Abstract
Pain is a frequent and important symptom in cancer patients. Among the available strong opioids, transdermal buprenorphine has been licensed in Europe since 2002, and results from a few clinical studies suggest that it may be a good alternative to the other oral or transdermal opioids. To assess the best available evidence on its efficacy and safety, we carried out a systematic literature review with the aim of pooling relevant studies. We identified 19 eligible papers describing 12 clinical studies (6 randomized controlled trials and 6 observational prospective studies), including a total of about 5000 cancer patients. Given the poor quality of reports and the heterogeneity of methods and outcomes, pooling was not feasible as the type of data was not appropriate for combining the results statistically. A meta-analysis based on individual data is ongoing in the context of the Cochrane Collaboration. In conclusion, although the narrative appraisal of each study suggests a positive risk benefit profile, well designed and statistically powered controlled clinical trials are needed to confirm this preliminary evidence.
Introduction
Advances in prevention, diagnosis, and therapy have extended the life expectancy of cancer patients, but improvement in the survival is still disappointing for most common tumors, mostly when the disease is diagnosed at advanced or metastatic stages. Despite the availability of new and innovative anti-cancer drugs, metastatic patients are indeed unlikely to have important benefit from these treatments either in terms of quantity and quality of life. For most of these patients, the later part of their lives is impaired by pain, fatigue, depression, and other symptoms related to the disease and treatments, which become prominent contributors to suffering. Pain, in particular, is one of the most important problems. Most patients with advanced or metastatic cancer experience pain during their lifeCitation1,Citation2 and despite effective treatments being available, undertreatment has been documented in nearly one of two patients with cancer pain, with a few differences according to geographical, economic, and cultural factors.Citation3 The use of analgesic pharmacotherapy is the mainstay of all guidelines on pain management which are available since 1986.Citation4–Citation7 The World Health Organization (WHO) suggested that the choice of analgesics should be based on pain intensity, and not simply on its etiology; the preferred route of administration should be oral, for drugs ranging from paracetamol and NSAID to strong opioids, morphine being the first choice.Citation4 Since then, a broad spectrum of analgesics has become available, such as morphine, methadone, hydromorphone, oxycodone, fentanyl, and buprenorphine, which have been shown to be effective in treating moderate to severe cancer-related pain. In addition to the traditional analgesics that are deliverable orally or parenterally, a few transdermal delivery systems (TDS) containing fentanyl or buprenorphine have been introduced onto the market, suggesting a potential for improvement in view of their advantages over the oral and parental routes, in terms of non-invasiveness and slow and continuous release of the compound. A recent review has pointed out the advantages and disadvantages of available TDS for cancer pain, focusing on the problem of transdermal dosing conversion.Citation8 Another very recent review updated the pharmacological properties of transdermal buprenorphine with a focus on its ceiling effect,Citation9 and also qualitatively summarized the clinical efficacy and tolerability of TDS containing buprenorphine, using results from clinical studies including cancer patients, as primary evidence. As this work combined the early and late randomized placebo and comparative trials with retrospective and prospective studies, it was difficult to obtain an overall quantitative estimate of the yield of TDS containing buprenorphine in terms of efficacy and safety.
Our paper is based on a systematic review of published literature with the aim of assessing the best available evidence for the effect of TDS containing buprenorphine in chronic cancer pain. We have searched, identified, appraised, selected, and integrated all relevant publications on this topic and attempted to combine statistically the valid studies. The results and comments are presented according to the study design (randomized clinical trials vs observational studies) and are based on 19 papers reporting data from 12 prospective studies involving more than 5000 patients.
Materials and Methods
Synthetic profile of buprenorphine
Excellent profiles of the pre-clinical and clinical pharmacology of buprenorphine have been reported elsewhere.Citation9–Citation11
Briefly, buprenorphine is a semi-synthetic compound derived from tebaine, a natural opium alkaloid, which is structurally similar to morphine, even if several molecular differences confer a higher lipophilicity and a higher pharmacological potency.
Pharmacokinetically, buprenorphine shows very low bioavailability (about 15%) after oral administration,Citation12,Citation13 because of an intense metabolic degradation at the intestinal and hepatic level (first pass). After transdermal administration, the plasma concentration remains more or less steady as a consequence of continuous delivery of the drug. A very high percentage of available buprenorphine is strongly bound to plasma proteins,Citation14 and only the unbound fraction that consists of 3% to 5% of the total plasma buprenorphine may cross the blood–brain barrier. Transit across the blood–brain barrier is regulated by several factors, the most important being the free fraction of the drug, and lipophilicity. Among the opioids, buprenorphine has an intermediate to high lipophilic property. Once buprenorphine has crossed the blood–brain barrier, it must reach its biological target, the opioids receptors (ORs). Buprenorphine presents a high selectivity for the MOR (μ-ORs). In an in vitro study,Citation15 the Ki values of buprenorphine were 0.08 for the MOR, 0.11 for the KOR, and 0.42 for the DOR. For pharmacological efficacy, buprenorphine presents 60% to 65% with respect to MOR (partial agonist).Citation16–Citation22 These results confirm that the analgesic activity of buprenorphine is mainly mediated by MOR; its partial agonist action reflects the potential existence of a ceiling effect that, in clinical practice, has been estimated to be about 15 to 25 mg daily,Citation23 a dosage that may not be compatible with the doses usually prescribed in clinical practice.
In terms of pharmacokinetics, two different metabolic pathways have been observed to work in parallel: the first consists of N-dealkylization by means of CYP3A4, and the second produces three glycuronized compounds. The first metabolic pathway generates nor-buprenorphine, the only molecule having some biological activity. Buprenorphine is eliminated through two routes: unchanged molecules are excreted mainly via the biliary system, whereas metabolites are eliminated via renal excretion, but metabolite accumulation is of minimal importance given that these agents are substantially inactive substances.Citation24 For these reasons buprenorphine may be considered as a safe opioid in cases of reduced renal function, which is a frequent clinical condition in cancer patients, especially in the far advanced phase.Citation9
Transdermal buprenorphine in pain management
Buprenorphine was first synthesized in the late 1960, and introduced in clinical practice for parenteral and sublingual (SL) administration in 1978 and 1981, respectively. In the late 1990s, it was introduced as a transdermal formulation, contained in a matrix patch that can be applied to the skin for a duration of up to 4 days, which was indicated for moderate to severe cancer and chronic pain unrelated to cancer. The continuous release from the matrix across the skin and then into the systemic circulation is regulated mainly by the concentration gradient across the skin and the patch.Citation25 In the matrix patch, the drug is an integral part of the polymer structure, making the delivery system more robust than the reservoir patch. This feature prevents “dose-dumping” and potential overdosing either intentionally or unintentionally, as damaging the patch does not interfere with the controlled release of medication. TDS containing buprenorphine is available with release rates of 35, 52.5, and 70 μg/hour (Transtec®) that correspond to 0.8, 1.2, and 1.6 mg/day of buprenorphine or 60, 90, and 120 mg/day equivalent of oral morphine, respectively.Citation25,Citation26 Recently, low-dose patches (5 to 20 μg/hour released for 7 days) have been marketed in a few countries.Citation26
Transtec® has been available since 2002 in 18 European countries including Russia and three South American countries.
Search strategy and study selection
MEDLINE (from 1966 to April 2009), EMBASE, and the Cochrane Library were searched, and only papers written in English were taken into account for data extraction; the main search terms were: ‘pain’, ‘neoplasms’, ‘buprenorphine’, ‘transdermal’, and ‘randomized controlled trial’ (RCT), as well as combinations of these terms. The complete search strategy can be obtained from the authors on request. References in the published articles, reviews, meta-analyses, and relevant organization websites were checked. No systematic attempt was made to identify unpublished studies.
Data extraction and synthesis
The eligibility assessment of the titles and abstracts was performed in a standardized manner by one reviewer (SD). Two reviewers (SD and GA) independently screened the full papers. Disagreements were resolved by discussion and consensus. Details of the study design, participants, inclusion criteria, clinical setting, study duration, definition of outcomes and endpoints, and results were recorded and summarized. Given the heterogeneity of the studies and the difficulty of extracting quantitative disaggregated information from each study, we undertook a narrative synthesis of each study. Eligible studies were then grouped based on the study design (randomized vs observational). Data from the studies reporting the use of standardized measures of pain intensity were grouped, described, and integrated. In this case, an attempt to summarize the results of the studies reporting the use of standardized measures of pain intensity has been attempted using appropriate statistical methods.Citation27
Results
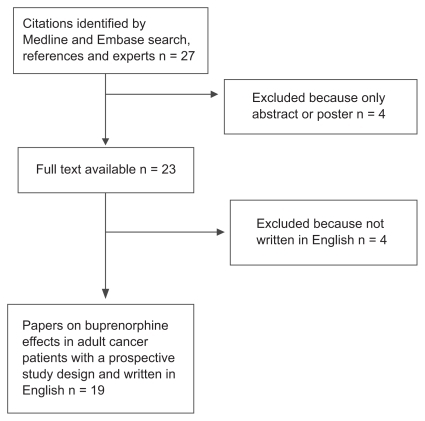
The search of MEDLINE and EMBASE with the review of titles and abstracts, integrated by scanning the references and consulting experts in the field, yielded 27 relevant articles. Four articles were excluded because they were published as abstracts and/or full text was not available, and the other four were excluded because they were written in languages other than English. presents a flowchart of manuscript selection.
The main characteristics of the remaining 19 papersCitation28–Citation46 are reported in . The 19 published articles described 16 different studies: RadbruchCitation29 presented the aggregated data from Bohme,Citation28 Sittl,Citation31 and Sorge;Citation32 Evans reported efficacy and safety data by pooling the 3 placebo-controlled RCTs;Citation25 and RadbruchCitation30 and ApoloneCitation35 reported the preliminary information later published by GriessingerCitation33 and Apolone,Citation46 respectively. Nine were clinical trialsCitation28,Citation31,Citation32,Citation37,Citation39–Citation42,Citation44 and 7 were observational (non-intervention) studies.Citation33,Citation34,Citation36,Citation38,Citation43,Citation45,Citation46
Table 1 List and characteristics of the 19 papers selected
Most of the papers reported data from a mixed population including both cancer and non-cancer patients (12/19).
Three experimental studies were subsequently excluded because they were early-phase feasibility trials: a rotation feasibility trial describing cross-over administration of two drugs,Citation41 a pilot study describing the effect of pre-planned rotation administration of several drugs,Citation39 and a dose escalation administration trial.Citation37 The characteristics of the remaining 6 studies,Citation28,Citation31,Citation32,Citation40,Citation42,Citation44 involving 499 cancer patients (range 9 to 189) are reported in .
Table 2A Characteristics of 6 randomized clinical trials investigating buprenorphine TDS effects in adult cancer patients
One observational study was also excluded, because it was focused on assessing the effect of buprenorphine TDS across pre-planned age groups, to test its effectiveness in a special population:Citation43 the characteristics of the remaining 6 studies (4599 cancer patients, range 6 to 3690) are reported in .
Table 2B Characteristics of 6 observational prospective studies investigating buprenorphine TDS effects in adult cancer patients
RCTs
Six RCTs were available for analysis and discussion. Details of the papers and the studies are summarized in and . Studies may be classified into 3 types: 3 preliminary pivotal studies comparing TDS buprenorphine vs placebo, 2 testing different schedules and a maintenance regimen, respectively, and 1 comparative trial vs morphine.
Table 3 Description of pain intensity outcomes and endpoints in 6 randomized clinical trials and 2 prospective observational studies investigating buprenorphine TDS effects in adult cancer patients
The first Phase III studies were 3 multicenter, randomized, double-blind clinical trials vs placebo (Bohme,Citation28 Sittl,Citation31 Sorge)Citation32 in patients with chronic cancer and non-cancer pain, initiated and sponsored by the industry marketing in Europe the drug under evaluation. In the first and the third study, only patients whose pain was satisfactorily relieved after a run-in phase with buprenorphine SL were randomized. In the study by Sorge,Citation32 only 35 μg/hour dose strength was allowed; the 3 dose strengths (35, 52.5, and 70 μg/hour) were used by BohmeCitation28 and Sittl.Citation31 In the first 2 studies, the primary outcome was the responder’s status defined as ‘patients whose pain relief was at least satisfactory at all determination points (excluding the final examination) and who took a mean of 0.2 mg/day or less of SL buprenorphine on days 7–12’ (Bohme)Citation28 and ‘any patient who required no more than 1 SL tablet of buprenorphine as rescue medication per day from day 2 until the end of the study and who recorded at least satisfactory pain relief at each application of a new patch’ (Sittl).Citation31 The third study, on the contrary, used only the rescue therapy as the primary outcome, in terms of the number of buprenorphine SL tablets required each day. BohmeCitation28 reported a percentage of responder patients of 34% for 35 μg/hour, 37% with for 52.5 μg/hour, and 50% for 70 μg/hour, but these response rates failed to reach statistical significance. The proportion of patients reporting good to complete pain relief, none to mild pain on a verbal rating scale (VRS), and an uninterrupted sleep for more than 6 hours increased during the double-blind phase, but the authors do not report a test of statistical significance vs placebo. Overall, 23% of the patients reported adverse events, with no statistically significant differences between the treatment groups. In the study by Sittl,Citation31 the percentage of responders was very similar to that in the BohmeCitation28 study (ie, 36.6%, 47.5%, and 33.3% for the 3 dosages); also, in this case, the difference vs placebo failed to reach statistical significance in the 70 μg/hour patch. Buprenorphine seemed to give better results than the placebo also in terms of rescue therapy (P = 0.002), pain relief, pain intensity, and duration of sleep uninterrupted by pain (P value not reported). The percentage of patients reporting at least 1 adverse event was high for both buprenorphine (85.4%, 80.5%, 75.7% for the 3 patches) and placebo group (73.7%) with no statistical difference. In the study by Sorge,Citation32 the mean daily requirement of buprenorphine SL tablets during the double-blind phase was lower than that required during the run-in for the buprenorphine TDS group (−55.4%, dose-dependent) than for placebo (−45.1%, P = 0.01), but was similar in terms of mean number of daily dose (in mg) consumed (0.5 for all patients, 0.4 vs 0.6 for cancer patients). Pain intensity, pain relief, and sleep quality evaluation suggested a better effect of buprenorphine, although a statistical significance was not reached or it was not evaluated or reported. Adverse events were reported in 54.4% of the buprenorphine TDS group and 42.6% of the placebo group with no significant difference. RadbruchCitation29 reported the aggregated results of these 3 RCTs and classified the patients according to the disease: among the cancer patients, 36% of those with 35 μg/hour patch and 42% and 40% of those with the 52.5 and 70 μg/hour patch were classified as responders vs 23% of the placebo.
LikarCitation40 compared the efficacy and tolerability of the 2 different delivery schedules in subjects who have already responded to buprenorphine: patients (only 9 with cancer) were randomized to a treatment sequence in which they changed the buprenorphine patch every 3 days during the first step of the study (3-day regimen) or every 4 days (4-day regimen). In the second phase, the patients crossed over to the alternative regimen. The 2 regimens failed to show any statistical difference in terms of patient’s and physician’s satisfaction, pain intensity assessed with a numerical rating scale (NRS) (3.73 for 3-day regimen and 3.88 for 4-day regimen), pain relief, and tolerability. The latter findings were presented only in an aggregated way, making it impossible to evaluate the effect of the 2 interventions in cancer patients.
To date, the study by PaceCitation42 is the only comparative trial available. It compared the effects of buprenorphine of 35 μg/hour with the oral SR morphine of 60 mg/day in an open-label study. The patients treated with transdermal buprenorphine experienced significantly greater improvement in pain intensity (−2.5 for buprenorphine, −1.4 for morphine), quality of sleep, and quality of life. Adverse drug reactions (ADRs) were not reported and the side effects that were significantly more frequent for morphine than for buprenorphine, were vertigo, constipation, and nausea.
Finally, more recently, another RCT vs placebo was conducted to evaluate the maintenance of efficacy (Poulain)Citation44 in severe cancer pain. Opioid-tolerant patients were converted to buprenorphine TDS during a run-in phase and then randomized for the 2-week maintenance phase; of the 289 patients initially recruited, only 189 entered the second phase, because 100 discontinued the treatment for adverse events or lack of efficacy. The primary efficacy outcome was the proportion of the responder patients (defined as patients completing at least 12 days of the double-blind period, with an average pain intensity score of <5 on the numerical rating scale during the last 6 days of treatment, and not using more than 2 tablets of rescue medication per day). More patients receiving transdermal buprenorphine of 70 μg/hour responded (74.5%) compared with those receiving placebo (50%) (P = 0.0003). Pain intensity was significantly lower in the TDS containing buprenorphine group (1.8) compared with placebo (2.7). In addition, the daily consumption of SL tablets remained stable during the double-blind phase for buprenorphine, but increased from 0.6 to 1.7 tablets for the placebo group. The incidence of adverse events was slightly higher for buprenorphine than for the placebo group, and the most commonly reported symptoms were nausea, vomiting, and constipation (P value not reported).
Observational prospective studies
The first observation prospective study was a post-marketing surveillance in Germany in 13,179 cancer and non-cancer patients with unsatisfactory pain relief or unacceptable side effects with previous therapy.Citation33 Patients with cancer-related pain were about 30% of the total sample. Seventy percent of them started with 35 μg/hour patch, 22% with 52.5 μg/hour, and 7% with 70 μg/hour; patch strength was changed at visit 1 (median 14 days) in 16% of the patients and at visit 2 (median 40 days) in 2% of the subjects. ‘Good’ or ‘very good’ pain relief was obtained in 84% of the cancer patients. Twenty-seven percent of the cancer patients experienced 1 adverse event (21.6% experienced serious adverse events).
MurielCitation34 in Spain followed up for 3 months the cancer and non-cancer patients who were beginning buprenorphine for moderate to severe pain who had not responded to non-opioid analgesics. Patients began buprenorphine TDS at the lowest possible dosage, and the daily median amount at the baseline was 35 μg/hour (all the patients). At the end of the study, 22% of all the patients had been changed to a higher dosage. The study included 207 patients with cancer (17%). Sixty-five percent of these reported ‘good’ or ‘very good’ pain relief after 1 month, and 57% after 3 months. Other outcomes and adverse events were not reported for the cancer population alone: 63% of all the patients reported and improvement in the quality of sleep after 1 month, and 56% after 3 months, and the quality of life also improved, from a mean EQ-5D score of 40.6 at baseline to 56.8 at the end of the study (P < 0.001); 42% of the patients experienced at least 1 adverse event during the follow-up.
Some patients recruited in the 3 registered clinical trials mentioned earlier,Citation28,Citation31,Citation32 were subsequently included in a follow-up study (Likar)Citation36 where they continued the treatment with 35 μg/hour patch and SL tablets (0.2 mg). There were 134 cancer patients who were followed up for a mean of 119 days, but 107 discontinued after the first 2 months, mainly because of death and insufficient pain relief. Among the cancer patients, 86.6% reported an ‘at least satisfactory’ pain relief throughout the study period. Eighty-four cancer patients reported adverse events; 31 events were probably drug-related and 26 were ADRs (the more frequent were nausea and vomiting).
CambaCitation38 summarized 3 longitudinal multicentre studies conducted by the Sociedad Española del Dolor. The third study involved 164 cancer patients with moderate to severe pain, who did not respond to previous analgesics, and who were followed up for 8 weeks after the first administration of buprenorphine TDS. Fifteen percent of the patients started with 17.5 μg/hour and 82% started with 35 μg/hour of the drug. Mean pain intensity, measured with a visual analogue scale (VAS), at baseline was 7.4 and decreased to 3.2 at week 8 (P < 0.001). This study also included a quality of life questionnaire and parameters such as physical fitness, social and daily activities, and how they felt significantly improved during the course of the observation period. Fifty-one patients discontinued the study; the results of adverse events were not reported for the cancer study alone.
WirzCitation45 conducted a controlled trial with the oral sustained-release hydromorphone, transdermal fentanyl, and buprenorphine on randomly selected 174 cancer patients, to assess the difference in terms of gastrointestinal symptoms in long-term treatment (all patients were pretreated with their current opioid therapy for more than 28 days). Patients were selected for participation by random selection from a sample of outpatients undergoing pain therapy with one of the study medications. Apart from a significantly higher incidence of stool-free periods of > 72 hour for transdermal opioids, there were no differences in the effects of the 3 drugs in terms of nausea, emesis, and constipation.
ApoloneCitation46 followed up 1801 advanced cancer patients with persistent pain of any degree, using several outcomes and endpoints, including pain intensity measured with a NRS, satisfaction, and quality of life: among the patients recruited, 257 were consuming buprenorphine at baseline, with a mean TDS dose of 43.2 μg/hour (median 35) and an average increase to 50.0 at day 28. All the outcome measures consistently improved over time, in terms of statistical significance and clinical relevance. For example, the worst pain differences were −1.4 points (95% confidence interval −1.1/−1.8, P < 0.0001). The effect-size estimates indicated that on average, the endpoints based on pain intensity were more responsive (range 0.2 to 0.6) than those measuring pain relief, satisfaction, or quality of life (range 0.2 to 0.3), and the best measure was worst pain difference. About 34% of the patients had an improvement of at least 2 points in worst pain, nearly 48% improved less or were stable, 15% had a 20% improvement in pain relief, and 40% reported an increase in satisfaction. The most frequent side effects were constipation (56%) and sedation (51%), but the frequency of the symptoms rated by patients as ‘a lot/very much’ was always less than 25%.
Estimate of the overall treatment effect on pain
When studies were evaluated according to the type of outcomes and endpoints used to find a measure to pool studies and estimate the overall treatment effect, a substantial heterogeneity emerged that did not allow statistical pooling.
In the 6 RCTs, 3 studiesCitation28,Citation31,Citation44 shared the same primary outcome (the responder status), an endpoint that may be considered valid and relevant in this setting, but it was actually operationally defined in different ways (mentioned previously). The other primary outcomes were rescue therapy,Citation32 patient satisfaction,Citation40 and pain intensity.Citation42 When we expanded this assessment to the secondary outcomes, among the wide list of measures used (from indicators of pain intensity to safety and quality of life), the only one used in all 6 RCTs was pain intensity, which became our candidate for a formal attempt for a statistical combination.
In the 6 observational studies, the same evaluation again yielded a very heterogeneous result: in 3 cases the primary endpoint was pain relief,Citation33,Citation34,Citation36 in 2 it was pain intensity,Citation38,Citation46 and in 1 it was safety.Citation45
As 8 studies, 6 clinical trialsCitation28,Citation31,Citation32,Citation40,Citation42,Citation44 and 2 observational studies,Citation38,Citation46 have used patients’ reported pain intensity, we focused on this subsample. presents the details of the studies that were candidates for a pooling analysis.
A deeper analysis of the studies’ characteristics and efficacy estimates reported in the papers showed that in this subsample also, the type of data was not appropriate for combining the results. For example, although all the studies used some standardized measures of pain intensity, the assessment tools were different: in 4 cases NRS was used,Citation40,Citation42,Citation44,Citation46 in 3 cases VRS was used,Citation28,Citation31,Citation35 and in 1 case VAS was used.Citation38 In addition, for NRS different anchors were actually used to identify the least and worst pain intensity. In 4 casesCitation28,Citation31,Citation32,Citation40 it was not possible to distinguish the results between cancer and non-cancer patients. Finally, the period of time chosen by the authors to estimate the difference of pain intensity attributable to the intervention varied from 1028,Citation32 to 56 days.Citation38
Estimate of overall safety and tolerability
After the narrative description of safety and tolerability given for each paper and study, we tried to summarize and integrate these important characteristics; however, papers describing the 12 clinical studies reported the safety and tolerability of the drugs under evaluation using different and heterogeneous methods and terms. Most authors reported side effects in terms of adverse events, ADR, or simply using a list of most frequent symptoms reported by patients. Sometimes, adverse events and ADR were also classified as severe. Some authors reported side effects that were attributable to opioid administration only, while others classified them according to the system or organ involved, such as skin, central nervous system, or according to the type, such as nausea, vomiting, and constipation. Given the large variability of the methods used, we tried to classify them into the following groups: adverse events, ADR, gastrointestinal (including nausea, vomiting, constipation), central nervous system (including confusion, dizziness), and skin (including pruritus and local erythema). shows the results of such reclassification and synthesis. Most of the times, it was not possible to identify the exact quantity and type of the side effects, as the authors did not report the estimates according to the type of patients (cancer vs non-cancer). In addition, the unit of analysis was not homogeneous across the studies (as the authors reported either the number of events or the number of patients with at least 1 event), yielding a quite unreliable classification of the phenomenon.
Table 4 Local and systemic safety and tolerability adverse events and adverse drug reactions from 12 selected studies
Discussion
Buprenorphine TDS has been available in most European countries since 2002, to treat moderate to severe chronic pain. The TDS formulation that allows for a slow release by minimizing the typical opioid side effects makes it a good alternative to the other oral opioids in clinical practice. The results from 3 clinical pivotal trialsCitation28,Citation31,Citation32 including only 453 cancer patients, documented its analgesic efficacy, at least in terms of the responders’ status, and the safety profile that was typically opioid in nature and not different from placebo in terms of incidence of reported events; local adverse events associated with TDS application were erythema and pruritus, while the most frequent systemic adverse events were nausea and vomiting. Pharmacokinetics and pharmacodynamics data suggest that buprenorphine TDS may be safely used in aged patients and in renally impaired individuals, although the small sample size of the studies and the restriction in inclusion criteria did not allow any subgroup or interaction analysis. Post-marketing and outcome research of the prospective studies yielded satisfactory results in terms of effectiveness, despite the presence of a large variability in inclusion/exclusion criteria and outcomes assessment. Safety and tolerability results were often reported together for both cancer and non-cancer patients, making it difficult to extract and summarize the findings across the individual original studies. Despite these limitations, the type of adverse events was in line with that expected, given the opioid nature of the drug, and the reported incidence from observational studies, when evaluable, was low, mostly in terms of serious events.
The evidence for comparative efficacy and safety is indeed scanty, as most of the efficacy data are from placebo trials. Only one RCTCitation42 was designed to compare it with morphine. This trial had an open-label design and a very small sample size. As pointed out by other researchers,Citation9 well-designed and statistically powered controlled clinical trials focusing exclusively on treating cancer patients suffering from pain using buprenorphine TDS are still lacking. In addition, our analysis based on a systematic review that included published papers up to April 2009 confirmed that the quality of the 19 reports describing 16 studies was poor and did not allow for safe data pooling. Studies, although stratified according to the study design, were very heterogeneous in terms of population included, interventions studied, comparators evaluated, and outcomes assessed. Even when the same outcome was formally assessed (eg, the responders’ status or the patients’ reported pain intensity), the differences in terms of operational definition of endpoints, timing of the assessment, or simply the type of measures used (NRS vs VRS vs VAS), made it impossible to identify a subsample for formal data synthesis and statistical pooling.
The results of this systematic review confirm the potential of this drug when delivered through a TDS, but it may be considered as a lost opportunity for a meta-analysis. The studies that we retrieved were very few, very heterogeneous, and reported results in a way that prevented an appropriate data extraction and data pooling. This fact might explain why TDS buprenorphine has not yet been recommended by national and international guidelines, and sometimes is not included in the list of reimbursable drugs at national or local levels.
In fact, the poor quality of pain studies is a well-known phenomenon throughout the field, across the type of interventions, countries, and clinical settings. A recent literature review carried out by the European Palliative Care Research Collaborative Group to assess the quality of pain assessment in palliative care documented that in the 230 papers retrieved and evaluated, the methods and tools used were very heterogeneous and did not meet the criteria identified by experts as minimal standards.Citation47,Citation48
In conclusion, buprenorphine TDS has been observed to have good intrinsic (pharmacological) characteristics and promising clinical results, suggesting the need to appropriately review the evidence base for its utility. The results from this systematic literature review are not conclusive and suggest the need for 3 sequential initiatives: 1) a meta-analysis carried out on individual data to minimize the problems that we have found working on aggregated data; 2) an international initiative to find a consensus on definitions, methods, and measures of pain assessment in clinical research; and 3) a well-designed, large, multicenter, international comparative study to produce final evidence about the relative effectiveness and safety of the available third-level WHO opioids. These above-mentioned actions are are now being planned.
For the Cochrane Collaboration, one group has published a protocol titled ‘Buprenorphine for cancer pain’. The authors planned to carry out a systematic review based on an electronic search of 5 databases, including the contact of the original authors of the primary studies for clarification, or to obtain missing information or individual data.Citation49
A joint initiative of 3 non-profit organizations (Center for the Research and Evaluation of Pain, Norwegian University of Science and Technology, Italian National Cancer Institute) planned an expert panel meeting on the issues related to cancer-pain assessment in clinical research. The first meeting involving experts from the USA and Europe, including representatives of FDA and EMEA, will be held in Milan, Italy, in September 2009.
Finally, on the basis of the results of this review and according to the preliminary results of a prospective outcome research study carried out in Italy on 1800 cancer patients with pain,Citation50 a multicenter RCT to compare the efficacy of 4 strong opioids on cancer pain (buprenorphine, morphine, fentanyl, and oxycodone) will be launched at the end of this year, involving about 80 centers and 1000 patients.
Disclosures
Dr Apolone and Dr Corli have received consulting and lecture fees from Grunenthal-Italy.
References
- HearnJHigginsonIJBrueraEDPortenoyRKCancer pain epidemiology: a systematic reviewCancer Pain, Assessment and ManagementCambridgeCambridge University Press2003
- van den Beuken-van EverdingenMHde RijkeJMKesselsAGSchoutenHCvan KleefMPatijnJPrevalence of pain in patients with cancer: a systematic review of the past 40 yearsAnn Oncol2007181437144917355955
- DeandreaSMontanariMMojaLApoloneGPrevalence of undertreatment in cancer pain. A review of published literatureAnn Oncol2008191985199118632721
- World Health OrganizationCancer Pain Relief2nd edGenevaW.H.O1996
- JacoxACarrDBPayneRNew clinical-practice guidelines for the management of pain in patients with cancerN Engl J Med19943306516557508094
- SIGN: Scottish intercollegiate guideline networkControl of pain in patients with cancerA national clinical guideline SIGN publication number 44, June 2000
- HanksGDe ConnoFChernyNfor Expert Working Group of the Research Network of the European Association for Palliative CareMorphine and alternative opioids in cancer pain the EAPC recommendationsBr J Cancer20018458759311237376
- SkaerTLTransdermal opioids for cancer painHealth Qual Life Outcomes200631424
- KressHGClinical update on the pharmacology, efficacy and safety of transdermal buprenorphineEur J Pain20091321923018567516
- McQuayHJMooreRACowanALewisJWBuprenorphine kinetics in humansBuprenorphine: Combating Drug Abuse with a Unique OpioidNew YorkWiley-Liss1995137147
- WeinbergDSInturrisiCEReidembregBSublingual absorption of selected opioid analgesicsClin Pharmacol Ther1988443353422458208
- GarrettERChandranVRPharmacokinetics of morphine and its surrogates: bioanalysis, solvolysis kinetics, solubility, pKa values and protein binding of buprenorphineJ Pharmaceut Sci198574515524
- HuangPKehnerGBCowanAComparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonistJ Pharmacol Exp Ther200129768869511303059
- CowanACowanALewisJWupdate on the general pharmacology of buprenorphineBuprenorphine: Combating Drug Abuse with a Unique OpioidNew YorkWiley-Liss19953147
- TraynorJRNahorskiSRmodulation by mu-opioid agonists of guanosine-5’-0-(3-(35S)thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cellsMol Pharmacol1995478488547723747
- SelleyDALiuQChildersSRSignal transduction correlates of mu-opioid agonist intrinsic efficacy: receptor-stimulated [35S]GTP gamma S binding in mMOR-CHO cells and rat thalamusJ Pharmacol Exp Ther19982854965059580589
- TollLBerzetei-GurskeIPPolgarWEStandard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medicationsNIDA Res Monogr19981784404669686407
- LeeKAkilHWoodsJHNovel binding properties of oripavines at a cloned mu-opioid receptorEur J Pharmacol199937832333010493109
- RomeroDVPartillaJSZhengQXOpioid peptide receptor studies. Buprenorphine is a potent and selective mu/kappa antagonist in the [35S]-GTP-gamma-S functional binding assaySynapse199934839410502307
- ZhuJLuoLYChenCActivation of the cloned human kappa opioid receptor by agonists enhances [35S]GTPgammaS binding to membranes: determination of potencies and efficacies of ligandsJ Pharmacol Exp Ther19972826766849262330
- DahanAYassenARombergRBuprenorphine induces ceiling in respiratory depression but not in analgesiaBr J Anaesth20069662763216547090
- RothmanRBCowanALewisJWBuprenorphine: a review of the binding literatureBuprenorphine: Combating Drug Abuse with a Unique OpioidNew YorkWiley-Liss19951929
- VilligerJWTaylorKMBuprenorphine: characteristics of binding sites in the rat central nervous systemLife Sci198129269927086276633
- SidhuBSKhichySSinghKHComparative evaluation of intramuscular buprenorphine, pentazocine and nefopam in postoperative pain reliefJ Ind Med Assoc199391288289
- EvansHCEasthoperSETransdermal buprenorphineDrugs2003631999201012962515
- JohnsonREFuadalaPJPayneRBuprenorphine: considerations for pain managementJ Pain Symptom Manage20052929739615781180
- HigginsJPTGreenSCochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]The Cochrane Collaboration2008
- BöhmeKLikarREfficacy and tolerability of a new opioid analgesic formulation, buprenorphine transdermal therapeutic system (TDS) in the treatment of patients with chronic pain. A randomized, double-blind, placebo-controlled studyPain Clinic200315193202
- RadbruchLVielvoye-KerkmeerABuprenorphine TDS: the clinical development- rationale and resultsJ Clin Pract Suppl20031331528
- RadbruchLBuprenorphine TDS: use in daily practice, benefits for patientsInt J Clin Pract Suppl2003133192212665120
- SittlRGriessingerNLikarRAnalgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: a multicenter, randomized, double-blind, placebo-controlled trialClin Ther20032515016812637117
- SorgeJSittlRTransdermal buprenorphine in the treatment of chronic pain: results of a Phase III, multicenter, randomized, double-blind, placebo-controlled studyClin Ther2004261808182015639693
- GriessingerNSittlRLikarRTransdermal buprenorphine in clinical practice- post-marketing surveillance study of 13,179 patientsCurr Med Res Opin2005211147115616083522
- MurielCFaildeIMicòJANeiraMSanchez-MagroIEffectiveness and tolerability of the buprenorphine transdermal system in patients with moderate to severe chronic pain: a multicenter, open-label, uncontrolled, prospective, observational clinical studyClin Ther20052745146215922818
- ApoloneGManganoSCompagnoniAfor Cancer Pain Outcome Research GroupA multidisciplinary project to improve the quality of cancer pain management in ItalyJ Ambulatory Care Manage20062933234116985391
- LikarRKayserHSittlRLong-term management of chronic pain with transdermal buprenorphine: a multicenter, open-label, follow-up study in patients from three short-term clinical trialsClin Ther20062894395216860176
- MercadanteSFerreraPVillariPIs there a ceiling effect of transdermal buprenorphine? Preliminary data in cancer patientsSupport Care Cancer20071544144417106658
- CambaMRodriguez-LopezMJMurielCBuprenorphine TDS in the treatment of chronic nociceptive, neuropathic and cancer-related painJ Appl Ther Res20076313
- FreyeEAnderson-HillemacherARitzdorfILevyJVOpioid rotation from high-dose morphine to transdermal buprenorphine (Transtec®) in chronic pain patientsPain Practice2007712312917559481
- LikarRLorenzVKorak-LeiteMKagerISittlRTransdermal buprenorphine patches applied in a 4-day regimen versus 3-day regimen: a single-site, phase III, randomized, open-label, crossover comparisonClin Ther2007291591160617919542
- MercadanteSPorzioGFulfaroFSwitching from transdermal drugs: an observational “N of 1” study of fentanyl and burenorphineJ Pain Symptom Manage200753253817629666
- PaceMCPassavantiMBGrellaEBuprenorphine in long-term control of chronic pain in cancer painFront Biosci2007121291129917127381
- LikarRVadlauEMBreschanCKagerIKorak-LeiterMZiervogelGComparable analgesic efficacy of transdermal buprenorphine in patients over and under 65 years of ageClin J Pain20082453654318574363
- PoulainPDenierWDoumaJEfficacy and safety of transdermal buprenorphine: a randomized, placebo-controlled trial in 289 patients with severe cancer painJ Pain Symptom Manage20083611712518411010
- WirzSWittmannMSchenkMGastrointestinal symptoms under opioid therapy: a prospective comparison of oral-sustained release hydromorphone, transdermal fentanyl, and transdermal buprenorphineEur J Pain200810.1016/j.ejpain.2008.09.005
- ApoloneGCorliONegriEManganoSMontanariMGrecoMTon behalf of the writing protocol committee and the Cancer Pain Outcome Research Study Group (CPOR SG) InvestigatorsEffects of transdermal buprenorphine on patients-reported outcomes in cancer patients. Results from the Cancer Pain Outcome Research (CPOR) Study GroupClin J PainIn press2009
- HjermstadMJGibbinsJHaugenDFCaraceniALogeJHKaasaSfor EPCRC European Palliative Care Research CollaborativePain assessment tools in palliative care: an urgent need for consensusPalliat Med20082289590318799513
- KaasaSLogeJHFayersPSymptom assessment in palliative care: a need for international collaborationJ Clin Oncol2008263867387318688054
- ZeppetellaGWiffenPJBuprenorphine for cancer painCochrane Database of Systematic Reviews20074 Art. No: CD00676610.1002/14651858.CD006766
- ApoloneGCorliOCaraceniAfor Cancer Pain Outcome Research Study Group (CPOR SG) InvestigatorsPattern and quality of care of cancer pain management. Results from the Cancer Pain Outcome Research Study Group (CPOR SG)Br J Cancer20091001566157419401688