Abstract
Objectives:
The significance of early ischemic changes (EIC) on computed tomography (CT) within 3 hours after stroke onset remains controversial. The semi-quantitative Alberta Stroke Program Early CT Score (ASPECTS) is found to have prognostic value in early stroke. This study assesses factors associated with the presence of EIC and the relation between EIC and clinical outcome.
Materials and methods:
CT scans from 61 consecutive patients receiving thrombolytic therapy were reviewed by 3 experienced stroke neurologists, assessing EIC (ASPECTS) and vascular signs (hyperdense middle cerebral artery stem and/or branches). Short-term outcome was assessed with the National Institute of Health Stroke Scale at 24 hours and long-term outcome with the modified Rankin Scale score after 3 months.
Results:
The prevalence of EIC was 54% and the agreement between assessors was good (kappa 0.52–0.67). EIC was independently associated with younger age and absence of diabetes mellitus. Neither EIC nor vascular signs were associated with 3-months outcome.
Conclusions:
ASPECTS is as simple, systematic approach to assessing EIC, and the inter-observer agreement is good. Patient age and diabetes mellitus influence the presence of EIC.
Introduction
Intravenous recombinant tissue plasminogen activator (tPA) improves outcome after acute ischemic stroke (CitationNINDS 1995; CitationHacke et al 2004). Outcome is influenced by a number of factors like age, treatment delay, diabetes mellitus, hypertension, and possibly also early ischemic changes (EIC) on baseline computed tomography (CT). EIC may represent cytotoxic edema and irreversible injury (Citationdel Zoppo et al 1998) and are associated with unfavorable outcome (CitationBarber et al 2000). It has been suggested that early major ischemic changes on CT should preclude use of tPA (Citationvon Kummer 2003), but controversies on this topic remain (CitationLyden 2003; CitationDemchuk et al 2005). Appropriate patient selection for thrombolysis is important and needs to be refined. The Alberta Stroke Program Early CT Score (ASPECTS) is a reproducible grading system and a potentially valuable tool for studying early ischemic changes (CitationBarber et al 2000; CitationCoutts et al 2004a). The aim of this study was to assess factors associated with the presence of early ischemic changes and their relation to good clinical outcome, and to assess inter-observer agreement among experienced stroke neurologists using the ASPECTS system.
Materials and methods
From May 1998 to October 2003, 67 patients with acute ischemic stroke affecting the middle cerebral artery (MCA) territory received thrombolytic treatment with 0.9 mg/kg recombinant tissue plasminogen activator (tPA) (alteplase; Actilyse®, Boehringer Ingelheim) in accordance with the National Institute of Neurological Disorders and Stroke (NINDS) tPA Stroke Study protocol (CitationNINDS 1995) and the European Stroke Initiative (EUSI) guidelines (CitationHacke et al 2003). The diagnosis MCA stroke was determined on clinical ground and later verified by CT in all cases.
Computed tomographic (CT) scans (Philips Tomoscan, General Electric Highspeed, or General Electric Lightspeed plus) were done at baseline before treatment. CT scans were acquired using 5-mm slices of the basal parts of the brain and 10-mm slices otherwise. The CT scans were assessed retrospectively on plain film image by 3 senior consultant stroke neurologists with more than 10 years of experience from both European Co-operative Acute Stroke Studies (ECASS and ECASS II), including ECASS CT reading training sessions (Citationvon Kummer 1998). The assessors had knowledge of the affected side, but not of the stroke severity. The assessment was first performed separately and thereafter using a consensus approach. Early ischemic changes were assessed using the ASPECT score (CitationBarber et al 2000). The affected MCA territory is allotted 10 points, and 1 point is subtracted for an area of ischemic changes, ie, loss of grey-white discrimination and/or focal edema (effacement of cortical sulci or reduced space of the sylvian fissure), or hypoattenuation. An ASPECT score of 10 points indicates a normal CT scan, whereas a score of 0 points indicates extensive MCA ischemia. The ASPECT score was calculated from two standard axial cuts, one at the level of the thalamus and the basal ganglia, and one just rostral to the ganglionic structures. We recorded any early ischemic sign and did not differentiate between “subtle ischemic changes” (focal swelling) and obvious hypodensity (CitationLyden 2003; CitationButcher et al 2007). We chose a cut-off for ASPECTS of 7 based on prior studies. The presence of a pathologic vascular condition, ie, a hyperdense proximal MCA (HMCA) sign and/or a hyperdense distal MCA “dot” (MCAD) sign in the sylvian fissure (CitationBarber et al 2001), was recorded.
The baseline and 24 h stroke severity was graded using the National Institute of Health Stroke Scale (NIHSS). Long term outcome was assessed by a structured telephone interview with the patients or their caregivers after 3 months using the modified Rankin Scale (mRS; grade 0–6). All interviews were performed by the same stroke nurse, blinded to the ASPECT score and clinical data. Favorable outcome was defined as mRS grade 0–2, ie, independence. If there was uncertainty about the mRS outcome group, the patient was placed in the less favorable of the two possible groups.
Statistical analysis
Pairwise inter-observer agreement was measured by kappa statistics. Short-term outcome (24 h after stroke onset) was analyzed based on linear regression, with NIHSS score after 24 h as the dependent variable. Chi-square test, Mann-Whitney test and Fisher’s exact test were performed when appropriate. Variables possibly associated with the consensus ASPECT score were evaluated by linear regression analysis using the consensus ASPECT score as the dependent variable. The analysis was performed with Statistical Package for the Social Science 11.0.1 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Six (9%) out of 67 patients were excluded from the study due to poor quality CT scans which did not allow an exact assessment. The ASPECT score was obtained in the remaining 61 patients based on CT scans performed within three hours (mean 104 minutes) of stroke onset. Patient demographics are shown in for patients with ASPECT score ≤7 versus >7.
Table 1 Demographics of ischemic stroke patients with ASPECTS scores ≤7 and >7 based on initial CT before receiving thrombolysis
shows the results of the linear regression analysis with the ASPECT score as the dependent variable. The linear regression analysis showed that higher ASPECT score was independently associated with old age (P = 0.004), low NIHSS score on admission (P = 0.006), presence of diabetes mellitus (P = 0.018), and short time from stroke onset to CT (P = 0.044).
Table 2 Linear regression analysis with the ASPECTS score as the dependent variable
Unfavorable short-term outcome (based on the NIHSS score 24 hours after stroke onset) was independently associated with low ASPECTS score (partial correlation = −0.367, P = 0.005) and diabetes mellitus (partial correlation = 0.267, P = 0.043). Long-term unfavorable functional outcome (mRS ≥ 3) at 3 months was associated with high NIHSS score on admittance (P < 0.001, Mann-Whitney test). Any early ischemic sign (ie, ASPECTS <10 vs. 10) was not associated with unfavorable functional outcome at 3 months (P = 0.56, Fisher’s exact test), neither was HMCA sign (P = 0.37) nor MCAD sign (P = 1.00).
Six (9%) patients suffered cerebral hemorrhagic complications, of which two (3%) were symptomatic. There was no association between ASPECTS and hemorrhage.
The kappa (κ) statistics between 3 neurologists as to ASPECT score >7 or ≤7 ranged pair-wise from 0.52 to 0.67 (P < 0.001). Based on the consensus score, the prevalence of early ischemic changes was 54%. Fourteen (23%) patients had ASPECT score ≤7 and 47 (77%) patients ASPECTS score >7. shows the distribution of the ASPECT score.
Figure 1 The distribution of ASPECT scores on initial CT scan within 3 hours after stroke onset in 61 ischemic stroke patients before undergoing thrombolysis.
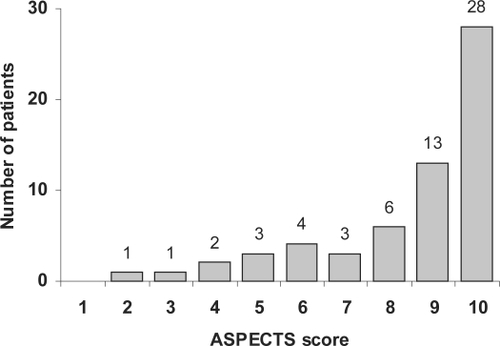
The prevalence of vascular pathology signs was 43%, 18% had a hyperdense proximal MCA (HMCA) sign, 18% a hyperdense distal MCA “dot” (MCAD) sign, and 7% had both. The κ-statistics between 3 neurologists as to HMCA sign ranged pair-wise from 0.44 to 0.70 (P = 0.001). The corresponding κ-statistics as to MCAD sign ranged from 0.35 to 0.61 (P = 0.005).
Discussion
Early ischemic changes (EIC) on CT within 3 hours were independently associated with younger age and absence of diabetes mellitus in this study (). It is conceivable that cerebral pathology such as leukoaraiosis and atrophy associated with diabetes and old age makes it difficult to detect minor EIC, leaving younger patients with an apparently higher prevalence of ischemic changes. It is possible that younger patients show a better grey – white distinction in normal brain tissue making EIC easier to detect. The higher prevalence of EIC among younger patients may cause underutilization of thrombolysis in this age group. Supporting our findings, a relationship between younger age and CT changes has been found also in a small study assessing CT scans within 12 hours after stroke onset (CitationJaillard et al 2002). By contrast, in the NINDS rtPA Stroke Study, age was not correlated with ASPECTS (CitationDemchuk et al 2005).
Based on the ECASS trials many centers exclude patients with early ischemic changes exceeding 1/3 third of the MCA area (Citationvon Kummer 2003), even though this was not shown to impact outcome in the NINDS trial or the Australian Streptokinase Trial (CitationGilligan et al 2002). Although we did not assess this question, our findings suggest that the >1/3 exclusion criteria might lead to a relatively more frequent exclusion of younger than older ischemic stroke patients. This selection bias may have contributed to the reported better outcome of younger ischemic stroke patients treated with tPA compared with older patients.
We found that short time from stroke onset to CT, and low NIHSS score on admittance were independently associated with high ASPECTS in a three hour time window (). These results were as expected.
The prevalence of EIC in this study was 54%. This is comparable with other studies reporting a prevalence of EIC between 41% and 77% within the 3-hour time window (CitationGrotta et al 1999; CitationBarber et al 2000; CitationBarber et al 2001; CitationDemchuk et al 2005). One reason for the varying prevalence could be technical, as newer generation CT scanners with improved tissue contrast and spatial resolution may show more EIC than older ones. Another reason may be differences in time delay until baseline CT, as EIC become increasingly visible over time even within the 3-hour time window (CitationPatel et al 2001). A possible reason may also be that there is a general lack of definitions for EIC (CitationWardlaw and Mielke 2005).
Although high ASPECT score was independently associated with favorable short-term prognosis at 24 h, we did not find any association between EIC and long-term outcome at three months. One possible explanation for this discrepancy may be that some EIC are reversible (CitationJaillard et al 2002). Subtle changes like focal swelling may reflect mild reversible tissue edema (CitationLyden 2003; CitationButcher et al 2007) or vasodilatation (CitationJaillard et al 2002) and may thus correlate more to short-term neurological dysfunction than to neuronal injury and long-term prognosis. More obvious hypodensity may reflect irreversibly damaged ischemic brain tissue and may thereby have long-term prognostic value. We also found no association between vascular signs (HMCAS, MCADS) and outcome at 3 months. This is in accordance with earlier data (CitationBarber et al 2001). The lack of association between ASPECTS and prognosis in this study is in accordance with earlier studies on EIC (CitationPatel et al 2001; CitationGilligan et al 2002; CitationDzialowski et al 2006) but in contrast to other studies of ASPECTS and tPA treatment within the 3 hour time window (CitationBarber et al 2000; CitationCoutts et al 2004b; CitationDemchuk et al 2005). Exclusion of patients for thrombolysis based on ASPECT score or vascular signs within the first three hours after stroke onset, is therefore not supported by our study. Caution in the use of ASPECTS at predicting the outcome of individual patients is warranted (CitationWeir et al 2006). Clear definitions of EIC with differentiation between subtle changes and obvious hypodensity and a well-defined time axis may improve the prognostic accuracy of the ASPECT score in future studies.
In this study the κ statistics for agreement between 3 neurologists was good (0.52–0.67). This is compatible with κ values for inter-observer agreement in other studies (κ 0.20–0.88) assessing early CT changes within 3 hours after stroke onset (CitationGrotta et al 1999; CitationBarber et al 2001; CitationFiebach et al 2002). In a systematic review of 15 studies of observer reliability (CitationWardlaw and Mielke 2005) the range of κ values was 0.14–0.78 for any early sign of infarction within 6 hours after stroke onset. The highest levels of agreement were those for the HMCAS, for which the κ values ranged from 0.36 to 1.00. This was also found in our study (κ 0.44–0.70). The ECASS trial investigators (Citationvon Kummer 1998) and others (CitationWardlaw and Mielke 2005) have shown that training results in a significant improvement in the detection of early ischemic changes. The good inter-observer agreement among trained assessors in this study supports the value of systematic training. The assessors had knowledge of the affected side, which might influence the results. However, in previous studies this knowledge did not change inter-observer agreement significantly (CitationTomsick et al 1990; Citationvon Kummer et al 1996; CitationWardlaw et al 1999).
In conclusion, this study shows that the ASPECTS is a systematic, robust, and practical method for evaluating early ischemic changes on CT scans when performed by experienced neurologists. High ASPECT score was associated with favorable very short-term outcome, but this association disappeared on 3 months follow-up in patients treated with intravenous tPA. A relationship between younger age and CT changes exists and a lower ASPECT score among younger patients may cause underutilization of thrombolysis in this age group.
Acknowledgements
This work was supported by the Frank Mohn Foundation, Bergen, Norway. The authors report no conflicts of interest.
References
- BarberPADemchukAMHudonME2001Hyperdense sylvian fissure MCA “dot” sign: A CT marker of acute ischemiaStroke3284811136919
- BarberPADemchukAMZhangJ2000Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT ScoreLancet3551670410905241
- ButcherKSLeeSBParsonsMW2007Differential prognosis of isolated cortical swelling and hypoattenuation on CT in acute strokeStroke38941717272776
- CouttsSBDemchukAMBarberPA2004aInterobserver variation of ASPECTS in real timeStroke35e103515073381
- CouttsSBLevMHEliasziwM2004bASPECTS on CTA source images versus unenhanced CT: added value in predicting final infarct extent and clinical outcomeStroke352472615486327
- del ZoppoGJvon KummerRHamannGF1998Ischaemic damage of brain microvessels: inherent risks for thrombolytic treatment in strokeJ Neurol Neurosurg Psychiatry65199667553
- DemchukAMHillMDBarberPA2005Importance of early ischemic computed tomography changes using ASPECTS in NINDS rtPA Stroke StudyStroke3621101516166579
- DzialowskiIHillMDCouttsSB2006Extent of early ischemic changes on computed tomography (CT) before thrombolysis: Prognostic value of the Alberta Stroke Program Early CT Score in ECASS IIStroke3797397816497977
- FiebachJBSchellingerPDJansenO2002CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic strokeStroke3322061012215588
- GilliganAKMarkusRReadS2002Baseline blood pressure but not early computed tomography changes predicts major hemorrhage after streptokinase in acute ischemic strokeStroke3322364212215593
- GrottaJCChiuDLuM1999Agreement and variability in the interpretation of early CT changes in stroke patients qualifying for intravenous rtPA therapyStroke3015283310436095
- HackeWDonnanGFieschiC2004Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trialsLancet3637687415016487
- HackeWKasteMBogousslavskyJ2003European Stroke Initiative recommendations for stroke management - update 2003Cerebrovasc Dis163113714584488
- JaillardAHommelMBairdAE2002Significance of early CT signs in acute stroke. A CT scan-diffusion MRI studyCerebrovasc Dis13475611810011
- LydenP2003Early major ischemic changes on computed tomography should not preclude use of tissue plasminogen activatorStroke34821212624317
- NINDS; NINDS rt-PA Stroke Study Group1995Tissue plasminogen activator for acute ischemic strokeN Engl J Med333158177477192
- PatelSCLevineSRTilleyBC2001Lack of clinical significance of early ischemic changes on computed tomography in acute strokeJAMA2862830811735758
- TomsickTABrottTGChambersAA1990Hyperdense middle cerebral artery sign on CT: efficacy in detecting middle cerebral artery thrombosisAJNR Am J Neuroradiol1147372112309
- von KummerR1998Effect of training in reading CT scans on patient selection for ECASS IINeurology51S5029744835
- von KummerR2003Early major ischemic changes on computed tomography should preclude use of tissue plasminogen activatorStroke34820112624316
- von KummerRHolleRGizyskaU1996Interobserver agreement in assessing early CT signs of middle cerebral artery infarctionAJNR Am J Neuroradiol17174388896631
- WardlawJMDormanPJLewisSC1999Can stroke physicians and neuroradiologists identify signs of early cerebral infarction on CT?J Neurol Neurosurg Psychiatry67651310519873
- WardlawJMMielkeO2005Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment – systematic reviewRadiology2354445315858087
- WeirNUPexmanJHWHillMD2006How well does ASPECTS predict the outcome of acute stroke treated with IV tPA?Neurology675161816894120