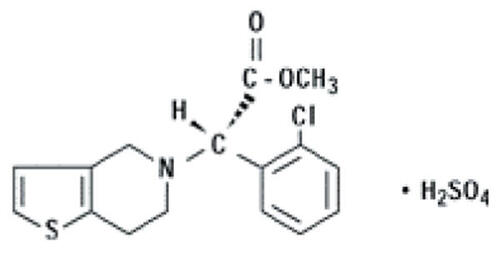
Abstract
Peripheral arterial disease (PAD) is a condition typified by decreased arterial blood flow in the non-coronary branches of the aorta as a result of chronic atherosclerosis. Despite the higher prevalence of PAD compared with other cardiovascular entities such as myocardial infarction and stroke, far less import is given to its diagnosis and treatment. In this review, we highlight principal diagnostic and therapeutic considerations in the management of PAD and its complications. We particularly emphasize the role of clopidogrel in the reduction of risks associated with PAD.
Introduction
For many years, peripheral arterial disease (PAD) has been identified as and limited to occlusive arterial disease in the lower extremities. The more accurate and preferred definition of PAD is atherosclerotic stenosis, occlusion, or aneurysmal disease of the aorta and its non-coronary branches (CitationHirsch et al 1996). PAD in the vast majority of patients is asymptomatic. Symptomatic manifestations vary depending on the location and extent of the disease, the acuity or chronicity of the disease, and whether it leads to stenosis or aneurysms of the arteries afflicted.
In the lower extremities, PAD can manifest as intermittent claudication, characterized as pain or fatigue in the lower extremities during ambulation. In its most severe form, patients may suffer from rest leg pain, non-healing leg ulcers, or frank gangrene leading to limb amputation (CitationCavendish and Safani 2004). In the aorta, atherosclerosis leads to stenoses of the renal, mesenteric and common iliac arteries. The atherosclerosis encroaches on the ostia of theses arteries and causes subsequent renal insufficiency and hypertension, mesenteric ischemia, or severe claudication. Aneurysmal formation can develop in many arteries secondary to atherosclerosis. The aorta, iliac, and popliteal arteries are more commonly affected. Atherosclerosis also affects the brachiocephalic arteries and is a significant cause of stroke when plaque rupture occurs in the carotid or vertebral arteries.
PAD is thought to affect up to 12 million Americans. Numerous epidemiological studies have shown that the prevalence of PAD increases with age (CitationCriqui et al 1985; CitationNewman et al 1993; CitationAronow et al 2002). Symptomatic PAD affects up to 30% of elderly patients as shown in one nursing home cohort (CitationAronow et al 2002). The need for increasing awareness of diagnosis and treatment of PAD is underscored by statistics showing that patients with PAD have a five-fold increased risk of dying from an atherothrombotic cardiovascular event (CitationCriqui et al 1992). Additionally, atherothrombosis, in its varying clinical presentations, is the leading cause of mortality worldwide (CitationHirsch et al 1996; CitationLopez and Murray 1998; CitationFuster et al 2005). Therefore, diagnosing PAD and prevention of atherothrombotic events with aggressive risk factor modification and antiplatelet therapy will have a great impact on the survival of patients with PAD.
Awareness and treatment of PAD has been notably poor over the past two decades. Enthusiasm has increased particularly in light of recent endovascular advances in the treatment of peripheral arterial stenoses. As medical device companies have developed balloons, stents, cryoablation, and atherectomy instruments, a surge to screen, diagnose, and treat these patients has begun. Board certification in vascular medicine is currently available through the American Board of Vascular Medicine, which offers certification examinations in general vascular medicine and endovascular therapy. The publication of the American College of Cardiology (ACC) and American Heart Association (AHA) Guidelines on the Management of Patients with PAD further advances awareness and education with appropriate recommendations for the evaluation and treatment of patients with PAD. (CitationHirsch et al 1996) These guidelines are specific for lower extremity arterial occlusive disease, renal artery stenosis, mesenteric ischemia, and aneurysmal disease of abdominal aorta, its branch vessels, and the lower extremities. The guidelines not only outline appropriate indications for endovascular or surgical revascularization of stenosed arteries, they also clearly and concisely recommend the appropriate work up and include recommendations for aggressive risk factor modification, with lipid-lowering therapy, smoking cessation, walking programs, diet, and antiplatelet therapy (). The ACC/AHA Guidelines for PAD is the source document for all practitioners who care for patients with PAD.
Figure 1 ACC/AHA Guidelines for Cardiovascular Risk Reduction in Patients with peripheral arterial disease. Reproduced from CitationHirsch AT, Haskal ZJ, Hertzer, HR, et al. 2006. ACC/AHA Guidelines for the Management of Patients with Peripheral Artery Disease: Executive Summary. J Am Coll Cardiol, 47:1239–312. Copyright © 2006 with permission from Elsevier.

Pathophysiology of atherothrombosis
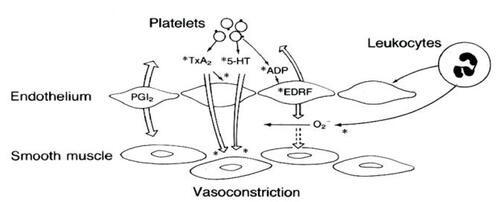
Atherosclerosis appears to be initiated as a result of endothelial injury and dysfunction. A cascade of events ensues over the course of months to years as inflammatory cells, oxidized LDL, and smooth muscle cells form the true atherosclerotic plaque (CitationHirsch et al 1996; CitationFuster et al 2005). The vulnerability of an atherosclerotic plaque has been the subject of intense research. Therapies to decrease potential plaque rupture have had a tremendous impact on clinical outcomes. However, ruptured atherosclerotic plaques continue to cause more morbidity and mortality throughout the world than any other disease. Antiplatelet therapy has been shown to decrease events as the platelet plays a pivotal role in atherothrombosis (CitationCAPRIE 1996; CitationClopidogrel Trial 2001; CitationATC 2002; CitationVorchheimer and Becker 2006). Platelet adhesion, activation, and ultimately aggregation occur immediately following disruption of the endothelium. Platelet adhesion occurs through binding of von Willebrand’s factor and glycoprotein (GP) receptors, along with receptors for fibronectin and collagen. Platelet adhesion leads to activation of the platelet with the liberation of a variety of cytoplasmic granules including adenosine diphosphate (ADP), thromboxane A2 (TXA2), collagen, serotonin, epinephrine, and thrombin (). Platelet adhesion is highly enhanced by the release of these substances. The platelet is then further stimulated to release more of these substances as well as to conformationally change and express GP IIB/IIIA receptors. GP IIB/IIIA receptors bind fibrinogen connecting together millions of platelets forming the platelet plug, the core of an arterial thrombus (CitationLopez and Murray 1998). Thienopyridines are ADP receptor antagonists which irreversibly inhibit platelet activation. Ticlopidine was the first-generation thienopyridine that was essentially replaced by clopidogrel (). Clopidogrel has been tested in thousands of patients and is effective at decreasing platelet activation and subsequent risk of an atherothrombotic event ().
Figure 2 Platelet interactions with diseased endothelium. Endothelial dysfunction, a consequence of a variety of disease states, has important implications for platelet aggregation and vascular tone. When platelets aggregate, they release thromboxane A2 (TxA2), 5-hydroxytryptamine (5-HT, or serotonin), and ADP.
Diagnosis of PAD
Diagnosing PAD is straightforward when patients present with complaints or overt manifestations of an atherothrombotic event. In the asymptomatic patient, a thorough history and review of symptoms, inquiring particularly about exertional symptoms in the upper or lower extremities, is important in diagnosing PAD. The classic symptoms of intermittent claudication are exertional pain or cramping in the legs, brought on by the same degree of exertion and relieved with rest. Recently, more atypical features of claudication have been described and include lower extremity heaviness, generalized pain, or fatigue (CitationMcDermott et al 2001). The presence of symptoms assists in classifying the patient with lower extremity PAD () (CitationDormandy and Rutherford 2003). A review of risk factors for developing PAD (previous or current tobacco use, diabetes mellitus, hypertension, obesity, and dyslipidemia) is also important when diagnosing patients. Diabetic patients are at the highest risk of progression of disease and need for limb-saving surgery. The importance of diagnosis and treatment of PAD cannot be highlighted any more than by noting the fact that in patients with symptomatic PAD, the relative risk of death from cardiac causes is increased almost six-fold (CitationCriqui et al 1992). Diagnosis of PAD is best aided by a comprehensive physical examination. ACC/AHA guidelines give a Class I recommendation for a comprehensive pulse examination for patients at risk for lower extremity PAD (CitationHirsch et al 2006). Blood pressure measurements in both arms should be performed on all patients suspected of having PAD. Left subclavian artery stenosis is under diagnosed and often asymptomatic until the patient develops a severe high grade stenosis. Auscultation for bruits in the neck, supraclavicular area, abdomen, flank, and groins must be done as well. In addition to a thorough history and physical examination, the ankle brachial index is a powerful, yet simple office-based tool to diagnose and determine the severity of lower extremity PAD. Moreover, it is highly accurate in predicting the associated risk of future cardiovascular events such as fatal myocardial infarction (MI), stroke (CVA), and vascular deaths (CitationSikkink et al 1997). The Ankle Brachial-Index measurement (ABI) can be easily performed in an office setting with the use of Doppler, gel, and a sphygmomanometer (CitationHiatt 2001). A normal ABI is between 0.9 and 1.2. An ABI less than 0.90 is 99% specific and 95% sensitive for the diagnosis of PAD (CitationMcDermott et al 2002). An ABI less than 0.9 but above 0.6 is representative of mild to moderate disease. ABIs of 0.4 to 0.6 will result in severe claudication symptoms. Lower ABIs correlate with even more severe disease as rest pain and critical ischemia can be reflected in ABIs of 0.25 to 0.4. If PAD is suspected but the resting ABI is normal, exercise ABIs should be performed. Pulse volume recordings, segmental limb arterial blood pressures and Doppler waveforms also can be performed to provide further information regarding arterial disease. When revascularization is imminent, duplex ultrasonography, MR, and CT angiography can be obtained to map arterial disease anatomically. Conventional angiography is not indicated in patients with claudication symptoms and normal exercise ABIs (CitationHirsch et al 2006). Diagnosis via conventional angiography should be reserved for patients presenting with acute or critical limb ischemia at risk for limb loss and in need of urgent revascularization (CitationHirsch et al 2006).
Table 1 Classificaton of peripheral arterial disease
Management of PAD
The primary goal in treating patients with PAD is two fold: the prevention of future cardiovascular events in addition to maximal secondary preventive measures using antiplatelet therapy and smoking cessation, and treating hypertension, hyperlipidemia, and diabetes mellitus. These steps will lower the risk of future atherothrombotic events as these patients have a significant increased risk of events (). Adhering to a daily regimen of exercise and modifying the dietary intake of fat must be included in this therapeutic plan. The ACC/AHA practice guidelines () give a Class I indication for the use of statins to treat LDL to a goal of less than 100 mg/dL with less than 70 mg/dL as a therapeutic option in the very high risk patients (CitationHirsch et al 2006). Hypertensive patients should be treated aggressively to achieve a systolic less than 130 mmHg and a diastolic less than 80 mmHg. Weight reduction, dietary restrictions and complete tobacco cessation are necessary as well.
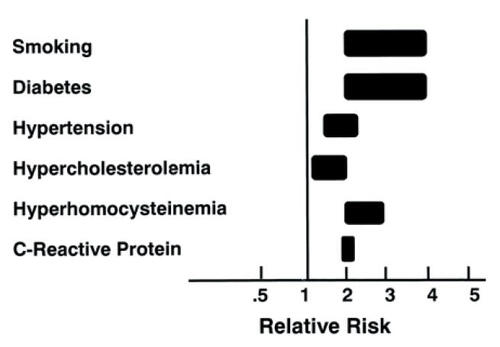
Figure 5 Risk of developing lower extremity peripheral arterial disease. The range for each risk factor is estimated from epidemiologic studies (see text). The relative risks take into consideration current smokers vs. former smokers and nonsmokers; the presence vs the absence of diabetes and hypertension; and the highest vs. the lowest quartile of homocysteine and C-reactive protein. The estimate for hypercholesterolemia is based on a 10% risk for each 10 mg/dL rise in total cholesterol. Adapted from CitationDormandy JA, Rutherford RB. 2000. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic InterSociety Consensus (TASC). J Vasc Surg, 31:S1-S296. Copyright © 2000 with permission from Elsevier.
Antiplatelet therapy has been shown in multiple studies and meta-analyses to prevent the risk of occlusive arterial disease in patients with PAD (CitationATC 2002). The most widely used antiplatelet agent is aspirin, which has been used for centuries. Aspirin blocks platelet activation by irreversibly inhibiting cyclo-oxygenase; this reduces the production of the potent prothrombotic and vasoconstrictive agent, thromboxane A2. To date, the largest meta-analysis studying the use of aspirin was conducted by the Antithrombotic Trialists’Collaboration, which showed marked reduction in vascular events in patients treated with aspirin (CitationATC 2002). This meta-analysis combined data from 287 studies with over 13,500 high-risk patients with cardiovascular disease and compared the efficacy of anti-platelet therapy against control. The use of anti-platelet agents aspirin and ticlopidine resulted in a 22% reduction in adverse cardiovascular events defined as MI, CVA, or vascular death (CitationATC 2002). Of the 287 studies, 42 were randomized reports of nearly 10,000 patients with PAD. The use of aspirin in this subset of patients resulted in a significant 23% decrease in the incidence of non-fatal MI or CVA and fatal vascular death (CitationATC 2002).
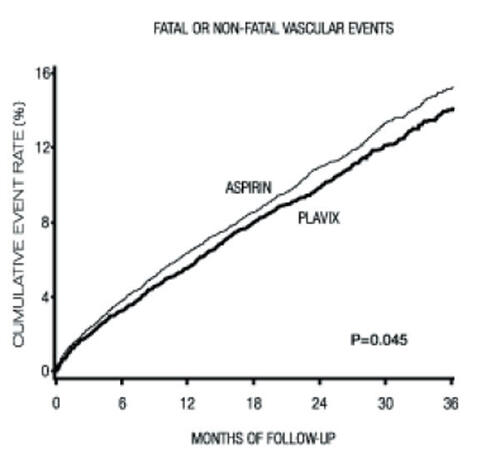
Clopidogrel, a thienopyridine derivative that inhibits platelet aggregation, has been widely studied in the treatment of patients at risk for cardiovascular events (). It inhibits platelet aggregation by selectively inhibiting the binding of the adenosine 5’-diphosphate molecule to the P2Y12 receptor on the platelet surface (CitationMills et al 1992). This property of clopidogrel leads to inhibition of platelet activation and aggregation, thereby making it an important agent in the prevention of thrombotic complications especially in acute vascular ischemic events. The use of clopidogrel was studied in the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk for Ischemic Events) trial, a randomized double blind controlled study of almost 20,000 patients, nearly 6000 with PAD (CitationCAPRIE 1996). This randomized trial compared clopidogrel 75 mg daily with aspirin 325 mg daily with the primary endpoint of vascular death, non-fatal myocardial infarction, or CVA. At 1.9-year follow-up, the incidence of primary endpoint was 3.7% in the clopidogrel arm versus 4.9% in the aspirin arm, equating to a significant 24% relative risk reduction (CitationCAPRIE 1996). Clopidogrel treatment in patients with symptomatic PAD was more effective than aspirin in preventing ischemic events (CitationCAPRIE 1996).
Much focus has been given to the use of dual anti-platelet therapy in the prevention of ischemic events in generally high-risk patients. As mentioned, clopidogrel and aspirin work by different mechanisms to irreversibly block platelet activation. Hypothetically, the combination of aspirin and clopidogrel should yield an even stronger antiplatelet effect producing even more protection against thrombotic complications of acute ischemic events. The combination of aspirin and clopidogrel therapy versus aspirin alone was recently reported in the CHARISMA (Clopidogrel and Aspirin versus Aspirin Alone for the Prevention of Atherothrombotic Events) trial, a multicenter, randomized placebo-controlled, double blind trial of over 15,000 patients (CitationBhatt et al 2006). The study looked at a broad group of patients, with a large percentage at lower risk for atherothrombotic events who did not have established cardiovascular disease. Additionally, this study included a high risk subgroup with established cardiovascular disease and nearly 3000 patients with known PAD. Overall, no benefit was seen when clopidogrel was added to aspirin versus aspirin alone in reducing the primary efficacy endpoint of MI, CVA, or vascular death in the overall population of patients (CitationBhatt et al 2006). However, the study period was relatively short with a follow up of only 30 months, which could account for the lack of benefit seen in a population of largely low-risk patients. Conversely, the higher-risk subgroup of patients with established cardiovascular disease appeared to derive a benefit from the combination of aspirin and clopidogrel. Also, the suggestion of increased harm in the low-risk group was made. The question of whether dual antiplatelet is for all patients at risk for atherothrombosis has likely been answered in the CHARISMA. Patients with established and diffuse atherosclerosis, particularly with PAD, remain at the highest risk for atherothrombosis and should strongly be considered candidates for dual antiplatelet therapy with 81 mg of aspirin and 75 mg of clopidogrel (CitationBhatt et al 2006).
The secondary goal in the management of PAD involves the treatment of lifestyle and the limitation of symptoms in order to improve quality of life. PAD patients with intermittent claudication account for the largest group of symptomatic patients. Exercise therapy is recommended for all patients with claudication symptoms. A supervised exercise training program that is monitored by trained personnel has been studied in numerous trials. Increase in walking abilities has been shown in large meta-analyses from the Cochrane Collaboration (CitationLeng et al 2000). Drug therapy with cilostazol is indicated to improve walking time and reduce painful lower extremity claudication. Although the true mechanism of its action is unknown, cilostozol has numerous actions including anti-platelet activity, a vasodilatory effect, and improvement in HDL levels (CitationIgawa et al 1990). Use of this agent is limited by its side-effects of headache, nausea, palpitations, and dizziness. Due to its inhibition of phosphodiesterase III, the FDA has a black-box warning for use of cilostazol in patients with heart failure and left ventricular dysfunction (CitationHirsch et al 2006). Trental, a rheolytic agent with effects on platelet aggregation, can be considered as a second alternative to cilostazol. Due to its unpredictable effectiveness, the ACC/AHA practice guidelines give it a Class IIb indication (CitationHirsch et al 2006).
Revascularization via surgery or endovascular procedures is reserved for patients that have lifestyle-altering disabilities due to the severity of PAD or intermittent claudication. Although a detailed review of revascularization procedures is beyond the scope of this article, numerous methods to include percutaneous transluminal angioplasty (PTA) with balloon dilation, atherectomy and stents have been implemented successfully in the treatment of claudication. These procedures, including surgical revascularization, can effectively decrease or entirely alleviate the symptoms of PAD. The majority of patients who are treated with either surgical or endovascular revascularization should be treated with aspirin and clopidogrel for at least a month following the procedure and, often, indefinitely.
Complications of PAD
MI and CVA remain the most serious complications assocoiated with PAD. Other complications are related to the area of the body supplied by the artery involved. Whether it is mesenteric ischemia, renovascular hypertension, or intermittent claudication, the end result of untreated severe arterial stenoses is progression to possible occlusion of the artery and infarction of the tissue it supplies.
Lower extremity critical limb ischemia, occurring when limb perfusion becomes critically low to the point that limb tissue viability is jeopardized, can lead to tissue loss and gangrene. Acute limb ischemia is a severe complication of PAD due to arterial embolism or acute arterial thrombosis. The classic hallmark signs of acute critical limb ischemia include the 5 “Ps”: pain, pulselessness, palor, paresthesias, and paralysis. Suggestions to add a 6th “P”, polar, describing the coolness of the affected extremity have been made (CitationHirsch et al 2006). Acute treatment consists of re-establishing flow to peripheral tissue beds by recanalizing the occluded artery. This can be performed with either catheter-based thrombolysis or mechanical thrombectomy or surgical thrombectomy or bypass. In the worst case, primary amputation should be considered when vast areas of necrosis are already encountered in the late presentation of critical limb ischemia. One-year mortality rates are greater than 20% for patients with chronic limb ischemia (CitationDormandy and Rutherford 2000). Furthermore, morbidity is even higher, approaching 40% for those patients who have disease that is not amenable to peripheral or surgical intervention and will eventually require limb amputation. Therefore, the treatment and prevention of progression of PAD is critically important. All measures to control the progression of atherosclerosis, decrease modifiable risk factors, and treat the hyperactive platelet state, needless to say, should be aggressively implemented. The development and rupture of abdominal aortic aneurysms (AAA) is a catastrophic complication. Screening measures and early treatment are outlined in the ACC/AHA guidelines (CitationHirsch et al 2006). Atherosclerotic renal artery stenosis causes renal insufficiency and uncontrolled hypertension. This can be effectively treated with renal artery stenting. Subclavian steal from severe stenosis in the subclavian or innominate arteries can cause vertebrobasilar insufficiency with patients who present with dizziness, syncope, and vertigo; these patients can also have claudication of the upper extremity. If the stenosis is distal to the left vertebral artery, the patient will present only with left arm claudication. Significant disease in the innominate artery can also lead to subclavian steal syndrome with often very dramatic symptoms if reversal of flow occurs in the right vertebral and carotid arteries. The coronary-subclavian steal syndrome occurs in patients with severe left subclavian stenosis causing reversal of flow in the left internal mammary graft to the left anterior descending coronary artery (LAD). These patients often present with angina, particularly if they are using their left arm.
Safety and tolerability
Millions of prescriptions have been written for clopidogrel over the past few years. Clopidogrel was the third most prescribed medication by US cardiologists in 2005. The most experience with clopidogrel comes from the patients who undergo coronary artery stent implantation and subsequent treatment with clopidogrel. These patients are treated with the combination of clopidogrel 75 mg daily along with aspirin 325 mg for 1 month, following bare metal stent implantation, 3 months with sirolimus-eluting stents and 6 months with paclitaxel-eluting stents. Many interventional cardiologists treat patients with aspirin and clopidogrel for up to a year and often indefinitely when drug eluting stents are placed. Patients discharged after admission for acute coronary syndromes are generally treated for at least a year with the aspirin and clopidogrel combination (CitationSmith et al 2006). Patients with peripheral artery disease and carotid artery stent implantation constitute a smaller population of patients in whom the aspirin and clopidogrel combination is being used. The most data accumulated recently have involved the CHARISMA, COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial), and CLARITY-TIMI 28 (Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation) trials where major safety endpoints were not statistically different in comparing aspirin and clopidogrel with aspirin alone (CitationChen et al 2005; CitationSabatine et al 2005; CitationBhatt et al 2006).
The most common side-effect seen in patients taking clopidogrel has been a hypersensitivity drug eruption. The onset can occur days to weeks after the initiation of the medication and generally requires discontinuation of clopidogrel. Acute treatment involves antihistamines, and sometimes steroids are needed. The cessation of the drug and replacement with ticlopidine is often required. Rarely, patients can be treated through their drug eruption or they can undergo clopidogrel desensitization, which has been shown to be safe and successful in a number of case series (CitationCamara and Almeda 2006).
The least likely side-effect to occur with clopidogrel use is thrombotic thrombocytopenic purpura (TTP). TTP has been reported by CitationBennett et al (2000) as a serious life-threatening complication of taking clopidogrel. The incidence of clopidogrel associated TTP is felt to be less than 4 cases per 1 million (CitationBristol-Myers Squibb and Sanofi-Synthelabo 2002). However, it has been argued that these patients may develop TTP coincidentally with recent initiation of clopidogrel and that no causal relationship exists at all (CitationMajhail and Lichtin 2003).
Serious bleeding from clopidogrel is certainly a concern but is likely related to concomitant use with other anti-platelet and antithrombotic medications. Dual or triple antiplatelet therapy or antiplatelet therapy combined with antithrombotic therapy increases the likelihood of major and minor bleeding. CitationKhurram et al (2006) reported the combined use of aspirin, clopidogrel, and warfarin had a significantly increased risk of bleeding compared with the dual antiplatelet group of aspirin and clopidogrel. Major bleeding was 6.6% in the triple therapy group versus 0% in the dual antiplatelet group; minor bleeding was 14.9% versus 3.8%, respectively (CitationKhurram et al 2006). In the PAD population studied in CAPRIE, the incidence of bleeding with clopidogrel versus aspirin was not statistically different (CitationCAPRIE 1996). In CURE, however, the risk of both major and minor bleeding was significantly higher with the combination of aspirin and clopidogrel versus aspirin alone (CitationClopidogrel Trial 2001). Although as the dose of the aspirin given increased, the risk of major and minor bleed increased equally in both arms (CitationClopidogrel Trial 2001).
Hemorrhagic complications are increased with all antiplatelet and antithrombotic medications. Gastrointestinal erosions and bleeding with aspirin are well recognized phenomena. Clopidogrel does not appear to induce lesions and cause bleeding but likely increases the risk of some underlying pathology to be more prone to bleed. Another theory is clopidogrel impairs the long-term healing of patients with prior gastrointestinal ulcers. A 2005 study by CitationChan et al (2005) found patients with recent gastrointestinal (GI) bleeding, aspirin, and esomeprazole was better than clopidogrel alone for recurrent GI bleeding. This report had a number of flaws and was criticized as a small study without an arm looking at esomeprazole and clopidogrel. In CAPRIE, with patients receiving clopidogrel, GI hemorrhage occurred at a rate of 2.0%, and hospitalization was required in 0.7% (CitationCAPRIE 1996). In patients receiving aspirin, the corresponding rates were 2.7% and 1.1%, respectively (CitationCAPRIE 1996). In CURE, the Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial, the incidence of major GI bleeding was 1.3% vs 0.7% (Clopidogrel + aspirin vs placebo + aspirin, respectively) (CitationClopidogrel Trial 2001). Clopidogrel should be used with caution in patients who are at risk for bleeding, have had peptic ulcer disease, NSAID induced gastritis or bleed and those patients chronically taking NSAIDS. Proton pump inhibitors should be used concomitantly to reduce the risk of GI bleed. Patients with hepatic or renal impairment are at potentially higher risk of hemorrhagic complications with clopidogrel. Use in these patients is limited and close monitoring and patient education regarding increased risk is necessary.
Intracranial and other life-threatening bleeding is uncommon. The incidence of intracranial hemorrhage is 0.4% for clopidogrel compared with 0.5% for aspirin. Surgical bleeding is increased if patients do not stop taking clopidogrel at least 5 days prior to the surgery. Current recommendations are to hold clopidogrel at least 5 to 7 days prior to any surgery.
Patient perspectives
Patient complaints of easy bruising are frequent with most antiplatelet or antithrombotic medications. The combination of aspirin and clopidogrel increases those complaints. In the triple therapy, patients in whom warfarin is also needed, the risk of hemorrhagic complications is significant, requiring meticulous attention to the international normalized ratio, maintaining it at its lowest possible threshold and using the lowest possible dose of aspirin. Measuring platelet aggregation and thromboelastography may be quite beneficial in managing these patients. Other than easy bruising and a small incidence of drug eruptions with initiation of therapy, clopidogrel is very well tolerated and effective at reducing atherothrombotic risk. Patient satisfaction is generally very good as is adherence.
Conclusion
Antiplatelet therapy in some form is required in all patients with vascular disease. Clopidogrel in patients with PAD is a critically important therapy, particularly in the higher risk patients. Clopidogrel carries the only FDA-labeled indication for cardiovascular risk reduction in patients with established PAD. Those patients with diffuse, severe atherosclerosis, or those who have had multiple atherothrombotic events, are at the highest risk for recurrent events; dual antiplatelet therapy is likely beneficial in this group of patients. Combination therapy is also recommended in patients who have had an event on aspirin as they likely need more platelet inhibition. Risk reduction with aspirin is far less expensive but slightly less effective as seen in CAPRIE (CitationCAPRIE 1996). The combination of aspirin and clopidogrel may increase the possibility of bleeding in lower risk patients, however, in higher risk patients, the benefits likely outweigh the risk (CitationBhatt et al 1006). The CHARISMA trial did not show a benefit in the total population studied (CitationBhatt et al 1006). However, if a greater portion of patients studied were the highest risk patients and followed for a longer duration of time, there might have been a significant benefit of the combination of aspirin and clopidogrel versus aspirin alone.
Antiplatelet therapy, particularly with clopidogrel, is a mainstay of therapy to prevent atherothrombotic events in high risk patients. Aggressive risk reduction with the addition of clopidogrel is mandatory for patients who experience atherothrombotic events while already receiving aspirin, for patients who are aspirin intolerant and for patients with established cardiovascular disease in multiple vascular beds. Additionally, patients with a history of atherothrombosis who still smoke, or patients who are statin intolerant or whose cholesterol cannot be optimally reduced, are also ideal candidates for more aggressive antiplatelet therapy with clopidogrel and/or the combination of clopidogrel and low-dose aspirin in order to reduce their risk for future atherothrombotic events.
Declaration
The views, opinions, and assertions contained in this work are those of the authors and are not to be construed as official or as reflecting the views of the US Navy, Department of Defense, or the United States Government.
Acknowledgements
We greatly appreciated Ms. Waine MacAllister’s expertise and assistance in preparing this manuscript.
Disclosures
JJC is on the Speakers Bureau for BMS/Sanofi-Aventis.
References
- AronowWSAhnCGutsteinHPrevalence and incidence of cardiovascular disease in 1160 older men and 2464 older women in a long-term health care facilityJ Gerontol A Biol Sci Med Sci200257AM45611773211
- [ATC] Antithrombotic Trialists’ CollaborationCollaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patientsBMJ2002324718611786451
- BennettCLConnorsJMCarwileJMThrombotic thrombocytopenic purpura associated with clopidogrelN Engl J Med20003421773710852999
- BhattDLFoxKAHackeWClopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic eventsN Engl J Med2006354170617 Epub 2006 Mar 1216531616
- SquibbBristol-MyersSanofi-SynthelaboClopidogrel (Plavix) [package insert]2002May 2002New York, NY
- CamaraMGAlmedaFOClopidogrel (Plavix) desensitization protocolCatheter Cardiovasc Interv20066915417139677
- CAPRIE Steering CommitteeA randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE)Lancet19963481329398918275
- CavendishJJSafaniMRole of antiplatelet therapy in cardiovascular disease III: Peripheral arterial diseaseCurr Med Res Opin2004201851515537486
- ChanFKLChingJYLHungLCTClopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleedingN Engl J Med20053522384415659723
- ChenZMJiangLXChenYPOn Behalf of COMMIT (ClOpidogrel and Metoprolol in Myocardial Infarction Trial) Collaborative GroupLancet200536616072116271642
- The Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial InvestigatorsEffects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevationN Engl J Med200134549450211519503
- CriquiMHFronekABarrett-ConnorEThe prevalence of peripheral arterial disease in a defined populationCirculation198571510153156006
- CriquiMHLangerRDFronekAMortality over a period of 10 years in patients with peripheral arterial diseaseN Engl J Med199232638161729621
- DormandyJARutherfordRBManagement of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic InterSociety Consensus (TASC)J Vasc Surg200031S1S29610666287
- FusterVMorenoPRFayadZAAtherothrombosis and high-risk plaque.I. Evolving conceptsJ Am Coll Cardiol2005469375416168274
- HiattWRMedical treatment of peripheral arterial disease and claudicationN Engl J Med2001344160821 1608-21 (158a)11372014
- HirschATHaskalZJHertzerHRACC/AHA Guidelines for the Management of Patients with Peripheral Artery Disease: Executive SummaryJ Am Coll Cardiol200647123931216545667
- IgawaTTaniTChijiwaTPotentiation of anti-platelet aggregating activity of cilostazol with vascular endothelial cellsThromb Res1990576176232158154
- KhurramZChouEMinutelloRCombination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleedingJ Inv Cardiol2006181624
- LengGCFowlerBErnstEExercise for intermittent claudicationCochrane Database Syst Rev2000CD00099010796572
- LopezADMurrayCCThe global burden of disease, 1990–2020Nat Med19984124112439809543
- McDermottMMGreenlandPLiuKLeg symptoms in peripheral arterial disease. Associated clinical characteristics and functional impairmentJAMA2001286159960611585483
- McDermottMMGreenlandPLiuKThe ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation StudyAnn Intern Med200213687388312069561
- MajhailNSLichtinAEClopidogrel and thrombotic thrombocytopenic purpura: no clear case for causalityCleveland Clin J Med20037046670
- MillsDCPuriRHuCJClopidogrel inhibits the binding of ADP analogues to the receptor mediating inhibition of platelet adenylate cyclaseArterioscler Thromb1992124304361558834
- NewmanASiscovickDSManolioTAAnkle-arm index as a marker of atherosclerosis in the Cardiovascular Health StudyCirculation199388837458353913
- SabatineMSCannonCPGibsonCMAddition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevationN Engl J Med200535211798915758000
- SikkinkCJvan AstenWNvan’tHofMADecreased ankle/brachial indices in relation to morbidity and mortality in patients with peripheral arterial diseaseVasc Med19972169739546965
- SmithSCAllenJBlairSNAHA/ACC Guidelines for Secondary Prevention for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2006 UpdateJ Am Coll Cardiol2006472130916697342
- VorchheimerDABeckerRPlatelets in atherothrombosisMayo Clin Proc200681596816438480