Abstract
Chemotherapy-induced nausea and vomiting (CINV) are among the most feared and distressing symptoms experienced by patients with cancer. The knowledge of the pathogenesis and neuropharmacology of CINV has expanded enormously over the last decades, the most significant discoveries being the role of 5-hydroxytryptamine (5-HT)3- and neurokinin (NK)1 receptors in the emetic reflex arch. This has led to the development of two new classes of antiemetics acting as highly selective antagonists at one of these receptors. These drugs have had a huge impact in the protection from chemotherapy-induced vomiting, whereas the effect on nausea seems to be limited. The first NK1 receptor antagonist, aprepitant, became clinically available in 2003, and casopitant, the second in this class of antiemetics, has now completed phase III trials. This review delineates the properties and clinical use of casopitant in the prevention of CINV.
Introduction
Historically, chemotherapy-induced nausea and vomiting (CINV) are among the most feared and distressing symptoms experienced by patients with cancer.Citation1 Patients’ ranking of side effects have changed over time. In a work by Coates and colleagues in 1983, patients receiving cancer chemotherapy reported vomiting and nausea as the two most severe side effects.Citation1 In another work ten years later, the same group found that patients experienced nausea as the most severe side effect of chemotherapy, and vomiting was now ranked fifth.Citation2 With the introduction of high-dose metoclopramide in 1981 the antiemetic outcome markedly changed, providing significant reduction in cisplatininduced emesis.Citation3 In the 1980s a new class of antiemetic agents, the 5-hydroxytryptamine (5-HT)3 receptor antagonists, were developed and became clinically available in the early 1990s. This further improved protection from emesis, and the efficacy was potentiated by addition of dexamethasone.Citation4 Patients perception of side-effects in 1993 emphasises, that the 5-HT3-receptor antagonists are more preventive of vomiting than nausea, and in particular management of delayed nausea and vomiting remains a challenge.Citation5 The latest approach towards elimination of CINV was made with the appearance of the neurokinin (NK)1 receptor antagonists. When added to the standard antiemetic regimen until 2004 (a 5-HT3-receptor antagonist and a corticosteroid), the NK1 receptor antagonist, aprepitant, significantly improved the percentage of patients with complete response (CR), defined as no emetic episodes and no rescue therapy on days 1 to 5 after the initiation of chemotherapy. Even a significant reduction in delayed emesis was demonstrated.Citation6–Citation9 In 2003, the first NK1 receptor antagonist, aprepitant, became commercially available. The second drug in this class, casopitant (casopitant mesylate), has now completed phase II and phase III trials, demonstrating efficacy in the same magnitude as demonstrated with aprepitant. Previous indications for casopitant, overactive bladder, social anxiety disorder, major depressive disorder, insomnia, and fibromyalgia have been revoked, and remaining indications are CINV and postoperative nausea and vomiting (PONV). This review delineates the properties and clinical use of casopitant in the prevention of chemotherapy-induced nausea and vomiting. It should be noted, that most of the clinical data is availably in abstract form only.
Neuropharmacology of emesis
Insight in the complex human emetic pathway has been achieved primarily on the basis of animal models.Citation10 It is a general assumption, that the vomiting centre (VC) in the medulla oblongata, the chemoreceptor trigger zone (CTZ) in the area postrema (AP) on the caudal margin of the IVth ventricle, the visceral afferent neurons and abdominal vagal afferent neurons, form the central emetic pathway.Citation11 The VC is representing anatomical structures at the level of the nucleus tractus solitarius (NTS) and the visceral and somatic motor nuclei. Another acceptation is that CINV results from directly activation of the NTS by visceral afferent neurons and/or by inputs from the CTZ. The CTZ, in turn, may be stimulated by abdominal vagal afferent activation by release of serotonin (5-HT3) from the enterochromaffin cells (EC cells) in the gut. The reflex activation of the NTS and the CTZ further extends to the visceral and somatic motor nuclei giving rise to physiological changes, resulting in nausea and vomiting.Citation12 Radioligand binding studies have identified the binding affinity of several neurotransmitters to receptors such as dopamine (D2), muscarine cholinergic, histamine (H1) and serotonin (5-HT3), involved in the emetic response to chemotherapy.Citation10 The fundamental role for serotonin and 5-HT3-receptors in the emetic pathway was discovered in the mid 1980s, giving rise to the development of 5-HT3- receptor antagonists.Citation13 Recently, the role of substance P and the neurokinin1 (NK1) receptors in the emetic pathway has been investigated, resulting in development of the NK1 receptor antagonists.
Substance P and NK1 receptor antagonists
Substance P (SP) was isolated in 1931 but not purified and sequenced until 1970.Citation14,Citation15 SP is a member of a family of small peptides, the mammalian tackykinins (TKs).Citation16 Three receptors for TKs (NK1, NK2, and NK3) have been cloned, with SP being the preferred agonist at NK1 receptors.Citation16 The role of SP in emesis dates back to 1984, when Carpenter and colleauges demonstrated that systemic administration of the neuropeptide caused emesis in dogs.Citation17 In 1993, Andrews and Bhandari suggested that resinferatoxin exerts its potent antiemetic activity by depleting SP at a central site in the emetic pathway, possibly in the NTS.Citation18 Penetration of the blood-brain barrier is essential for the antiemetic activity of systemically administered NK1 receptor antagonists, a quality that the peptide-based NK1 receptor antagonists did not exhibit.Citation19,Citation20 Hence a milestone was reached, when the first nonpeptide NK1 receptor antagonist, CP-96,345, with high affinity for the NK1 receptor, was developed in 1991.Citation21 This finding was followed by a number of experimental studies confirming a broad-spectrum antiemetic activity of nonpeptide NK1 receptor antagonists.Citation22–Citation25 These studies led to the development of the latest class of antiemetic agents, with aprepitant being the first clinically available NK1 receptor antagonist. Casopitant has now completed phase III trials for the prevention of CINV from moderately and highly emetogenic chemotherapy.
Metabolism, pharmacokinetics, and interactions
Preclinical studies
Only sparse information about the ADME (absorption, distribution, metabolism, and excretion) properties of casopitant (oral and intravenous formulation) has been published. Casopitant is a piperazine derivative [1-piperidinecarboxamide, 4-(4-acetyl-1-piperazinyl)-N-((1R)-1(3,5-bis(trifluorometh yl)phenyl)-ethyl)-2-(4-fluoro-2-methylphenyl-N-methyl- (2R,4S)-; GW679769] ().Citation26 In a ferret-model of cisplatin- induced emesis, GW679769 (casopitant) inhibited retching and vomiting and reduced nausea-like behaviours in a dose-dependent manner.Citation27–Citation29 Several clinical trials have assessed safety, potential interactions and pharmacokinetic properties of casopitant; however many data is available in abstract form only.
The pharmacokinetics and brain penetration of casopitant were studied in the ferret-model of cisplatin-induced emesis. Following a single intraperitoneal dose, radioactive labeled casopitant ([Citation14 C] casopitant) was rapidly absorbed, with plasma and brain concentrations being approximately equal at two hours post-dosing. [Citation14 C] casopitant was found in the brain as the parent compound and two major oxidative metabolites (M1 and M2), accounting for approximately 76%, 19%, and 3% of the radioactivity, respectively; suggesting that the pharmacologic activity of casopitant in the ferret is largely attributable to the parent compound.Citation26 An in vitro receptor binding affinity study describes, that casopitant possesses a high affinity for brain NK1 receptors in the ferret.Citation26
Because casopitant is intended to be administered in combination with a 5-HT3-receptor antagonist and because therapeutic synergy has been observed with this combination in the ferret, a drug interaction study was conducted.Citation28 Following co-administration of ondansetron and casopitant in ferrets, no alteration of disposition of either agent was seen. A synergistic antiemetic activity was demonstrated, proposing complementary mechanisms of pharmacologic actions of the two agents.Citation30 No information about animal toxicity was described in the studies above.
Clinical studies
Pharmacokinetic and pharmacodynamic aspects (PK/PD) of casopitant were assessed in two phase II trials (2802 PK samples from 765 subjects) in patients undergoing treatment with moderately and highly emetogenic chemotherapy (MEC and HEC). In addition to ondansetron and dexamethasone, patients received placebo; 50-, 100-, or 150 mg daily of oral casopitant for three days; or a single oral dose of 150 mg casopitant, starting prior to chemotherapy on day 1. The distribution of casopitant follows a two-compartment first-order model, and the oral absorption was in general rapid, however 30% of subjects exhibited delayed and slow oral absorption. Oral clearance was 17.4 L/h/70 kg, displaying a large intersubject variability (72%). Body weight was identified as a significant covariate of casopitant clearance and central volume of distribution. Further, it was shown that low casopitant area under the curve (AUC) in patients receiving HEC increased the risk of emesis in some patients, suggesting that high concentrations of casopitant during the first 24 h may be important for adequate pharmacological response. Oral casopitant administered as a single dose of 150 mg on day 1, or followed by 50 mg doses on days 2 and 3, seem to provide adequate receptor occupancy and prevention of CINV associated with MEC and HEC.Citation31
A PK/PD study analyzed data (1637 PK samples from 562 subjects) from a phase II trial in which casopitant was evaluated for the prevention of PONV. Patients were female and undergoing surgery and at high risk for PONV. In addition to ondansetron, patients received placebo; 50, 100, or 150 mg single oral doses of casopitant prior to surgery. In this study oral clearance was 24.4 L/h/70kg, displaying moderate intersubject variability (48%). Body-weight was also identified as a significant covariate of casopitant central volume of distribution, but not of clearance. For the treatment of PONV in high-risk patients, a dose of 50 mg casopitant is suggested to be the minimally effective dose.Citation32
Casopitant is a substrate and weak-to-moderate inhibitor of CYP3A4.Citation33 Based on the role of CYP3A4 in the metabolism of several antiemetic drugs, pharmacokinetic interactions between casopitant, dexamethasone (substrate and inducer of CYP3A4) and ondansetron (mixed CYP substrate) were assessed in a two-part, three-period, single-sequence phase I study in 44 healthy adult subjects. The study aimed at investigating possible changes in bioavailability of casopitant, ondansetron and dexamethasone, when these agents are co-administered. In Part 1, which was representative of a three-day regimen for the prevention of CINV resulting from HEC, subjects received oral casopitant (150 mg, day 1; 50 mg, days 2–3) in regimen A; oral dexamethasone (20 mg, day 1; 8 mg twice daily, days 2–3) and IV ondansetron (32 mg, day 1) in regimen B; and oral casopitant (150 mg, day 1; 50 mg, days 2–3), a reduced dose of oral dexamethasone (12 mg, day 1; 8 mg once daily, days 2–3), and IV ondansetron (32 mg, day 1) in regimen C. In Part 2, which was representative of a three-day regimen for the prevention of CINV resulting from MEC, subjects received oral casopitant (150 mg, day 1; 50 mg, days 2–3) in regimen D; IV dexamethasone (8 mg, day 1; 8 mg twice daily, days 2–3) and oral ondansetron (8 mg twice daily, day 1) in regimen E; and oral casopitant (150 mg, day 1; 50 mg, days 2–3), IV dexamethasone (8 mg, day 1; 8 mg twice daily, days 2–3), and oral ondansetron (8 mg twice daily, day 1) in regimen F. Blood samples for PK analysis were collected at fixed times. The pharmacokinetic results of the Part 2 regimens demonstrated a 28% increase in mean casopitant AUC on day 1, when casopitant was co-administered with 12 mg oral dexamethasone and 32 mg ondansetron compared to casopitant administered alone. Further, it was shown that on Day 1, the lower dose of dexamethasone (12 mg) as used in regimen C resulted in a lower mean dexamethasone AUC and maximum concentration (Cmax), by 17% and 35%, respectively, when compared to dose regimen B (20 mg of dexamethasone). Dose normalization of the pharmacokinetic parameters showed that casopitant increased the AUC of oral dexamethasone by 39%. After three days of co-administration, AUC resulting from 8 mg once daily of oral dexamethasone combined with 50 mg oral casopitant was similar to that resulting from 8 mg twice daily of oral dexamethasone alone. Plasma exposures of 32 mg ondansetron were not affected by co-administration with casopitant. The results of Part 2 showed a 16% increase in mean casopitant AUC on day 1, when casopitant was co-administered with 8 mg oral dexamethasone and 8 mg ondansetron twice daily, compared to casopitant administered alone. As to dexamethasone an increase in AUC day 1 by 21% was observed when dexamethasone (8 mg) was co-administrered with 150 mg casopitant and 8 mg ondansetron twice daily. The pharmacokinetics of ondansetron in Part 2 was not altered by co-administration with casopitant. All dose regimens were generally well tolerated, with headache and dizziness being the most commonly reported adverse events (AEs). In conclusion, the study suggests a reduction in dexamethasone dose of 40%–50%, when repeat-dose oral dexamethasone is to be co-administered with oral casopitant, whereas there is no need to change the dose of ondansetron or casopitant.Citation34
In another phase I, two-part, two-period study, the effect of casopitant on the pharmacokinetics of two 5-HT3-RAs, dolasetron and granisetron, was investigated. Dolasetron is reduced to its active metabolite, hydrodolasetron, which is metabolized by CYP2D6 with minor involvement of CYP3A4. Plasma exposures of hydro-dolasetron are usually increased approximately threefold in CYP2D6 poor metabolizers (PMs) as compared to extensive metabolizers (EMs). For CYP2D6 PMs, CYP3A4 is likely to play a larger role in the clearance of hydrodolasetron, and these subjects may be more sensitive to co-administration of inhibitors of CYP3A4, such as casopitant. Granisetron is primarily metabolized by CYP3A4 with a minor contribution from CYP1A1. A total of 18 subjects, (nine were CYP2D6 EMs and nine were CYP2D6 PMs), received oral dolasetron 100 mg days 1–3 (period 1), and 5–14 days later the same dose of dolasetron combined with oral casopitant 150 mg day 1, and 50 mg days 2–3 (period 2). The granisetron cohort (19 subjects) received oral granisetron 2 mg days 1–3 (period 1), and 5–14 days later combined with oral casopitant 150 mg day 1, and 50 mg days 2–3 (period 2). Blood samples for PK analysis were collected at fixed times. The largest changes in hydrodolasetron exposure after coadministration with casopitant were seen in CYP2D6 EMs, with a 24% increase in hydrodolasetron AUC on day 1 and 30% increase in Cmax on days 1 and 3. All other changes in hydrodolasetron exposure were <20%, and granisetron exposure was not altered to any relevant extent (<11%). None of the changes observed are considered clinically meaningful. Coadministration of casopitant with dolasetron or granisetron was well tolerated.Citation33
In a phase I trial, the effect of casopitant on the PK and PD of steady-state warfarin in healthy adults was studied. In vitro studies had shown that casopitant is a dose and durationdependent inhibitor of CYP3A4, and a moderate inducer of CYP2C9. These enzymes are important in the metabolism of warfarin. Subjects received warfarin and were randomized to receive either casopitant, 150 mg day 1, 50 mg days 2 and 3, and warfarin, days 1–10, or casopitant, 60 mg daily, and warfarin, days 1–14. When casopitant was administered for three days, there was no significant alteration in steady-state Cmax and AUC of R- and S-warfarin. In the other regimen R- and S-warfarin AUC was increased 1.31- and 1.27-fold, respectively. However, steady-state international normalized ratio (INR) was not significantly affected with either regimen.Citation35
A number of other studies have addressed the role of CYP-enzymes and drug-interactions with casopitant. These studies enrolled a limited number of patients, but it seems likely that casopitant can be administered safely with drugs metabolized by CYP3A4, such as cyclophosphamide and docetaxel.Citation36,Citation37 Vinorelbine and etoposide, (likely to be co-administered with cisplatin), are metabolized by CYP3A, potentially leading to increased plasma levels of these agents when co-administered with casopitant. Oral contraceptives are also metabolized by CYP3A-enzymes. Co-administration of casopitant and oral contraceptives may result in lower levels of the hormones, causing the oral contraceptives to be an uncertain method of contraception when co-administred with casopitant. No data on these potential interaction risks have been published.
Ketoconazole is a strong CYP3A4 inhibitor. A phase I study characterized the effect of ketoconazole on the PK of casopitant, demonstrating a four- to six-fold increase in casopitant exposure. However no safety concerns were noted.Citation38 Yet another phase I study was conducted to investigate the potential of casopitant to prolong the QTc interval in supratherapeutic doses and when combined with ketoconazole. Compared with placebo, no significant impact on QTc was observed.Citation39
No available data has been published as concerns; oral absorption fraction, influence of concomitant food consumption, half-life, or percentage of NK1 receptor occupancy needed for optimal efficacy of casopitant.
Clinical development
The clinical development of casopitant in preventing CINV comprises phase I, phase II, and phase III trials. In the section above phase I trials were described. Phase II and phase III trials handle documentation for casopitant in the prevention of CINV in patients receiving MEC and HEC, respectively.
Phase II trials
Patients treated with MEC
A large phase II, multicenter, randomized, double-blind, placebo-controlled, dose-ranging, parallel group trial, evaluated the addition of casopitant to standard prophylaxis (ondansetron plus dexamethasone) in patients receiving MEC.Citation40 Primary endpoints were rates of complete response (CR), defined as no vomiting or retching, no use of rescue antiemetics and no premature withdrawal during the first 120 hours after initiation of MEC; and no significant nausea (SN), during the first 120 hours after initiation of MEC. The MEC regimens used were: cyclophosphamide (500–1500 mg/m2) with another unspecified MEC; cyclophosphamide (750–1500 mg/m2) alone; oxaliplatin (≥85 mg/m2); doxorubicin (≥60 mg/m2); epirubicin (≥90 mg/m2); or carboplatin AUC ≥ 5. Patients were stratified by gender and taxane use. 719 patients were randomized to six arms; arms 5 and 6 being exploratory. In addition to ondansetron 8 mg twice-daily day 1–3 and dexamethasone 8 mg IV day 1, Patients in arms 1–5 received either placebo, casopitant 50 mg daily days 1–3, casopitant 100 mg daily days 1–3, casopitant 150 mg daily days 1–3, or casopitant 150 mg day 1. Patients in arm 6 received ondansetron 16 mg daily days 1–3, dexamethasone 8 mg day 1 and casopitant 150 mg daily days 1–3. CR (120 h) was achieved in 81% of patients receiving 50 mg casopitant, compared to 70% in the control arm (p = 0.0410). CR (120 h) rates for the 100 mg and 150 mg casopitant arms were 79% and 85%, respectively (p = 0.1092, 0.0124). There were no significant differences among groups in the rates of CR (24 h) or no SN. The exploratory arm 5, casopitant 150 mg day 1, was of particular interest, with CR (120 h) rate and no SN rate of 80% and 66%, respectively, in arm 6 the figures were 84% and 70%. The antiemetic regimens were well tolerated, with nausea, fatigue and neutropenia being the most common side effects.Citation40
A subgroup analysis from the phase II trial examined the antiemetic efficacy in patients (n = 124) with gastrointestinal cancers receiving oxaliplatin ≥85 mg/m2. The majority were diagnosed with colorectal cancer (90%). CR was achieved in 83%, 76%, and 86% in the three casopitant arms (50 mg, 100 mg, and 150 mg, respectively), compared to 61% in the control arm. Although one should be cautious with interpretation of data of a small subgroup analysis, CR rates were similar with those of the complete dataset.Citation41
Safety and efficacy of casopitant in women with breast cancer, was considered in another subgroup analysis of the phase II trial. Patients (n = 176) received one or more of the following regimens: cyclophosphamide (C) (500–1500 mg/m2) with another unspecified MEC; cyclophosphamide (750–1500 mg/m2) alone or with another minimally emetogenic agent; doxorubicin (A) (≥60 mg/m2); or epirubicin (E) (≥90 mg/m2). The majority received a combination of AC or EC (n = 102) or a taxane (n = 37). This subgroup analysis found that CR rates were similar to the overall response profile. The antiemetic regimens were generally well tolerated. Nausea (24%), alopecia (17%), neutropenia (16%), anorexia (13%), and fatigue (12%), were the most commonly reported side effects in this patient group.Citation42
Patients treated with HEC
Another phase II trial examined the antiemetic efficacy of casopitant in addition to standard antiemetic prophylaxis in patients receiving cisplatin (≥70 mg/m2) day 1.Citation43 It was conducted as a multicenter, randomized, double-blind, placebo-controlled, dose-ranging, parallel group study. Patients (n = 493) were stratified by gender and randomized among six arms. All patients received ondansetron 32 mg IV day 1 and dexamethasone PO day 1–4. The casopitant doses in arms 2, 3, and 4 were the same as in the phase II, MEC study (50 mg, 100 mg, and 150 mg daily days 1–3) and compared to placebo in arm 1. Arms 5 and 6 were exploratory with casopitant 150 mg day 1 and aprepitant (125 mg day 1 and 80 mg day 2–3), respectively. As in the MEC study, results demonstrated that casopitant significantly improved the CR (120 h) rates. CR (120 h) was achieved in 76% of patients receiving 50 mg casopitant compared to 60% in the control arm. CR (120 h) rates for the 100 mg and 150 mg casopitant arms were 86% and 77%, respectively. Again it was revealed that casopitant administered as a single dose, 150 mg day 1, resulted in a similar high response rate, 75%. The CR (120 h) was 72% for the three-day aprepitant regimen. The prevention of emesis in the 24 hours after cisplatin was similar in all groups with CR rates in the range of 86%–96%. Again the addition of casopitant to standard prophylaxis was well tolerated. Neutropenia, nausea and hiccups (≤17%) were the most common side effects reported.Citation43
The phase II studies indicated that a single dose of casopitant 150 mg was as good as a three-day regimen. Furthermore no evident dose-efficacy correlation was observed (although casopitant 50 mg and 150 mg was superior to casopitant 100 mg in the phase II MEC trail, as the latter was insignificant). Emergence of the intravenous formulation of casopitant and the results from the phase II trials contributed to the design of the phase III trials.
Phase II trials
Patients treated with MEC
The phase III, MEC study was a multinational, double-blind, placebo-controlled trial.Citation44,Citation45 Patients (n = 1933, 95% diagnosed with breast cancer) received a regimen consisting of an anthracycline plus cyclophosphamide (AC). In addition to antiemetics in the control arm (dexamethasone 8 mg IV day 1 and ondansetron 8 mg twice daily PO day 1–3) patients were randomized to receive either placebo (control) or one of three dosing regimens: casopitant 150 mg PO day 1; casopitant 150 mg PO day 1 and 50 mg PO day 2–3; or casopitant 90 mg IV day 1 and 50 mg PO day 2–3 (). Therapy was continued for up to four cycles. The primary endpoint was CR rate (120 h), defined as no vomiting or retching, no use of rescue antiemetics and no premature withdrawal during the first 120 hours after initiation of chemotherapy. All casopitant arms were superior to the control arm as concerns CR rates. In the group of patients receiving the single oral dose of casopitant, CR (120 h) was 73% as compared to 59% in controls (p < 0.0001 for all treatment arms) in the first treatment cycle (), and the improvement appeared to be maintained through cycles two to four. An improvement was also demonstrated for the secondary endpoint, no vomiting. Similar observations were noticed for the group receiving casopitant 150 mg PO day 1 and 50 mg PO day 2–3, with CR (120 h) at 73%.Citation44 Finally, patients in the IV/PO group experienced improvement of emesis over the first 120 hours after MEC. CR (120) was increased to 74% versus 59% in the control group, and again improvement was maintained in repeat cycles of MEC.Citation45 No significant differences in protection from nausea was seen. There were no differences in distribution and occurrence of side effects among the groups. Neutropenia (with sequelae at 3% incidence were seen in all arms), alopecia, fatigue, leukopenia and constipation were the most commonly experienced sideeffects; there were only few reports of injection site reactions, and these were seen slightly more frequently in the IV/oral group.Citation44,Citation45 It is noteworthy that casopitant administered as a single oral dose exhibits similar efficacy as the three-day IV/oral dose regimen.
Table 1 Dose regimens, complete response (CR), and no emesis (EE) of phase II and phase III clinical trials in patients receiving MEC or HEC
Patients treated with HEC
The phase III, HEC trial was conducted to assess the impact of casopitant, when used in combination with ondansetron and dexamethasone as compared to ondansetron and dexamethasone alone.Citation46,Citation47 810 patients received cisplatin-based chemotherapy in a dose of ≥70 mg/m2 and participated in a maximum of six cycles. The study was designed as multinational, double-blind and placebo-controlled. The control regimen consisted of ondansetron 32 mg IV plus dexamethasone 20 mg PO day 1, and dexamethasone 8 mg PO twice daily day 2–4 (). Patients were randomized to the control regimen or one of two experimental arms: a single dose of casopitant 150 mg PO; a three-day IV/oral dose, consisting of casopitant 90 mg IV day 1 and casopitant 50 mg PO day 2–3. The primary endpoint was complete response in the first 120 hours (CR, 120 h) after initiation of HEC. In the casopitant 150 mg PO day 1 arm a statistically significant increase of 20% (86% vs 66% in controls, p = 0.0001) in the number of patients with CR (120 h) was obtained (), and this was maintained over multiple cycles. For the HEC regimen casopitant also demonstrated efficacy with regard to several secondary endpoints: CR (24 h) rates were 95% and 88% for the casopitant 150 mg and control groups, respectively (p = 0.0044); and interestingly improvements in no significant nausea (no SN), no nausea (NN) and no vomiting (NV) for both the acute and delayed phases, were observed as well.Citation46 Casopitant given as a three-day IV/oral dose regimen also demonstrated to be superior to the control arm. CR (120 h) was achieved in 80% of patients (p = 0.0004) and CR (24 h) was 94% in this group (p = 0.0165), moreover efficacy was maintained over multiple cycles. Again clinically meaningful improvements were observed for no SN, NN, or NV.Citation47 Casopitant was generally well tolerated. The most frequently reported side effects were neutropenia, leukopenia and anemia occurring with similar frequency in the experimental- and control arms.Citation46,Citation47
Figure 2 Patients (%) with complete response (120 h) in the phase III, HEC trial.Citation46,Citation47
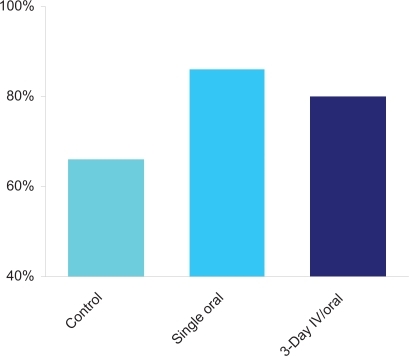
Table 2 Antiemetic regimens in the phase III, HEC trialCitation46,Citation47
Quality of life
Using the Functional Living Index – Emesis (FLIE) questionnaire, Citation48 a sub-study addressed quality of life in patients receiving HEC and the casopitant antiemetic regimens, as described in the phase III, HEC study. The primary end point was no impact on daily life (NIDL) defined as a total FLIE score >108. In the study 79% of patients in both casopitant arms met the NIDL criteria compared to 65% in the control arm (p = 0.0005 for single dose, p = 0.0003 for a three-day regimen), demonstrating that casopitant, when added to standard antiemetic prophylaxis, reduces the impact of both nausea and vomiting on daily life activities in patients receiving cisplatin-based HEC.Citation49
Conclusion
Casopitant is a novel NK1 receptor antagonist and second in the class of antiemetics that acts to antagonise the emetogenic effect of substance P. Casopitant has now completed phase III trials for prevention of CINV. The drug acts as a substrate and a weak-to-moderate inhibitor of CYP3A4.Citation33 Hence the early clinical development of casopitant is characterized by several phase I studies addressing potential drug–drug interactions and toxicity.Citation33–Citation39 Overall, casopitant co-administered with ondansetron and dexamethasone, warfarin, cyclophosphamide, docetaxel, dolasetron, or granisetron caused no toxicity.Citation33–Citation38 Nor the four- to six-fold increase in casopitant exposure when co-administered with the strong CYP3A4 inhibitor, ketoconazole, caused any safety concerns, but should of course be taken into consideration if co-administration of these drugs are indicated. Furthermore casopitant in this combination did not cause any significant impact on QTc interval.Citation38,Citation39 Although casopitant and dexamethasone is a safe combination, it is recommended that dexamethasone dose should be reduced by 50%, when repeat-dose oral dexamethasone is to be co-administered with casopitant.Citation33
The primary aim of the two phase II trials’ was finding of the appropriate dose of casopitant for phase III studies. In the phase II, MEC trial doses of casopitant 50 mg, 100 mg, and 150 mg on days 1–3 plus antiemetics as in the control arm, were compared to the control arm (ondansetron and dexamethasone). All dose regimens proved to be statistically significant superior to the control regimen. Furthermore the single oral dose of casopitant 150 mg (exploratory arm) seemed to possess antiemetic efficacy in the same size as the three-day regimens. Similar results were demonstrated in the phase II, HEC trial. All doses were well tolerated and the most common side-effects experienced by patients in the two phase II trials were; nausea, alopecia, neutropenia, nausea, anorexia, hiccups, and fatigue.Citation40–Citation43 An intravenous formulation of casopitant became available before initiation of phase III studies. Consequently, the two large phase III trials investigating patients receiving MEC and HEC, respectively, included both oral and intravenous dose regimens of casopitant. In both the phase III MEC and HEC studies, all casopitant arms (150 mg single oral dose, three-day IV/oral and three-day oral) demonstrated statistically significant improvement in complete response rates over the first 120 hours compared to ondansetron plus dexamethasone alone.Citation44–Citation47 An important finding was that the single oral dose of casopitant 150 mg proved to be as efficient as the threeday regimens, and all regimens seemed to protect patients against emesis in the same order as seen in previous studies with aprepitant.Citation6–Citation9
No unexpected side effects have been described in phase II–III studies (only abstract publications available). Of particular interest is the low degree of neutropenia with or without fever. As mentioned, a potential risk of febrile neutropenia will be co-administration of casopitant with agents like vinorelbine and etoposide, because these are metabolized through CYP3A4.
The role of the NK1 receptor antagonists is further established with the results of the casopitant studies. Drugs in this class of antiemetics are effective in reducing emesis induced by both MEC and HEC, and have a significant but less pronounced effect against nausea from HEC. Currently aprepitant is recommended by the guidelines for prevention of CINV in patients receiving HEC and in patients receiving MEC including an anthracycline plus cyclophosphamide. Citation50–Citation52 The antiemetic guidelines of the Multinational Association of Supportive Care in Cancer (MASCC) and those of the European Society for Medical Oncology (ESMO) will be updated in a meeting in June 2009. This update should take into consideration the results of the phase III casopitant trials.
Casopitant has also been investigated for the prevention of PONV.Citation53 Future trials should explore other indications for casopitant such as patients receiving radiotherapy with or without concomitant chemotherapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- CoatesAAbrahamSKayeSBOn the receiving end – patient perception of the side-effects of cancer chemotherapyEur J Cancer Clin Oncol1983192032086681766
- GriffinAMButowPNCoatesASOn the receiving end. V: Patient perceptions of the side effects of cancer chemotherapy in 1993Ann Oncol199671891958777177
- GrallaRJItriLMPiskoSEAntiemetic efficacy of high-dose metoclopramide: Randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomitingN Engl J Med19813059059097024807
- Italian Group for Antiemetic ResearchOndansetron + dexamethasone vs metoclopramide + dexamethasone + diphenhydramine in prevention of cisplatin-induced emesisLancet199234096991352024
- MartyMPouillartPSchollSComparison of the 5-hydroxytryptamine3 (serotonin) antagonist (GR 38032F) with high-dose metoclopramide in the control of cisplatin-induced emesisN Engl J Med19903228168212137902
- HeskethPJGrunbergSMGrallaRJThe oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin – the aprepitant protocol 052 study groupJ Clin Oncol2003214112411914559886
- Poli-BigelliSRodrigues-PereiraJCaridesADAddition of the Neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomitingCancer2003973090309812784346
- WarrDGHeskethPJGrallaRJEfficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapyJ Clin Oncol2005232822283015837996
- SchmollHJAaproMSPoli-BigelliSComparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatmentAnn Oncol2006171000100616524979
- PeroutkaSJSnyderSHAntiemetics: Neurotransmitter receptor binding predicts therapeutic actionsLancet1982182736586596121969
- BorisonHLMcCarthyLENeuropharmacology of chemotherapyinduced emesisDrugs198325Suppl 18176132805
- AndrewsPLRRapeportWGSangerGJNeuropharmacology of emesis induced by anti-cancer therapyTrends Pharmacol Sci1988993343413078093
- MinerWDSangerGJTurnerDHEvidence that 5-hydroxytryptamine3 receptors mediate cytotoxic drug and radiation-evoked emesisBr J Cancer19875621591623311109
- Von EulerUSGaddumJHAn unindentified depressor substance in certain tissue extractsJ Physiol (Lond)1931748716994201
- ChangMLeemanSEIsolation of sialogogic peptide from bovine hypothalamic tissue and its characteristic as substance PJ Biol Chem197024518:478447905456150
- MaggiCAThe mammalian tackykinin receptorsGen Pharmac1995265:911944
- CarpenterDOBriggsDBStromingerNPeptide-induced emesis in dogsBehav Brain Res19841132772816721920
- AndrewsPLRBhandariPResinferatoxin, an ultrapotent capsaicin analogue, has anti-emetic properties in the ferretNeuropharmacology1993328:7998068413843
- TattersallFDRycroftWFrancisBTachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in the ferretNeuropharmacology199635112111299121615
- DiemunschPGrélotLPotential of substance P antagonists as antiemeticsDrugs20006053354611030465
- SniderRMConstantineJWLoweJAIIIA potent nonpeptide antagonist of the substance P (NK1) receptorScience19912514354371703323
- BountraCBunceKDaleTAnti-emetic profile of a non-peptide neurokinin NK1 receptor antagonist, CP-99,994, in ferretsEur J Pharmacol1993249R3R47506663
- TattersallFDRycroftWHargreavesRJHillRGThe tachykinin NK1 receptor antagonist CP-99,994 attenuates cisplatin induced emesis in the ferretEur J Pharmacol1993250R5R68119305
- GardnerCJTwissellDJDaleTJThe broad-spectrum anti-emetic activity of the novel non-peptide tachykinin NK1 receptor antagonist GR203040Br J Pharmacol1995116315831638719790
- MinamiMEndoTYokotaHEffects of CP-99,994, a tachykinin NK1 receptor antagonist, on abdominal afferent vagal activity in ferrets: evidence for involvement of NK1 and 5-HT3 receptorsEur J Pharmacol200142821522011675038
- MinthornEMenckenTKingAGPharmacokinetics and brain penetration of casopitant, a potent and selective neurokinin-1 receptor antagonist, in the ferretDrug Metab Dispos2008361846185218556439
- KingAEvaluation of the NK1 receptor antagonist, GW679769, in cisplatin-induced acute and delayed emesis in the ferretSupport Care Cancer200513401483Abs 04–030.
- KingAComparison of anti-emetic and peri-emetic behaviours of NK1 receptor antagonist GW679769 and aprepitant in the ferretSupport Care Cancer200513401483Abs 04–031.
- KingAComplete rescue from established emesis by the NK1 receptor antagonist, casopitant mesylate, in cisplatin-induced delayed emesis in the ferretJ Clin Oncol200624Suppl 18Abs 18601.
- GagnonRCKingAGCo-administration of ondansetron and casopitant mesylate, a novel NK1 receptor antagonist, results in augmented anti-emetic activity in the ferretJ Clin Oncol200624Suppl 18Abs 18608.
- JohnsonBMHokeJFBandekarRLevinJRussoMWPharmacokinetics and pharmacodynamics (PK/PD) of casopitant, an NK1 receptor antagonist, in patients undergoing treatment with moderately and highlyemetogenic chemotherapyClin Pharmacol Ther200781Suppl 1Abs PIII30.
- JohnsonBMHokeJFBandekarRBlackburnLMLevinJRussoMWCasopitant for the prevention of post-operative nausea and vomiting (PONV): population pharmacokinetics and pharmacodynamics (PK/PD)Clin Pharmacol Ther200781Suppl 1Abs PIII29.
- AdamsLJohnsonBZhangKeYueLinKirbyLStoltzRMinimal impact of casopitant, novel neurokinin-1 receptor antagonist, on the pharmacokinetics of dolasetron and granisetronSupport Care Cancer2009DOI 10.1007/s00520-008-0572-4.
- JohnsonBAdamsLLuEImpact of casopitant, a novel NK1 antagonist, on the pharmacokinetics of ondansetron and dexamethasoneSupport Care Cancer2009DOI 10.1007/s00520-008-0571-5.
- BlumRAdamsLMJohnsonBMEffect of casopitant, a novel neurokinin-1 (NK1) receptor antagonist for prevention of chemotherapy induced nausea and vomiting (CINV), on the pharmacokinetics (PK) and pharmacodynamics (PD) of steady-state warfarinJ Clin Oncol200826Suppl 15Abs 20587.
- MacapinlacMBiggsDDGuarinoMJohnsonBMLebowitzPFSuS-FInterim results of an open-label, repeat dose, randomized, crossover study to investigate safety and potential pharmacokinetic interactions between the oral neurokinin-1 receptor antagonist casopitant mesylate and intravenous cyclophosphamide in cancer patientsProceedings of the 29th Annual San Antonio Breast Cancer Symposium2006; Abs 6088.
- AdamsLJohnsonBBaumanJEffect of oral (po) casopitant, a novel neurokinin-1 receptor antagonist, on the pharmacokinetics (PK) and safety profile of intravenous (iv) docetaxelProceedings of the 20th EORTC-NCI-AACR Symposium: Molecular Targets and Cancer Therapeutics2008; Abs 396.
- KirbyLCJohnsonBMAdamsLMImpact of strong inhibitors or inducers of CYP3A on the pharmacokinetics of casopitant, a novel NK1 receptor antagonistPoster presented at the American Society for Health-System Pharmacists meeting2008
- JohnsonBZhangKFangLUse of modeling and simulation in the QTc assessment of casopitantPhiladelphia, PAPoster presented at American College of Clinical Pharmacology 36th Annual MeetingSeptember 14–16, 2008
- ArpornwiratWAlbertIHansenVLMulticenter, randomized, double-blind, ondansetron (ond)-controlled, dose-ranging, parallel group trial of the neurokinin-1 receptor antagonist (NK1 RA) casopitant mesylate for chemotherapy-induced nausea/vomiting (CINV) in patients (pts) receiving moderately emetogenic chemotherapy (MEC)J Clin Oncol200624Suppl 18Abs 8512.
- HansenVLGabrailNLevinJBandekarRHerrstedtJAntiemetic efficacy of the novel neurokinin-1 (NK1) receptor antagonist, casopitant, in patients with GI cancers receiving oxaliplatin-based chemotherapy (OX): Subgroup analysis from a randomized, double-blind, placebocontrolled phase II trialProceedings of the ASCO Gastrointestinal Cancers Symposium2008Abs 359.
- HerrstedtJArpornwiratWAlbertIHansenVLBandekarRRLevinJGrunbergSMSafety and efficacy of the novel antiemetic neurokinin 1 (NK1) receptor antagonist, casopitant, in women with breast cancer (BC) receiving moderately emetogenic chemotherapy (MEC) – subgroup analysis from a randomized, double-blind, placebocontrolled phase II trialProceedings of the 6th European Breast Cancer Conference (EBCC6)2008Abs 492.
- RolskiJRamlauRDediuMRandomized phase II trial of the neurokinin-1 receptor antagonist (NK1 RA), casopitant mesylate with ondansetron (ond)/dexamethasone (dex) for chemotherapyinduced nausea/vomiting (CINV) in patients (pts) receiving highly emetogenic chemotherapyJ Clin Oncol200624Suppl 18Abs 8513.
- GrunbergSMAzizZShaharyarAPhase III results of a novel oral neurokinin-1 (NK1) receptor antagonist, casopitant: Single oral and 3-day oral dosing regimens for chemotherapy-induced nausea and vomiting (CINV) in patients (pts) receiving moderately emetogenic chemotherapy (MEC)J Clin Oncol200826Suppl 15Abs 9540.
- AzizZArpornwiratWHerrstedtJPhase III results for the novel neurokinin-1 (NK1) receptor antagonist, casopitant: 3-day IV/oral dosing regimen for chemotherapy-induced nausea and vomiting (CINV) in patients (Pts) receiving moderately emetogenic chemotherapy (MEC)J Clin Oncol200826Suppl 152008; Abs 20512.
- GrunbergSRussoMBandekarRWrightOCamlettIHerrstedtJPhase III Results of a single oral and a 3-day IV/oral dosing regimen for the novel neurokinin-1 (NK1) receptor antagonist, casopitant: in the prevention of chemotherapy-induced nausea and vomiting (CINV) In patients receiving highly emetogenic chemotherapy (HEC)Support Care Cancer200816619756Abs 01–008.
- StrauszJRolskiJAzizZPhase III results for the novel neurokinin-1 (NK1) receptor antagonist, casopitant: 3-day IV/oral dosing regimen for chemotherapy-induced nausea and vomiting (CINV) in patients (Pts) receiving highly emetogenic chemotherapy (HEC)J Clin Oncol200826Suppl 15Abs 20585.
- LindleyCMHirschJDO’NeillCVTransauMCGilbertCSOsterhausJTQuality of life consequences of chemotherapy-induced emesisQual Life Res199213313401299465
- HaideraliABandekarRGuckertMCasopitant, a novel neurokinin-1 (NK1) receptor antagonist, improves the quality of life of patients receiving highly emetogenic chemotherapy (HEC)Support Care Cancer200816619756Abs 01–009.
- The Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC)Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus ConferenceAnn Oncol200617202816314401
- KrisMGHeskethPJSomerfieldMRAmerican Society of Clinical Oncology Guideline for Antiemetics in Oncology: Update 2006J Clin Oncol2006242932294716717289
- NCCN Clinical Practice Guidelines in OncologyAntiemesis v.3. 2008Accessed on March 1, 2009. Available from: http://www.nccn.org/
- GlaxoSmithKlineCasopitant and zofran to prevent post operative nausea and vomiting in women. No results available. October 15, 2008. Accessed on March 1, 2009. Available from: http://ClinicalTrials.gov/show/NCT00334152