonist [abstract no P212]Ann Allergy Asthma Immunol200798A89
- SchoenwetterWFDupclayLJrAppajosyulaSEconomic impact and quality-of-life burden of allergic rhinitisCurr Med Res Opin2004203051715025839
- ShelleyWBTalaninNShelleyEDPolysorbate 80 hypersensitivityLancet19953451312137746084
- StanfordRPhilpotEFarisMFluticasone furoate nasal spray once-daily improves quality of life in subjects with seasonal allergic rhinitis (SAR) [abstract no P214]Ann Allergy Asthma Immunol2007a98A90
- StanfordRPhilpotEFarisMFluticasone furoate nasal spray once-daily improves nocturnal quality of life in subjects with seasonal allergic rhinitis (SAR) [abstract no P208]Ann Allergy Asthma Immunol2007b98A889
- SteinsvagSKBjerknesRBergOHEffects of topical nasal steroids on human respiratory mucosa and human granulocytes in vitroActa Otolaryngol1996116868758973724
- StuebnerPEffects of the novel intranasal glucocorticosteroid GW685698 (200 mcg once-daily) on seasonal allergic rhinitis (SAR) symptoms induced in the Vienna challenge chamber model (VCC) [abstract no 1232]J Allergy Clin Immunol2006117S319
- TaramarcazPGibsonPGIntranasal corticosteroids for asthma control in people with coexisting asthma and rhinitisCochrane Database Syst Rev20033CD00357014583983
- The International Study of Asthma and Allergies I Childhood (ISAAC) Steering committeeWorldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAACLancet19983511225329643741
- TogiasARhinitis and asthma: evidence for respiratory system integrationJ Allergy Clin Immunol200311111718312789212
- TripathyISterlingRClementsDLack of efect on hypothalamic-pituitary-adrenal (HPA) axis function by once-daily fluticasone furoate nasal spray (FFNS) 110 mcg in children with perennial allergic rhinitis [abstract no. 909]J Allergy Clin Immunol2007119S232
- UmlandSPNarhebneDKRazacBSThe inhibitory effects of topically active glucocorticoids on IL-4, IL-5, and interferon-[gama] production by cultured primary CD4+ cellsJ Allergy Clin Immunol199710051199338546
- UptonMNMcConnachieAMcsharryCIntergeneration 20 year trends in the prevalence of asthma and hay fever in adults: the Midspan family study surveys of parents and offspringBr Med J2000321889210884260
- van BavelJTolerTEllsworthAOnce-daily fluticasone furoate nasal spray (FFNS) improves quality of life (QoL) in subjects with seasonal allergic rhinitis (SAR) during the mountatin cedar pollen season [abstract no 903]J Allergy Clin Immunol2007119S230
- van CauwenbergePVan HoeckeHVandenbulckeLGlucocorticosteroids in allergic inflammation: clinical benefits in allergic rhinitis, rhinosinusitis, and otitis mediaImmunol Allergy Clin N Am200525489509
- VasarMHouleP-ADouglassJAA novel enhanced-affinity corticosteroid, once daily fluticasone furoate nasal spray (FFNS), provides 24-hour relief for the nasal symptoms of perennial allergic rhinitis (PAR) [abstract no 626]2007XXVI Congress of the European Academy of Allergology and Clinical Immunology 2007Jun 9-13Göteborg
- VerretDJMarpleBFEffect of topical nasal steroid sprays on nasal mucosa and ciliary functionCurr Opin Otolaryngol Head Neck Surg200513141815654209
- WeinerJMAbramsonMJPuyRMIntranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trialsBr Med J1998317162499848901
- YawnBPYungingerJWWollanPCAllergic rhinitis in Rochester, Minnesota residents with asthma: frequency and impact on health care chargesJ Allergy Clin Immunol19991035499893185