Abstract
Retapamulin is a novel semisynthetic pleuromutilin antibiotic specifically designed for use as a topical agent. The unique mode of action by which retapamulin selectively inhibits bacterial protein synthesis differentiates it from other nonpleuromutilin antibacterial agents that target the ribosome or ribosomal factors, minimizing the potential for target-specific cross-resistance with other antibacterial classes in current use. In vitro studies show that retapamulin has high potency against the Gram-positive bacteria (Staphylococcus aureus, Streptococcus pyogenes, and coagulase-negative staphylococci) commonly found in skin and skin-structure infections (SSSIs), including S. aureus strains with resistance to agents such as macrolides, fusidic acid, or mupirocin, and other less common organisms associated with SSSIs, anaerobes, and common respiratory tract pathogens. Clinical studies have shown that twice-daily topical retapamulin for 5 days is comparable to 10 days of oral cephalexin in the treatment of secondarily infected traumatic lesions. A 1% concentration of retapamulin ointment has been approved for clinical use as an easily applied treatment with a short, convenient dosing regimen for impetigo. Given the novel mode of action, low potential for cross-resistance with established antibacterial agents, and high in vitro potency against many bacterial pathogens commonly recovered from SSSIs, retapamulin is a valuable enhancement over existing therapeutic options.
Introduction
Damage to skin integrity compromises its barrier function and allows bacteria to pass into the subdermal environment where the moist, warm, and nutritious conditions are conducive to microbial colonization and proliferation. Traumatic skin lesions that can become infected include excoriations, lacerations, abrasions, and burns, as well as surgical sites involving incisions, sutured wounds, and skin-punch biopsies. Retapamulin is a new topical antibiotic that has been shown to be clinically effective in the management of skin and skin-structure infections (SSSIs).
Management of SSSIs
Three problems confront the clinical evaluation of patients with SSSIs: diagnosis, severity of infection, and the resistance patterns of pathogen-specific antibacterial agents. SSSIs have diverse causes that often reflect the epidemiological setting. Establishing a detailed history is a key step towards a differential diagnosis and may also indicate likely etiologies. Specific etiological diagnosis is difficult and generally unnecessary in patients with mild signs and symptoms of illness;Citation1 however, clinical assessment of the severity of the infection is essential. Patients with signs and symptoms of systemic toxicity (fever or hypothermia, tachycardia, and hypotension) should be investigated further for potentially severe, deep soft-tissue infection. In addition to biochemical and hematological tests, this may involve radiological procedures, emergency surgical exploration, and, if necessary, debridement for diagnostic and therapeutic reasons.Citation1
Practice guidelines issued by the Infectious Diseases Society of America (IDSA) recommend that minor or uncomplicated SSSIs, such as secondarily infected traumatic lesions (SITLs), may be empirically treated with anti-staphylococcal semisynthetic penicillins, first- or second-generation oral cephalosporins, macrolides, or clindamycin.Citation1 For impetigo, topical antibiotic therapy is recommended for patients with a limited number of lesions.Citation1
Reflecting the main sources of contamination for acute wounds – the environment, surrounding skin, and endogenous sources involving mucous membranes – traumatic lesions are susceptible to contamination and colonization by a wide variety of aerobic, facultative, and anaerobic micro-organisms.Citation2 Despite the established microbiological complexity of SSSIs, Staphylococcus aureus and β-hemolytic streptococci are widely viewed as common causes of a variety of these infections,Citation2 with S. aureus considered to be the predominant pathogen associated with infected traumatic lesions.Citation2 Increasing antibacterial resistance is a major problem in the treatment of these pathogens worldwide; the choice of empirical antibiotic treatment must include agents with activity against resistant strains.Citation1
Antibacterial resistance among strains of S. aureus has increased dramatically in recent years. Resistance to penicillin is almost universal among S. aureus owing to β-lactamase production, and high resistance to β-lactamase-stable β-lactams such as methicillin has followed with the acquisition of genes that alter penicillin-binding proteins, rendering the organism insusceptible to such β-lactams in isolates recovered from hospital as well as community infections.Citation3 Community-associated methicillin-resistant strains of S. aureus (CA-MRSA) are now reported to be an increasingly prevalent pathogen in SSSIs.Citation4 A USA study found that CA-MRSA in SSSIs increased from 9% to 21% during the period 2004 to 2006.Citation5 For uncomplicated SSSIs due to CA-MRSA, the IDSA practice guidelines recommend systemic vancomycin, linezolid, clindamycin, or daptomycin as empiric first-line agents with the use of others, such as doxycycline, minocycline, and trimethoprim–sulfamethoxazole, based on the results of susceptibility testing.Citation1,Citation6 Topical antimicrobial agents such as mupirocin are not without issues regarding resistance.Citation7,Citation8 However, the resistance pattern of S. aureus continues to broaden, with the emergence of CA-MRSA with reduced susceptibility to glycopeptide antibiotics,Citation9 and increasing resistance to mupirocin in both methicillin-resistant and methicillin-susceptible S. aureus.Citation8,Citation10
Most uncomplicated SSSIs are treated empirically, with antibacterial selection based on patient factors, pharmacologic considerations, and the likely bacteriologic involvement and antibacterial susceptibility pattern.Citation3 Selection of therapy is generally empiric, and is only rarely made based on microbial culture and susceptibility results. Although systemic antibacterial therapy is clearly essential for advancing infections and those that involve deeper tissues, lesions that show only localized signs of infection may be treated with topical agents.Citation2 Topical antibiotic treatment causes fewer side effects, in particular systemic ones such as gastrointestinal side effects, in comparison with oral treatments,Citation11 and also avoids the risk of resistance selection among the gut micro-flora.Citation12 Topical treatment also delivers a high antibacterial concentration at the site of infection with a relatively small amount of drug.Citation13
A recent addition to available topical antibiotics is retapamulin, a novel semisynthetic pleuromutilin antibiotic that has a unique mode of action and potent in vitro activity against a wide range of pathogens, particularly Gram-positive cocci.Citation14–Citation17 An ointment formulation of 1% retapamulin received regulatory approval from the US Food and Drug Administration for the treatment of impetigo and from the European Medicines Agency in 2007 for the short-term treatment of the following superficial skin infections: impetigo and infected small lacerations, abrasions, and sutured wounds. Retapamulin is the first new prescription topical antibiotic in more than 20 years.
Mode of action of retapamulin
Retapamulin exerts antibacterial activity by selective inhibition of protein synthesis through a unique interaction with the prokaryotic ribosome. By binding to bacterial ribosomes, retapamulin inhibits peptidyl transfer, blocks ribosomal P-site interactions, and inhibits normal ribosomal 50S subunit formation – three processes that are essential for protein synthesis. Many antibacterials that inhibit protein synthesis also bind to the 50S subunit and interact with the peptidyl transferase center; however, there are sufficient differences in retapamulin’s mode of drug–ribosome interactions for retapamulin to be differentiated from other antibacterial agents. The interaction of retapamulin with the bacterial ribosome not only differentiates retapamulin from other non-pleuromutilin ribosomal inhibitors in terms of mode of action, but also indicates an important difference regarding the low potential for development of resistance and the excellent in vitro activity of retapamulin against isolates with demonstrable resistance to other agents. Target-specific resistance to pleuromutilins is likely to emerge slowly through clinical use.Citation18–Citation20 No cross-resistance to retapamulin for subsets of organisms resistant to mupirocin, β-lactams, macrolides, or quinolones has been found.Citation16,Citation21,Citation22
In vitro activity of retapamulin
Global surveillance program data
The in vitro activity of retapamulin against a large and diverse collection of clinical isolates from SSSIs has been studied in a global multicenter surveillance program.Citation23 Retapamulin demonstrated excellent in vitro activity against the key Gram-positive species associated with SSSIs: S. aureus, coagulase-negative staphylococci, and Streptococcus pyogenes.Citation23 Minimum inhibitory concentrations (MIC) required to inhibit the growth of 50% (MIC50) of these three groups of organisms were 0.03 to 0.06 μg/mL, and to inhibit the growth of 90% (MIC90) of these three groups of organisms were 0.03 to 0.12 μg/mL.Citation23 Retapamulin, at concentrations of ≤0.5 μg/mL, was inhibitory to all isolates tested.Citation23 Against S. aureus, retapamulin was the most potent in vitro of 15 antibiotics tested, with a MIC90 value of 0.12 μg/mL, and 16-fold more active than fusidic acid, the most active of tested comparators against S. aureus. In vitro, retapamulin was also the most active agent against coagulase-negative staphylococci and S. pyogenes. For coagulase-negative staphylococci, retapamulin was 32-fold more potent than the next most active agent, linezolid.Citation23
In vitro activity of retapamulin: published studies
Retapamulin demonstrated favorable in vitro activity against anaerobes, including Propionibacterium species associated with acnes vulgaris, Fusobacterium species, and other anaerobic Gram-positive cocci,Citation14,Citation17,Citation24 and respiratory tract pathogens, including Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.Citation16 The in vitro activities of retapamulin against common pathogens associated with uncomplicated SSSIs reported in published studies correspond well with those found in the global surveillance program.Citation16,Citation21,Citation22,Citation25–Citation27 The range of MIC90 values reported were 0.12 to 0.25 μg/mL for S. aureus, 0.06 to 0.25 μg/mL for coagulase-negative staphylococci, and 0.03 to 0.06 μg/mL for S. pyogenes. The activity of retapamulin on bacterial biofilms has not been determined.
In vitro activity against antibiotic resistant strains
The global surveillance program also showed that the high in vitro potency of retapamulin was maintained against isolates of S. aureus resistant to methicillin, macrolides, fusidic acid, or mupirocin with MIC90 values of 0.12 μg/mL. The most active comparator agent tested, fusidic acid, was 16-fold less active than retapamulin against drug-resistant isolates of S. aureus.Citation23 The high potency of retapamulin against S. aureus and other organisms resistant to other antimicrobial agents has been confirmed by published studies, which have reported MIC90 values of 0.12 μg/mL for S. aureus resistant to erythromycin, mupirocin, and oxacillin; 0.06 to 0.12 μg/mL for coagulase-negative staphylococci (including Staphylococcus epidermidis) resistant to mupirocin and oxacillin; and ≤0.03 μg/mL for S. pyogenes resistant to erythromycin.Citation16,Citation21,Citation22 Against 664 S. aureus isolates from the UK, including many resistant to fusidic acid and/or highly resistant to mupirocin, retapamulin inhibited 663 (99.8%) isolates at ≤0.25 mg/L.Citation28
Propensity for the development of resistance to retapamulin in vitro
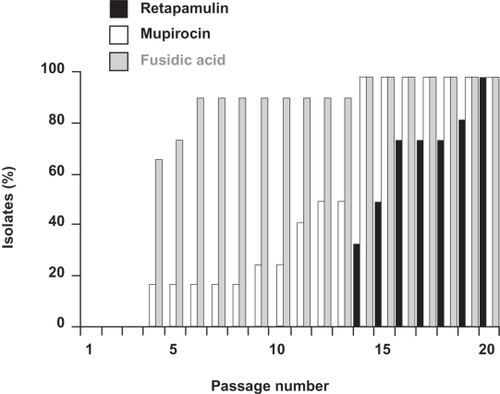
The distinct interaction between pleuromutilins and the 50S ribosome distinguishes their mode of action from those of other classes of antibiotics. This unique mode of action minimizes the potential for target-specific cross-resistance with other antibacterial classes in current use. This has been demonstrated through the finding that retapamulin is active in vitro against clinical isolates that are resistant to many other classes of antibiotics that act by inhibiting protein synthesis. Retapamulin also has activity against isolates resistant to antibiotics with very different modes of action, such as β-lactams and quinolones. Extensive preclinical testing has shown that retapamulin has a slow and gradual propensity for resistance development in S. aureus and a low potential for resistance selection in S. pyogenes.Citation19,Citation20 In multi-step passage studies, increased MIC values to retapamulin were seen among isolates of S. aureus and S. pyogenes, but required more passages and occurred at a lower magnitude compared with mupirocin or fusidic acid (). Furthermore, retapamulin had the lowest MIC values among the drugs tested.Citation19 This low potential for resistance selection limits concerns with regard to pathogens developing resistance to retapamulin through clinical use, which is a known problem with other topical antibiotics (eg, resistance to mupirocin or fusidic acid in S. aureus).Citation8,Citation29
Figure 1 In multi-step studies, increased minimum inhibitory concentrations (MICs) to retapamulin were seen among Staphylococcus aureus isolates, but required more passages compared with mupirocin or fusidic acid; figure shows a summary of the 12 S. aureus isolates tested. Drawn from data of Kosowska-Shick et al.Citation19
Efficacy of retapamulin in SITLs
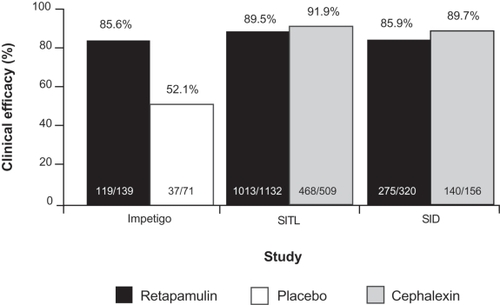
The efficacy, safety, and tolerability of topical retapamulin ointment, 1%, was demonstrated in two identical, randomized, double-blind, double-dummy, active-controlled, multicenter studies in 1904 patients with SITLs. In these studies, the clinical efficacy of retapamulin was high and comparable to that of cephalexin. Using a prespecified non-inferiority margin of 10%, retapamulin ointment, 1%, applied twice daily for 5 days, was shown to be non-inferior to oral cephalexin, 500 mg, twice daily for 10 days in both studies.Citation30 In the per-protocol (PP) analyses, the pooled clinical success rates at follow-up (7–9 days post-therapy) were 89.5% in patients receiving retapamulin, compared with 91.9% for patients treated with cephalexin (treatment difference: −2.5%; 95% confidence interval [CI]: −5.4%, 0.5%) ().Citation30 In patients with S. aureus or S. pyogenes at baseline, clinical success rates at follow-up for patients treated with retapamulin and cephalexin were 89.2% (365/409) and 92.6% (63/68), respectively.
Figure 2 Clinical success rates at follow-up in the per-protocol clinical populations from randomized controlled clinical trials comparing topical retapamulin, 1%, twice daily for 5 days, with placebo in the treatment of impetigo and oral cephalexin, 500 mg, twice daily for 10 days, in the treatment of secondarily infected traumatic lesions (SITLs) and secondarily infected dermatitis (SID). Drawn from data of Koning et al,Citation11 Free et al,Citation30 and Parish et al.Citation31
Retapamulin treatment was successful in the small numbers of patients with SITLs due to fusidic acid- or mupirocin-resistant strains of S. aureus (clinical success rates of 11/12 and 6/7, respectively [intent-to-treat (ITT) bacteriologic population]).Citation32 Against MRSA, clinical success was achieved by treatment with retapamulin in 37 of 57 isolates (64.9%) and by cephalexin treatment in 27 of 33 isolates (81.8%) (ITT bacteriologic population).Citation32 In these studies, SITLs included abrasions, lacerations, sutured wounds, and simple abscesses. If patients with abscesses were excluded, the clinical success rate against MRSA was 75.7% (28/37) in retapamulin-treated patients and 80.8% (21/26) in cephalexin-treated patients.Citation33
Safety and tolerability were similar for the two treatments (see ‘Safety and tolerability’ section below for more details). Non-compliance, defined as taking <80% of doses, was recorded in 8.0% (51/636) of patients receiving cephalexin, compared with 0.4% (5/1268) of patients receiving retapamulin.Citation30
Retapamulin for the treatment of secondarily infected dermatitis
In another randomized, double-blind, active-controlled study, the clinical efficacy and safety of topical retapamulin was compared with oral cephalexin for the treatment of secondarily infected dermatitis (SID), which included subjects with an underlying skin disease such as atopic dermatitis, psoriasis, or allergic contact dermatitis that had a secondary bacterial infection.Citation31 In this study, patients with SID were randomly assigned to treatment with retapamulin ointment, 1%, twice daily for 5 days, or oral cephalexin, 500 mg, twice daily for 10 days. At the follow-up visit (7–9 days post therapy) in the PP clinical population, the efficacy of retapamulin was comparable to that of cephalexin, with clinical success rates of 85.9% and 89.7%, respectively (treatment difference: −3.8%; 95% CI: −9.9%, 2.3%). Microbiologic success rates at follow-up were 87.2% for retapamulin and 91.8% for cephalexin. Retapamulin treatment was generally effective against the small numbers of fusidic acid-, methicillin-, or mupirocin-resistant S. aureus isolates (clinical success rates of 3/5, 5/5 and 4/5, respectively, in the PP bacteriological population).Citation32
Retapamulin for the treatment of impetigo
In the treatment of impetigo, retapamulin was compared with placebo in a randomized, double-blind, multicenter study in 213 patients who received either topical retapamulin or placebo ointment, twice daily for 5 days.Citation34 Based on the primary efficacy endpoint of clinical response at the end of therapy visit (day 2 post-therapy) in the ITT clinical population, retapamulin ointment was superior to placebo (success rate 85.6% vs 52.1%; p < 0.0001). Similar results were found in the PP clinical population and for those patients in the bacteriological population who had had a pathogen isolated at baseline.Citation34
In another clinical study, retapamulin, twice daily for 5 days, was compared with 2% fusidic acid ointment, three times daily for 7 days, in 519 adult and pediatric patients (aged at least 9 months) with impetigo.Citation35 In the PP clinical population, the clinical success rates at the end of therapy with retapamulin and fusidic acid were 99.1% and 94%, respectively (difference 5.1%, 95% CI: 1.1%, 9.0%, p = 0.003). In the ITT clinical population, clinical success rates at the end of therapy with retapamulin and fusidic acid were 94.8% and 90.1%, respectively (difference: 4.7%, 95% CI: −0.4%, 9.7%, p = 0.062).Citation35 In the analysis of patients who had a pathogen isolated at baseline, the bacteriologic efficacy of retapamulin was superior in both the PP and ITT populations. Retapamulin treatment was successful in the few fusidic acid-, methicillin-, or mupirocin-resistant isolates of S. aureus (clinical success rates of 9/9, 8/8, and 6/6, respectively).Citation35
Safety and tolerability of retapamulin in SSSIs
Phase III trials in the retapamulin clinical development program, in which 2115 patients received retapamulin, demonstrated the safety of this new antibacterial agent in patients with SITLs, SID, and impetigo.Citation30,Citation31,Citation36–Citation38 An adverse event (AE) was defined as any untoward medical occurrence that was temporally associated with the use of a medicinal product, whether or not it was considered by the investigator to be related to the use of that medicinal product.
The frequency of AEs of any type was low in patients receiving retapamulin, with a broadly similar incidence across studies and treatment groups.Citation39 Overall, the frequency of occurrence of all AEs varied little across age groups in patients receiving retapamulin. The majority of AEs were of mild or moderate intensity; AEs of severe intensity were reported by 28 (1.3%) patients across retapamulin groups in all studies. Serious AEs were reported by 0.5% of patients (11/2115) treated with retapamulin, none of which were considered by the investigator to be related to treatment. The most common AEs for retapamulin were application-site reactions; these occurred in 2.6% (56/2115) of patients for retapamulin, 0.9% (7/819) of patients for cephalexin, and 4.2% (3/71) of patients for placebo. Across all studies, very few patients treated with retapamulin withdrew because of an AE (1.2%, 26/2115).Citation39
The frequencies of AEs that were considered by the investigator to be related to use of the study medication were 5.5% (116/2115) for retapamulin, 6.6% (54/819) for cephalexin, 0.6% (1/172) for fusidic acid, and 2.8% (2/71) for placebo.Citation40 Across all studies, the only individual treatment-related AEs that were reported in at least 1% of patients were application-site irritation (1.4% [29/2115]) in the retapamulin group and diarrhea (1.7% [14/819]) in patients receiving cephalexin.Citation40
In the SITL treatment studies, the overall rates of AEs were comparable: 22.8% (289/1268) of patients who received retapamulin and 25.3% (161/636) of patients in the cephalexin group reported one or more AEs. The incidence of all treatment-related AEs was 5.3% (67/1268) in the retapamulin group and 7.7% (49/636) in the cephalexin group. The most common treatment-related AEs were application-site irritation (1.3% [17/1268]) in those who received retapamulin, and diarrhea (1.9% [12/636]) and nausea (1.6% [10/636]) in patients treated with cephalexin.Citation30
There were no notable changes in laboratory values obtained from routine hematology and clinical chemistry tests over the study period in patients randomly assigned to retapamulin in any of the clinical trials. Furthermore, for all laboratory clinical values, there were no large mean changes from baseline for the total retapamulin population. Across all treatment groups there were no notable changes in any of the age groups.Citation39
As with all topical antibiotics, application of retapamulin ointment directly on the infected area minimizes the likelihood of generalized AEs, such as gastrointestinal disturbances, that can occur with oral antibiotics.Citation11,Citation12,Citation41 In adult patients with SITLs treated with retapamulin, twice daily for 5 days, measurable plasma concentrations of the drug were detected in only 47 of 380 samples (range, 0.52–10.7 ng/mL). In children, measurable plasma concentrations were detected in only 9 of 136 samples (range, 0.54–18.5 ng/mL).Citation36
Patient preference and compliance
As with other therapeutic regimens, patient compliance is essential in the effectiveness of prescribed antibiotics. With poor compliance, therapeutic goals are less likely to be achieved, resulting in poorer patient outcomes.Citation42–Citation44 Poor compliance is associated with deteriorating health, the need for additional consultations, the emergence of bacterial resistance, extra drugs, additional hospital admissions, and increases in direct and indirect costs of healthcare management.Citation44–Citation46
In general, patients are more compliant with simple and less-frequent dosing regimens. Both the dosage schedule and the patient’s daily routine should be considered when prescribing antibiotics.Citation47 Topical agents may also be more attractive than oral therapy because they reduce the potential for systemic side effects, typically nausea and diarrhea, which are commonly associated with many systemic antibiotics.Citation12
Throughout the phase III clinical trial program for retapamulin, compliance was measured using diary cards that patients or their parents or guardians had completed. If doses were missed, the patient would have attained less than 100% compliance; if more treatment had been used than instructed, the patient would have exceeded 100% compliance. Taking the capsules/suspension or using the ointment within 80% to 120% of the desired dose was defined as compliance with treatment.
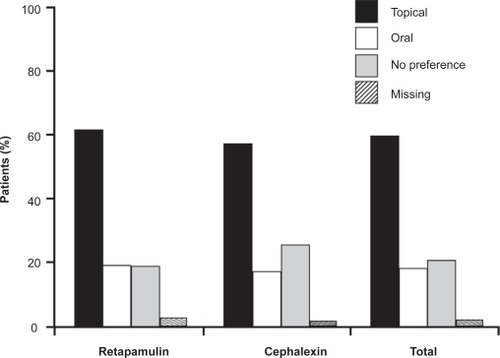
In the SITL clinical trials, compliance with the retapamulin regimen was greater than that for cephalexin (). In the ITT clinical population, 8.0% (51/636) of patients taking oral cephalexin took <80% of the prescribed dose, compared with 0.4% (5/1268) of patients applying retapamulin ointment.Citation30 In the SID study, compliance was similar for the two treatment arms.Citation31 Patients with SID were asked during their end-of-treatment visit whether they preferred oral or topical therapy, or had no preference for treatment. The majority of patients in each treatment group (60.6% and 56.8% in the retapamulin and cephalexin groups, respectively) preferred topical medication. Oral medication was preferred by fewer than 20% of patients in both treatment groups ().Citation31 The shorter treatment course for retapamulin compared with cephalexin – 5 days versus 10 days – may have influenced treatment preference. These results suggest that there is a preference for topical over oral antibacterial therapy for the treatment of SSSIs, a conclusion that has been reported by others.Citation48,Citation49
Figure 3 Compliance with retapamulin was greater than that for cephalexin in patients with secondarily infected traumatic lesions treated with topical retapamulin, 1%, twice daily for 5 days, or with oral cephalexin, 500 mg, twice daily for 10 days; figure shows pooled data from two identical randomized controlled clinical trials (Study 030A and 030B) for intent-to-treat patients who were 80%–120% compliant with study treatment.Citation30 For secondarily infected dermatitis (Study 032), there was no difference in compliance between the two treatment arms.Citation31 Patients ≥13 years of age received cephalexin capsules; those who were younger received cephalexin suspension. Drawn from data of GlaxoSmithKline.Citation36
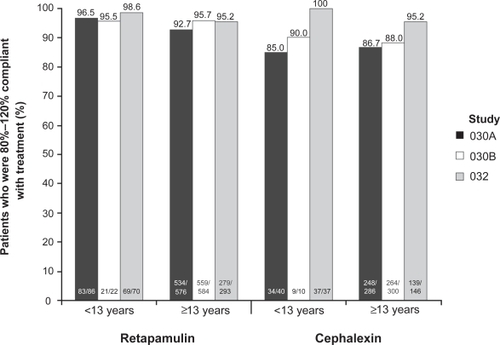
Figure 4 Patients with secondarily infected dermatitis treated with topical retapamulin, 1%, twice daily for 5 days, or with oral cephalexin, 500 mg, twice daily for 10 days, recorded a marked preference for topical therapy over oral therapy in both treatment arms. Drawn from data of Parish et al.Citation31
The ease of application and short, convenient dosing regimen of retapamulin is conducive to good compliance.Citation31 In clinical practice, patients are expected to benefit from the shorter duration of treatment and lower overall dosing frequency of retapamulin compared with other topical or oral antibiotics, as shorter treatment regimens and less frequent dosing are generally associated with improved compliance, patient preference, or both.Citation31,Citation42,Citation43 A short, convenient dosing regimen also reduces the potential for treatment failure and minimizes the development of antibacterial drug resistance.Citation31
Conclusions
Antibacterial resistance against many pathogens and several classes of antibacterial agents has increased markedly in recent years. This is clearly seen among the many bacterial species common to infected traumatic lesions, and with S. aureus in particular. Antibacterial resistance patterns are changing and should be taken into account in the choice of therapy. Topical antibiotic ointments are an effective treatment option for patients with limited or uncomplicated SSSIs, including SITLs.Citation1,Citation6 Topical agents may be more attractive than oral therapy because they reduce the potential for systemic side effects, such as nausea and diarrhea, and avoid resistance selection in the gut flora. In addition, application of the antibiotic directly to the infected lesion may result in higher local concentrations at the site of infection and, consequently, allow overall use of the drug to be reduced.Citation12 For uncomplicated skin infections not requiring systemic antimicrobial therapy, such as impetigo, SITLs, and SID, a topical antibiotic should ideally have a sufficiently broad spectrum of activity to be used as monotherapy, must not promote cross-resistance, and should be well tolerated with a low potential for AEs.
Retapamulin, the first new topical antibiotic in more than 20 years, has potent in vitro antibacterial activity against the most likely pathogens causing uncomplicated skin infections. Retapamulin has a unique mode of action that differentiates it from other non-pleuromutilin antibacterial agents that target bacterial protein synthesis, and minimizes the potential for target-specific cross-resistance with other antibacterial classes in current use. Topical treatment with retapamulin, twice daily for 5 days, has been shown in studies of impetigo to be superior to placebo, and in studies of SITLs and SID to be comparable to 10 days of oral cephalexin. Retapamulin offers a novel, effective, and convenient topical treatment for uncomplicated SSSIs.
Acknowledgements
The authors take full responsibility for the content of this paper and thank Neil McKendrick, supported by GlaxoSmithKline, for assistance in preparing the first draft of the manuscript and collating the comments of the authors.
Disclosures
All authors are employees of GlaxoSmithKline.
References
- StevensDLBisnoALChambersHFPractice guidelines for the diagnosis and management of skin and soft-tissue infectionsClin Infect Dis2005411373140616231249
- BowlerPGDuerdenBIArmstrongDGWound microbiology and associated approaches to wound managementClin Microbiol Rev20011424426911292638
- PereraGHayRA guide to antibiotic resistance in bacterial skin infectionsJ Eur Acad Dermatol Venereol20051953154516164705
- PatelMWaitesKBHoesleyCJStammAMCanuppKCMoserSAEmergence of USA300 MRSA in a tertiary medical centre: implications for epidemiological studiesJ Hosp Infect20086820821318289726
- GuptaKMacintyreAVanasseGDembryLMTrends in prescribing beta-lactam antibiotics for treatment of community-associated methicillin-resistant Staphylococcus aureus infectionsJ Clin Microbiol2007453930393417942648
- JacobsMRJonesRNGiordanoPAOral beta-lactams applied to uncomplicated infections of skin and skin structuresDiagn Microbiol Infect Dis20075755S65S17292581
- DeshpandeLMFixAMPfallerMAJonesRMEmerging elevated mupirocin resistance rates among staphylococcal isolates in the SENTRY Antimicrobial Surveillance Program (2000): correlations of results from disk diffusion, Etest and reference dilution methodsDiagn Microbiol Infect Dis20024228329012007448
- UptonALangSHeffernanHMupirocin and Staphylococcus aureus: a recent paradigm of emerging antibiotic resistanceJ Antimicrob Chemother20035161361712615862
- AppelbaumPCThe emergence of vancomycin-intermediate and vancomycin-resistant Staphylococcus aureusClin Microbiol Infect200612Suppl 1162316445720
- WalkerESVasquezJEDulaRBullockHSarubbiSAMupirocin-resistant, methicillin-resistant Staphylococcus aureus: does mupirocin remain effective?Infect Control Hosp Epidemiol20032434234612785407
- KoningSVerhagenAPvan Suijlekom-SmitLWMorrisAButlerCCvan der WoudenJCInterventions for impetigoCochrane Database Syst Rev2003CD003261
- GisbyJBryantJEfficacy of a new cream formulation of mupirocin: comparison with oral and topical agents in experimental skin infectionsAntimicrob Agents Chemother20004425526010639346
- BikowskiJSecondarily infected wounds and dermatoses: a diagnosis and treatment guideJ Emerg Med1999171972069950410
- GoldsteinEJCitronDMMerriamCVWarrenYATyrellKLFernandezHAComparative in vitro activities of retapamulin (SB-275833) against 141 clinical isolates of Propionibacterium spp., including 117 P. acnes isolatesAntimicrob Agents Chemother20065037938116377717
- PankuchGALinGHoellmanDBGoodCEJacobsMRAppelbaumPCActivity of retapamulin against Streptococcus pyogenes and Staphylococcus aureus evaluated by agar dilution, microdilution, E-test, and disk diffusion methodologiesAntimicrob Agents Chemother2006501727173016641442
- RittenhouseSBiswasSBroskeyJSelection of retapamulin, a novel pleuromutilin for topical useAntimicrob Agents Chemother2006503882388517065625
- OdouMFMullerCCalvetLIn vitro activity against anaerobes of retapamulin, a new topical antibiotic for treatment of skin infectionsJ Antimicrob Chemother20075964665117350985
- PringleMPoehlsgaardJVesterBLongKSMutations in ribosomal protein L3 and 23S ribosomal RNA at the peptidyl transferase centre are associated with reduced susceptibility to tiamulin in Brachyspira spp. isolatesMol Microbiol2004541295130615554969
- Kosowska-ShickKClarkCCreditoKSingle- and multistep resistance selection studies on the activity of retapamulin compared to other agents against Staphylococcus aureus and Streptococcus pyogenesAntimicrob Agents Chemother20065076576916436741
- GentryDRRittenhouseSFMcCloskeyLHolmesDJStepwise exposure of Staphylococcus aureus to pleuromutilins is associated with stepwise acquisition of mutations in rplC and minimally affects susceptibility to retapamulinAntimicrob Agents Chemother2007512048205217404009
- JonesRNFritscheTRSaderHSRossJEActivity of retapamulin (SB-275833), a novel pleuromutilin, against selected resistant gram-positive cocciAntimicrob Agents Chemother2006502583258616801451
- WilliamsLNorthwoodJCrowhurstNFelminghamDIn vitro activity of retapamulin, a novel pleuromutilin, against Staphylococcus spp. (n = 1413) and Streptococcus pyogenes (n = 503) from 26 European centresEuropean Congress of Clinical Microbiology and Infectious Diseases and International Congress of Chemotherapy31 March–3 April 2007Munich, GermanyPoster P790.
- GlaxoSmithKlineIn vitro activity of SB-275833 and comparators against 6,747 hospital- and community-acquired Gram-positive clinical isolates from skin and skin structure infections; data from the retapamulin global surveillance studyData on file2005
- MolitorisDFinegoldSMBolañosMIn vitro activity of retapamulin and five comparator agents against 226 anaerobic bacterial isolates45th Interscience Conference on Antimicrobial Agents and Chemotherapy6–19 December 2005Washington, DC, USAPoster F-2060.
- BrownSDTraczewskiMMRetapamulin: in vitro potency, spectrum of activity, MIC and disk breakpoints45th Interscience Conference on Antimicrobial Agents and Chemotherapy16–19 December 2005Washington, DC, USAPoster F-2065.
- DraghiDScangarellaNShawarRSahmDIn vitro activity of retapamulin and comparators against 1690 recent Gram-positive clinical isolates45th Interscience Conference on Antimicrobial Agents and Chemotherapy16–19 December 2005Washington, DC, USAPoster F-2052.
- FelminghamDWilliamsLJNorthwoodJGECrowhurstNAIn vitro activity of retapamulin, a novel pleuromutilin, against Streptococcus pyogenes, Streptococcus agalactiae and ‘viridans’ streptococci isolates from 26 centers in 14 European countries45th Interscience Conference on Antimicrobial Agents and Chemotherapy16–19 December 2005Washington, DC, USAPoster F-2056.
- WoodfordNAfzal-ShahMWarnerMLivermoreDMIn vitro activity of retapamulin against Staphylococcus aureus isolates resistant to fusidic acid and mupirocinJ Antimicrob Chemother2008619Epub ahead of print.
- HowdenBPGraysonLMDumb and dumber – the potential waste of a useful antistaphylococcal agent: emerging fusidic acid resistance in Staphylococcus aureusClin Infect Dis200639440016392088
- FreeARothEDalessandroMRetapamulin ointment twice daily for 5 days vs oral cephalexin twice daily for 10 days for empiric treatment of secondarily infected traumatic lesions of the skinSkinmed2006522423216957433
- ParishLCJorizzoJLBretonJJTopical retapamulin ointment (1%, wt/wt) twice daily for 5 days versus oral cephalexin twice daily for 10 days in the treatment of secondarily infected dermatitis: results of a randomized controlled trialJ Am Acad Dermatol2006551003101317097398
- GlaxoSmithKlineClinical and microbiological success rates at follow-up. Report number TOCMAA table 17.481Data on file2006
- GlaxoSmithKlineData on file – ABX208_UM2008-00159-00Collegeville, PAGlaxoSmithKline52008
- KoningSvan der WoudenJCChosidowOEfficacy and safety of retapamulin ointment as treatment of impetigo: randomized double-blind multicentre placebo-controlled trialBr J Dermatol20081581077108218341664
- OranjeAPChosidowOSacchidanandSTopical retapamulin ointment, 1%, versus sodium fusidate ointment, 2%, for impetigo: a randomized, observer-blinded, noninferiority studyDermatology200721533134017911992
- GlaxoSmithKlineProtocol SB-275833/030 – studies 030A and 030B: two identical double-blind, double-dummy, multicenter, comparative phase III studies of the safety and efficacy of topical 1% SB-275833, applied twice daily, versus oral cephalexin, 500 mg in adults, or 12.5 mg/kg (250 mg/5 ml) in children, twice daily, in the treatment of uncomplicated secondarily infected traumatic lesions (study SB-275833/030A)Data on file2005
- ChosidowOOranjeAPSacchidanandSRetapamulin as a novel treatment for impetigo: results of a phase III, randomized trial of efficacy and safety versus fusidic acidPresented at: 15th European Academy of Dermatology and Venereology4–8 October 2006Rhodes, GreecePoster P041.11.
- van der WoudenJCKoningSChosidowOEvaluation of retapamulin, a novel, broad-spectrum, topical antibacterial, for the treatment of impetigo: results of a placebo-controlled, randomized, double-blind, efficacy and safety trialPresented at: 15th European Academy of Dermatology and Venereology4–8 October 2006Rhodes, GreecePoster PO41.74.
- European Medicines Agency. European Public Assessment Report (EPAR) for Altargo (retapamulin). Revision 3. London, UK: European Medicines Agency [updated 2008 May 30; cited 2008 Sep 2]. Available from: http://www.emea.europa.eu/humandocs/Humans/EPAR/altargo/altargo.htm
- GlaxoSmithKlineSummary of drug-related adverse events in decreasing frequencyData on file2006
- GoldfarbJCrenshawDO’HoroJLemonEBlumerJLRandomized clinical trial of topical mupirocin versus oral erythromycin for impetigoAntimicrob Agents Chemother198832178017833149884
- PerchèreJCLaceyLOptimizing economic outcomes in antibiotic therapy of patients with acute bacterial exacerbations of chronic bronchitisJ Antimicrob Chemother200045192410719008
- BrixnerDIImproving acute otitis media outcomes through proper antibiotic use and adherenceAm J Manag Care200511SupplS20221016111443
- VrijensBUrquhartJPatient adherence to prescribed antimicrobial drug dosing regimensJ Antimicrob Chemother20055561662715772145
- KardasPPatient compliance with antibiotic treatment for respiratory tract infectionsJ Antimicrob Chemother20024989790312039881
- KardasPComparison of patient compliance with once-daily and twice-daily antibiotic regimens in respiratory tract infections: results of a randomized trialJ Antimicrob Chemother20075953153617289766
- CockburnJGibberdRWReidALSanson-FisherRWDeterminants of non-compliance with short term antibiotic regimensBr Med J19872958148183119056
- PakroohHA comparison of sodium fusidate ointment (‘Fucidin’) alone versus oral antibiotic therapy in soft-tissue infectionsCurr Med Res19775289294
- RistTParishLCCapinLRSulicaVBushnellWDCupoMAA comparison of the efficacy and safety of mupirocin cream and cephalexin in the treatment of secondarily infected eczemaClin Exp Dermatol200227142011952661