Abstract
In this narrative review of the current literature, we examine the traditional risk factors and patient profiles leading to cardiovascular disease and osteoporosis. We discuss the interrelationships between risk factors and common pathophysiological mechanisms for cardiovascular disease and osteoporosis. We evaluate the increasing evidence that supports an association between these disabling conditions. We reveal that vascular health appears to have a strong effect on skeletal health, and vice versa. We highlight the importance of addressing the risk benefit of preventative interventions in both conditions. We discuss how both sexes are affected by these chronic conditions and the importance of considering the unique risk of the individual. We show that habitual physical activity is an effective primary and secondary preventative strategy for both cardiovascular disease and osteoporosis. We highlight how a holistic approach to the prevention and treatment of these chronic conditions is likely warranted.
Introduction
Cardiovascular disease (CVD) and osteoporosis (ie, reduced bone mass and microarchitectural deterioration of bone) are major health problems in North America, whose incidence increases with advancing age (CitationWarburton et al 2006a). The socioeconomic and health care burden of osteoporosis and CVD on society is enormous (CitationJohnell 1997). Osteoporotic fractures (CitationBraithwaite et al 2003) and CVD-related events are key origins of morbidity and premature mortality in the elderly (CitationWarburton et al 2006a). Moreover, post-menopausal women appear to be particularly at risk for developing both osteoporosis and CVD. In fact, once a woman reaches menopause, the risk for both osteoporosis and CVD increases substantially. Moreover, CVD and osteoporosis are often observed in the same individual (CitationSchulz et al 2004).
Globally, CVD is a major cause of premature mortality accounting for approximately one-third of the cases of death. According to the World Health Organization approximately 17 million people die as a result of CVD each year (CitationWorld Health Organization 2006).
In the United States, approximately 10 million individuals have osteoporosis and approximately 34 million are at an increased risk owing to low bone mass (CitationNational Osteoporosis Foundation 2007). Approximately 80% of those affected with osteoporosis are women (CitationNational Osteoporosis Foundation 2007). In Canada, approximately 1.4 million suffer from osteoporosis (CitationOsteoporosis Canada 2007). In North America, 20%–25% of women over the age of 50 have osteoporosis (CitationOsteoporosis Canada 2007) and approximately 50% are estimated to have low bone mass (CitationNational Osteoporosis Foundation 2007).
For years, osteoporosis and CVD were thought to be independent chronic diseases that increased markedly with advancing age. However, increasing evidence now supports a direct association between these chronic conditions. Accordingly, in this article we review the risk factors and patient profiles leading to both CVD and osteoporosis. Moreover, we review the mounting evidence that reveals an association between these diseases. We also discuss methods of reducing the risk for each chronic condition and the effects of these interventions on the subsequent risk for each disease. We discuss the differential rates with which osteoporosis and CVD affect men and women, and highlight how a holistic approach to these chronic conditions is warranted.
Traditional risk factors and patient profiles
Cardiovascular disease
Several traditional major independent risk factors for CVD have been identified including non-modifiable (family history, male sex, and advancing age) and modifiable risk factors. The major modifiable risk factors for CVD include elevated blood pressure, cigarette or tobacco smoking, physical inactivity, abnormal lipid lipoprotein profiles (eg, high total cholesterol, LDL cholesterol and triglycerides levels, and low levels of HDL cholesterol), unhealthy diets, excessive alcohol use, obesity, and diabetes.
Recent research has also identified several “merging” or “novel” risk factors that are independent predictors of CVD and premature mortality. This includes factors such as vascular health (eg, arterial compliance, carotid intima media thickness, and pulse wave velocity), left ventricular mass, and a series of novel blood parameters (eg, lipoprotein (a), fibrinogen, C-reactive protein, and homocysteine). The measurement of “emerging” risk factors in combination with a traditional lipid panel is thought to provide “information on risk over and above that supplied by established risk factors” (CitationHeinrich and Assmann 1995).
The majority of CVD events are the result of atherosclerosis (CitationGrey et al 2003). The endothelium plays an important role in the process of atherosclerosis and thus has a significant effect on the long-term risk for CVD (CitationLeeson et al 1997). Endothelial dysfunction is thought to be an obligatory first step in the process of atherosclerosis and has been observed in patients with coronary atherosclerosis (CitationSorensen et al 1997) and individuals with risk factors for CVD (CitationCelermajer et al 1994). Therefore, a healthy endothelium is essential for the protection against atherosclerosis (CitationAnderson 2003). Endothelial dysfunction has been associated with multiple CVD risk factors and has also been reported to occur prior to the development of atherosclerosis (CitationCelermajer et al 1992; CitationWhitney et al 2004). It is important to note, that endothelial dysfunction has been observed in multiple chronic disease states including coronary artery disease, stroke, chronic heart failure, type 2 diabetes, hypertension, and obesity (CitationWarburton et al 2006a).
Cardiovascular disease places a significant burden upon health care systems worldwide. In Canada, CVD (including heart disease and stroke) is the leading cause of death (accounting for over one-third of all deaths) disability, and hospitalization accounting for approximately 17% of the total health care costs (CitationWilson and Wielgosz 1999; CitationHealth Canada 1999; CitationKatzmarzyk et al 2000). Cardiovascular diseases cost the Canadian economy $18 billion a year (CitationPublic Health Agency of Canada 2002). Fortunately, CVD is largely preventable.
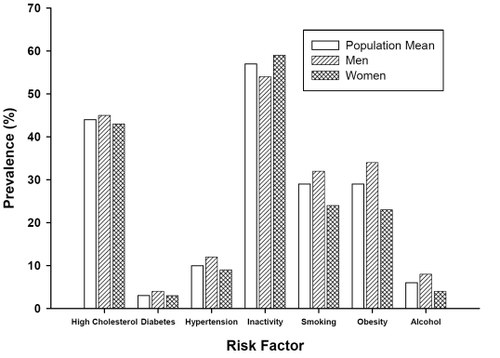
In developed countries, many adults have one or more risk factor for CVD. For instance, 80% of Canadians have one or more risk factor for CVD (CitationHealth Canada 2005). As illustrated in , the prevalence of modifiable risk factors for CVD in developed countries is alarming. Perhaps of more concern is the finding that many children and adolescents have risk factors for CVD. For instance, in a recent study we revealed that 58% of children (10–11 yr) had at least one elevated CVD risk factor and approximately 8% of the children had four or more elevated CVD risk factors (CitationMcKay et al 2004). Clearly, effective prevention and treatment interventions are required across the lifespan.
Figure 1 Prevalence of traditional risk factors for cardiovascular disease in Canadian society according to gender.
Source: Statistics Canada, National Population Health Survey, 1996/97 and the Heart and Stroke Foundation of Canada, The Changing Face of Heart Disease and Stroke in Canada 2000, October 1999 (CitationHeart and Stroke Foundation of Canada 2000, CitationStatistics Canada 1999b).
Osteoporosis
Several non-modifiable and modifiable risk factors have been identified for osteoporosis. Non-modifiable risk factors include female sex (women are at greater risk), advancing age, personal history of a fracture after age 40, family history of osteoporosis, European or Asian ancestry, and dementia. Potentially modifiable factors include low BMI, calcium and vitamin D intake deficiency, physical inactivity, certain medications (eg, excessive use of corticosteroids), estrogen deficiency, current cigarette smoking, excessive alcohol consumption, early menopause (before age 45), and prolonged pre-menopausal amenorrhea (<1 year) (CitationBrown and Josse 2002; CitationCanadian Multicentre Osteoporosis Study 2006).
Osteoporosis is a major health burden affecting millions of people worldwide (particularly in developed countries) (CitationWorld Health Organization 2003). In Canada approximately one in four women and one in eight men (over the age of 50) will have osteoporosis (CitationHanley and Josse 1996; CitationBrown and Josse 2002). In developed countries, it has been estimated that 40%–54% of 50 yr old women will have an osteoporosis-related fracture during their lifetime (CitationChrischilles et al 1991; CitationMelton et al 1992). The prevalence is higher amongst older adults; however, osteoporosis can present itself at any age (CitationCanadian Multicentre Osteoporosis Study 2006). The incidence of osteoporosis is positioned to increase markedly over the next few decades owing to the increasing numbers of older individuals worldwide (CitationBrown and Josse 2002; CitationCanadian Multicentre Osteoporosis Study 2006).
The burden osteoporosis places upon the individual, the family, and society as a whole is enormous. In Canada (1996 data) hip fractures were the second leading cause of hospital admission for women aged 65 years or older (CitationStatistics Canada 1999a). It has been estimated that without the creation of effective prevention and treatment strategies Canada will spend $1.9 billion per year to treat osteoporosis and related fractures (CitationOsteoporosis Canada 2007).
Health-related quality of life is often markedly reduced in persons with osteoporosis. It has been estimated that 50% of women who sustain a hip fracture will become functionally dependent during activities of daily living, and 20% will require long term care (CitationChrischilles et al 1991; CitationBrown and Josse 2002). Moreover, the incidence of premature mortality is 20% higher within a year of suffering a hip fracture (CitationChrischilles et al 1991; CitationBrown and Josse 2002). Fortunately, osteoporotic fractures are preventable (CitationBrown and Josse 2002).
The interrelationships between risk factors for cardiovascular disease and osteoporosis
In recent years, a growing body of literature (see ) has revealed an association between CVD and osteoporosis. In particular, epidemiological evidence has shown a relationship between vascular calcification and bone loss (as reviewed in ). For instance, bone mineral density has been inversely associated with coronary and/or aortic calcification (CitationUyama et al 1997; CitationBarengolts et al 1998; CitationBagger et al 2006), and directly associated with high-density lipoprotein cholesterol (HDL-C) (CitationYamaguchi et al 2002) ().
Table 1 Relationship between vascular and bone health
Clinical populations have also revealed a relationship between CVD and osteoporosis. For instance, accelerated bone loss has been observed in diabetics (CitationSchwartz et al 2005), a group that commonly exhibits marked vascular dysfunction (CitationRomney and Lewanczuk 2001; CitationMcGavock et al 2004). Furthermore, in cases where vascular function is affected differentially on opposite sides of the body (ie, asymmetric vascular disease in the lower limbs), the side that has the greatest vascular dysfunction also displays the lowest bone mineral content (CitationLaroche et al 1994; CitationLaroche et al 2003). Moreover, in elderly individuals who have experienced a fracture at the femoral neck, the blood vessels supplying the proximal femur are often atherosclerotic (CitationBocchi et al 1985; CitationBocchi et al 1987). The rate of bone loss at the hip has also been shown to be greater in women who have the greatest reductions in blood flow (as assessed by the ankle/arm index) (CitationVogt et al 1997a).
The potential relationship between these chronic conditions has important implications for the health of many individuals with (or at risk for) CVD and osteoporosis () (CitationTanko et al 2005). For instance, investigations with post-menopausal women have observed that there is an increased risk of cardiovascular-related and stroke mortality for each standard deviation decrease in bone mass (CitationBrowner et al 1991; Citationvon der Recke et al 1999; CitationKado et al 2000). For instance, CitationKado et al (2000) revealed that for each standard deviation decrease in bone mass there was a 1.2- to 1.3-fold increased risk of dying from coronary artery disease or other forms of atherosclerosis. Citationvon der Recke et al (1999) reported that the lowest quartile of bone mass was associated with a 2-fold increase in the risk for CVD-related death (vs. the highest quartile). CitationTanko et al (2005) revealed that osteoporotic women had a 3.9-fold increased risk for cardiovascular events than women with low bone mass. This increased risk could not be explained by common risk factors alone. It is important to note however that studies have also shown no relationship between coronary calcification and osteoporosis after controlling for age (CitationSinnott et al 2006).
It remains to be determined the key mechanism(s) responsible for the relationship between CVD and osteoporosis. As identified above, both chronic conditions share similar modifiable risk factors (such as physical inactivity, smoking, age, years since menopause, and excessive alcohol usage). However, researchers have shown a direct relationship between vascular disease and hip bone mineral density even after adjusting for a wide range of common risk factors (including age, years since menopause, BMI, level of education, current and previous smoking, and physical inactivity) (CitationTanko et al 2003). Therefore, CVD and osteoporosis also may be linked by common pathophysiological mechanisms (CitationTanko et al 2003). The available epidemiological evidence does not allow for the establishment of a causal link between CVD and osteoporosis. However, there are plausible biological pathways whereby diminished cardiovascular health can influence bone health and vice versa (CitationWhitney et al 2004). Several excellent reviews have been created recently on these topics Citation(Doherty et al 2003; CitationHamerman 2005; CitationRajzbaum and Bezie 2006).
Potential mechanisms
There is evidence that vascular calcification has several common features to bone formation at both the cellular and molecular level. As stated by CitationDoherty et al (2003) “calcified atherosclerotic arteries can contain tissue that is histomorphologically indistinguishable from bone.” Vascular calcium deposition is now thought to be an active, complex, and regulated process similar to new bone formation (or remodeling) that is not merely a consequence of aging (CitationDoherty et al 2003; CitationRubin and Silverberg 2004). Importantly, calcifying vascular cells appear to have the ability to experience osteoblast differentiation (CitationRubin and Silverberg 2004). Moreover, it has been hypothesized that there are arterial cells that can differentiate into mineral resorbing cells (ie, osteoclast-like cells) (CitationDoherty et al 2002). These cells are thought to be derived from hematopoietic precursors of the mononuclear phagocytic lineage (CitationDoherty et al 2002). CitationDoherty et al (2002) postulated that osteoclast-like cells may play a role in the delicate balance of mineral deposition and resorption within the arterial wall (similar to that seen in bone). These authors also considered arterial mineral deposition “as a localized failure of protective mechanisms that actively oppose mineral deposition within the disordered metabolic milieu of developing atherosclerotic plaque.” This supports a growing body of research that indicates that arterial calcification involves arterial osteoblast- and osteoclast-like cells (CitationDoherty et al 2003).
Both the artery wall and the osteon of cortical bone have a common endothelium lined lumen. Cancellous bone is also highly vascular. Moreover, calcified plaque has been shown to have numerous cellular and molecular elements that participate in bone formation including (but not exclusive to) bone morphogenetic protein-2, collagen 1, osteonectin, osteopontin, matrix Gla proteins, osteocalcin and osteoprotegerin (CitationDoherty et al 2003; CitationWhitney et al 2004; CitationHamerman 2005).
Several biological mechanisms (based primarily on animal models) have been proposed to explain the link between CVD and osteoporosis (see reviews of CitationDoherty et al 2003; CitationHamerman 2005; and CitationRajzbaum and Bezie 2006). Chronic inflammation is known to play a role in the development of both chronic conditions. For instance, proinflammatory cytokines (including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)) have been associated with vascular disease (CitationBlake and Ridker 2001) as well as increasing bone resorption (CitationCohen-Solal et al 1993). Also, both bone and the vasculature are affected by sex steroids (CitationStevenson 2004; CitationRossouw 2005). For example, estrogens play a role in CVD and osteoporosis via their effects on cytokines (including IL-6, osteoprotegerin (as discussed later), and TNF-α) (CitationBaldini et al 2005).
As outlined above, vascular calcifications and bone have several similarities (CitationHamerman 2005; CitationRajzbaum and Bezie 2006). Several compounds found within arterial wall calcifications have been implicated in the association between bone loss and CVD. For instance, osteoprotegerin (an important regulator of osteoclastgenesis) has recently received considerable attention. Osteoprotegerin is a member of the TNF receptor superfamily that serves as a bone resorption inhibitor by blocking the RANK-L/RANK interaction (CitationKiechl et al 2004; CitationRajzbaum and Bezie 2006). Animal and human studies have reported conflicting findings regarding the role of osteoprotegerin in CVD and osteoporosis. Mice that do not have the osteoprotegerin gene exhibit vascular calcifications and osteoporosis (CitationBucay et al 1998). The intravenous administration of osteoprotegerin into mice deficient in osteoprotegerin reversed the osteoporosis, but it did not correct the arterial calcification. Whereas, the transgenic osteoprotegerin gene delivered during gestation prevented the formation of arterial calcification in osteoprotegerin deficient mice (CitationMin et al 2000). In humans, a high level of osteoprotegerin has paradoxically been shown to be an independent risk factor for the progression of atherosclerosis and the development of CVD (CitationBrowner et al 2001; CitationKiechl et al 2004). Similarly, high levels of osteoprotegerin were observed in post-menopausal women with osteoporosis (CitationYano et al 1999). Based on animal literature, it would be anticipated that high osteoprotegerin levels would be associated with high bone mineral density Citation(Hamerman 2005). CitationYano et al (1999) postulated that the high osteoprotegerin levels (in osteoporotic post-menopausal women) may reflect a compensatory response to enhanced osteoclastic bone resorption.
Serum lipids (a well-established risk factor for atherogenesis) have been proposed as one common mechanism for CVD and osteoporosis. It has been postulated that oxidized lipid accumulation in the subendothelial space of arteries promotes arterial calcification and inhibits bone mineral formation (CitationParhami et al 2000). Lipids have been shown to have effects on osteoblasts (CitationParhami et al 1997, Citation2000, Citation2002) and osteoclasts (CitationTintut et al 2004). For instance, the same oxidized lipids that bring about osteoblast differentiation in calcifying vascular cells inhibit osteoblast differentiation in bone cells of mice (CitationParhami et al 1997). Moreover, in atherogenic susceptible and resistant mice exposed to a high fat or normal diet, a 35% decrease in both bone mineral content (BMC) and osteocalcin expression was found in the fat fed, atherogenic susceptible strain (CitationParhami et al 1999; CitationParhami et al 2001). Animal work also revealed that hyperlipidemia may lead to osteoporosis via an increase in osteoclastic bone resorption (CitationTintut et al 2004). Prolonged treatment with high-density lipoprotein has also been shown to inhibit the osteogenic activity of calcifying vascular cells. Moreover, HDL inhibited the osteogenic activity induced by inflammatory cytokines (CitationParhami et al 2002).
Epidemiological evidence in humans examining the role of lipids in the relationship between CVD and osteoporosis remains controversial. For instance, there is evidence that hyperlipidemia (eg, elevated low-density lipoprotein) is associated with reduced bone mineral density (CitationYamaguchi et al 2002). However, abnormal lipid lipoprotein levels have also been shown to be associated with increased bone mineral density in humans (CitationAdami et al 2004). Whereas, others have found no independent relationship between lipids and bone mineral density (CitationBagger et al 2007). For example, CitationBagger et al (2007) recently reported that there were no independent associations with lipid lipoprotein profile and bone mineral density of the hip or spine. Importantly, the authors also revealed that the severity of aorta calcification was independently associated with hip bone mineral density (with no contribution of lipids). The authors stated that these discrepancies provide evidence that the obstructive vascular disease (rather than lipid lipoproteins per se) facilitates the bone loss. The authors also postulated that lipids may act indirectly via the promotion of atherosclerosis which in turn can affect local bone metabolism. This effect is thought to be particularly evident at skeletal sites with end-arterial blood supply (CitationBagger et al 2007). This evidence supports the argument that a decrease in peripheral blood flow and supply could suppress bone cell function (CitationRubin and Silverberg 2004).
Endothelial function appears to be important for both vascular and bone health. The vascular endothelium provides a macromolecular barrier with multiple functions including: 1) anti-inflammation and pro-inflammation, 2) vasodilatation and vasoconstriction, 3) anti-thrombosis and pro-thrombosis, 4) anti-oxidation and pro-oxidation, and 5) growth inhibition and growth promotion (CitationWhitney et al 2004). When the integrity of the endothelium is disrupted, as with oxidized LDL-C, an imbalance in these roles occurs resulting in endothelial dysfunction. Endothelial dysfunction is thought to be an obligatory first step in the process of atherosclerosis. Owing to the intimate contact with endothelial cells, the health of the endothelium appears to also be of great significance for bone health.
A healthy vascular tone is maintained by the continual release of low levels of nitric oxide (NO). NO is generated from L-arginine by NO synthase isoenzymes. Three NO synthase isoforms have been identified including a neuronal form (nNOS), an endothelial form (eNOS), and an inducible form (iNOS). Recently, it has become apparent that NO is involved in the process of bone metabolism and as such the influence of NO on bone health has been the focus of several investigations. In bone, it has been established that nNOS is rarely expressed, while both eNOS and iNOS are expressed (CitationFox and Chow 1998). However, both the eNOS and nNOS isoforms affect bone differently. For instance, the eNOS isoform is expressed constitutively and is predominant in the osteoblast lineage (CitationFox and Chow 1998). Inflammation is associated with iNOS activity in osteoblast and osteoclast cells (CitationFox and Chow 1998; CitationArmour et al 1999; CitationArmour et al 2001b). For instance, CitationArmour et al (2001b) revealed that the activation of the iNOS pathway contributes to inflammation-mediated osteoporosis via suppressed bone formation and osteoblast apoptosis. Osteoclasts have also shown low levels of eNOS and iNOS (CitationFox and Chow 1998). eNOS negative (knock-out) mice have demonstrated reduced bone volume, bone formation rates, BMD, and osteoblast numbers (CitationAguirre et al 2001; CitationArmour et al 2001a; CitationSamuels et al 2001). eNOS knock-out mice have also revealed a blunted response to exogenous estrogen, supporting the important role of eNOS in mediating the stimulatory action of estrogen on bone formation (CitationArmour et al 2001a). Moreover, cells of osteoblast phenotype challenged with pulsatile fluid flow (as seen with exercise) have demonstrated increased eNOS production (CitationKlein-Nulend et al 1998). Researchers have also shown that L-arginine administration prevented bone loss and bone collagen breakdown in cyclosporin A-treated rats (CitationFiore et al 2000).
These findings (from animal and cell culture models) indicate that NO may contribute to the osteogenic pathway. However, exactly how eNOS and iNOS contribute to bone health remains to be determined, especially in humans. Prospective human investigations have evaluated NO and bone (CitationJamal et al 1998; CitationNabhan 2006). For instance, CitationJamal et al (1998) revealed that intermittent nitrate use increased hip and heel BMD compared to non-users. However, there was no difference in fracture free survival between groups. A recent randomized controlled trial (CitationNabhan 2006) revealed that isosorbide mononitrate (a NO donor) was effective in reducing a marker of bone resorption (urine N-telopeptide) and increasing a marker of bone formation (alkaline phosphatase) in post-menopausal women. The authors postulated that a NO donor may be effective in the prevention of post-menopausal osteoporosis. Additional research is required to clearly identify the roles that the specific NO isoforms have on bone health.
Other factors involved in arterial wall calcification have also been implicated in the relationship between CVD and osteoporosis. Potential factors include (but are not exclusive to) osteopontin, matrix Gla-proteins and osteocalcin, and leptin. It is recommended that interested readers consult reviews on this topic (CitationDoherty et al 2003; CitationHamerman 2005; CitationRajzbaum and Bezie 2006) for further information regarding the varied potential mechanisms explaining the relationship between CVD and osteoporosis.
Based on the above literature it is apparent that multiple pathophysiological mechanisms can be responsible for the observed relationship between CVD and osteoporosis. It is however important to note that many of the above findings provide evidence that vascular dysfunction plays a key role in the association between CVD and osteoporosis. Thus, impaired blood flow and a diseased vascular system may have negative effects on bone health, or diseased bone may impair vascular health (CitationWhitney et al 2004).
Differences between men, pre-and postmenopausal women
It is important to discuss the differences between men and women across the lifespan with respect to the risk for and prevalence of CVD and osteoporosis. Often CVD is thought to be a disease of primary concern for men and osteoporosis of primary concern for women owing to the differential prevalence rates for each condition amongst sexes. However, these statements must be tempered greatly owing to the unique sex-based differences in each condition.
With respect to CVD, men are more prone to atherosclerosis (approximately 3–4 fold greater) than women (CitationEaker et al 1993, CitationAmerican Heart Association 2006). With aging, this ratio declines to approximately 2 between the ages of 65–69, and to 1 by the age of 85 (CitationEaker et al 1993). The incidence of CVD increases markedly in women after menopause (CitationWitteman et al 1989). It has been estimated that the coronary heart disease rates increase 2–3 fold after menopause in women (increasing with aging) (CitationAmerican Heart Association 2006). Recent American data indicates that the prevalence of coronary heart disease in women 75 years or older was 10.3% in comparison to 1.6% between the ages of 45–54 (CitationAmerican Heart Association 2006).
The majority of sudden cardiac deaths occur in men (approximately 3–4 fold greater incidence); however, this inequality is also reduced with advancing age (CitationAmerican Heart Association 2006). Men have been shown to have a higher blood pressure than women until approximately age 45; thereafter, women have a higher blood pressure (CitationAmerican Heart Association 2006). This sex-based difference appears to be particularly pronounced in the elderly. For instance, in the United States the prevalence of hypertension is approximately 34% for both men and women aged 45–64. However, in the 75%+ cohort women have an increased prevalence of high blood pressure than men (83 vs 69%, respectively) (CitationAmerican Heart Association 2006). Although men (under the age of 75) have greater prevalence of coronary heart disease, women have a greater prevalence of CVD events due to heart failure (CitationAmerican Heart Association 2006).
The death rates associated with a myocardial infarction appear to be higher in women than men (CitationGreenland et al 1991; CitationGottlieb et al 2000). For instance, in the United States, 25% of men and 38% of women die within one year after have an initial myocardial infarction (CitationAmerican Heart Association 2006). This may be in part due to the fact that women are often older when they have a myocardial infarction. More American women (64%) than men (50%) who die suddenly due to CVD had no previous symptoms (CitationAmerican Heart Association 2006).
There is an increased incidence of osteoporosis in women (approximately 80%) (CitationNational Osteoporosis Foundation 2007). The incidence of osteoporosis increases markedly after the age of menopause with approximately 1 in 4 women over the age of 50 exhibiting osteoporosis (CitationOsteoporosis Canada 2007). This has led to a primary focus on elderly women in research within the field; however, approximately 30% of hip fractures occur in men. Men also have a higher risk of dying after a fracture (CitationCenter et al 1999). It has been estimated that the mortality rate in the first year after a fracture in men is twice that of women (CitationWright 2006).
Risk management of cardiovascular disease and osteoporosis
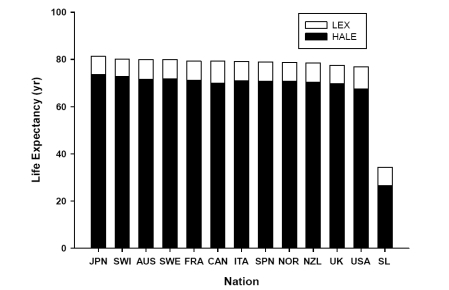
With the projected progressive increase in life expectancy it is clear that the incidence of both CVD and osteoporosis will increase for both males and females. Therefore, effective preventive interventions are required to offset the inevitable burden these chronic conditions will place upon society. It is also important to note, that with the global increase in life expectancy a major challenge for public health care systems will be the enhancement of overall quality of life, and not solely the prevention of chronic disease. Life expectancy does not take into account the years a person may live in a dependent or diseased state. Since overall quality of life is of utmost importance (especially as one ages) recent health agencies (such as the World Health Organization) have adopted new criteria to determine the number of years a person might live in a healthy state. For instance, the World Health Organization has recently introduced the Health-Adjusted Life Expectancy (HALE) scale that takes into account the anticipated years of ill-health to provide an estimate of years of healthy living ().
Figure 2 Life expectancy and health-adjusted life expectancy in representative nations.
Abbreviations: LEX, Life Expectancy; HALE, Health-Adjusted Life Expectancy; JPN, Japan; SWI, Switzerland; AUS, Australia; SWE, Sweden; FRA, France; CAN, Canada; ITA, Italy; SPN, Spain; NOR, Norway; NZL, New Zealand; UK, United Kingdom; USA, United States of America; SL, Sierra Leone.
The Health-Adjusted Life Expectancy (HALE) Takes into account the years of ill-health, weighted according to severity, and subtracted from the anticipated life expectancy to provide the equivalent years of healthy life.
Source: World Health Organization, World Health Report 2001. http://www3.who.int/whosis/hale/hale.cfm?path=whosis,hale&language=english (CitationWorld Health Organization 2001).
Health-related quality of life includes physiological, functional, emotional and spiritual well-being. We have discussed previously the physiological determinants of chronic disease. However, a holistic approach to health care should also include the evaluation of the other determinants of health-related quality of life. For instance, emotional and spiritual well-being are key factors in maintaining a high quality of life across the lifespan (CitationWarburton et al 2001a, Citation2001b). Moreover, the maintenance of functional capacity is fundamental to healthy aging (CitationWarburton et al 2001a, Citation2001b). Functional independence, especially as one ages, is related largely to one’s ability to perform activities of daily living. In fact, the capacity to carry out activities of daily living is thought to be of greater concern to the elderly than chronic disease (CitationEnsrud et al 1994; CitationWarburton et al 2001a, Citation2001b, Citation2006a). Therefore, preventative strategies should be developed that not only specifically target individual risk factors for chronic disease, but also address the emotional, physical and social well-being of the individual (CitationWarburton et al 2001a, Citation2001b; CitationPalacios et al 2005).
It is essential to stress that preventative strategies for both CVD and osteoporosis should not focus solely on the elderly at the expense of younger generations (CitationCenter et al 1999). It is clear that CVD starts in childhood and progresses with advancing age. It has been estimated that approximately one half of North American children exhibit one or more risk factor for CVD, with up to a third exhibiting at least one risk factor by the time they enter elementary school (CitationFreedman et al 1999). In fact, many children and adolescents exhibit multiple risk factors for CVD (CitationTroiano et al 1995; CitationBerenson et al 1998; CitationMcGill et al 2000). As discussed previously, we have recently shown that 8% of elementary children had four or more CVD risk factors (CitationMcKay et al 2004). This is of concern owing to the fact that the severity of underlying cardiovascular disease is greater with increasing numbers of CVD risk factors (CitationBerenson et al 1998) and these children may be more likely to develop CVD in adulthood (CitationReid et al 1999). Moreover, childhood and adolescence are important periods for the development of bone health. In fact, approximately 90% of adult bone mass is accrued by the end of adolescence (CitationMcKay et al 2005). Effective interventions (in particular interventions involving mechanical loading) can have marked effects upon bone metabolism and health during the growing years (CitationMcKay et al 2005). For instance, exercise interventions have been shown to have a greater effect when started in premenarche compared to postmenarche (CitationKannus et al 1995). It appears that benefits derived from interventions that optimize peak bone mass (such as weight-bearing exercise) may extend into adulthood (CitationKhan et al 2000). Therefore, preventative interventions must be devised that meet the needs of individuals from across the lifespan.
We feel that a preventative model across the lifespan is required to effectively address the high prevalence of CVD and osteoporosis, and to improve overall quality of life. Given the interrelationships between multiple chronic disease states a holistic approach to medicine should be advocated. In short, rather than focusing on the prevention and treatment of single disease states we should consider the treatment of the whole patient. Physical activity interventions have shown this strategy will hold great potential for addressing concurrently multiple chronic diseases, while improving overall quality of life.
Does minimizing the risk in one disease predispose an individual to risk in the other?
As discussed above, effective interventions can concurrently target multiple modifiable risk factors for both CVD and osteoporosis. For instance, the cessation of smoking and an increase in weight-bearing physical activity would each have a beneficial effect on both chronic conditions. However, clinicians must be aware that there is the potential for the reduction of risk in one disease to predispose the individual to an increased risk for the other condition. Perhaps the best example of this is weight reduction for the treatment of CVD. This is owing to the fact that a low BMI is an independent predictor of the risk for osteoporosis (CitationDe Laet et al 2005). For instance, CitationDe Laet et al (2005) revealed that a BMI of 20 kg/m2 was associated with a 2-fold increase in the risk for hip fracture in comparison to a BMI of 25 kg/m2. The risk for hip fracture however does not appear to be linear across BMI levels, as a BMI of 30 kg/m2 only conferred a 17% reduction in hip fracture risk in comparison to a BMI of 25 kg/m2. It appears that weight loss is also associated with an increased risk for osteoporosis (CitationEnsrud et al 2005; CitationMacdonald et al 2005). CitationMacdonald et al (2005) recommended that postmenopausal women (who are not taking hormone-replacement therapy) should be informed that low body weight or losing weight may worsen the degree of bone loss. Therefore, although losing weight confers health benefits for CVD, it may be counterproductive for osteoporosis. However, the level of weight loss often achieved in patients with CVD after lifestyle and/or pharmacological interventions is often not excessive and as such may confer limited additional risk for the development of osteoporosis. In fact, given the apparent relationship between CVD and osteoporosis it is likely that the changes in vascular health would be of great health benefit to the individual patient. It is important to note, that there are interventions (such as physical activity) that can meet the needs of both chronic conditions without markedly compromising the cardiovascular or skeletal health. This is particularly salient given the beneficial exercise-associated changes in bone and vascular health.
Hormone replacement therapy
Postmenopausal hormone therapy has been widely advocated for the prevention of osteoporosis and the treatment of postmenopausal symptoms (CitationBrown and Josse 2002). It was also anticipated that estrogen replacement therapy or combined estrogen-progestin therapy (hormone replacement therapy) would lower CVD risk (CitationAlexandersen et al 2006). In fact, during the early 1990s hormone therapy was widely promoted as an effective primary and secondary preventive strategy against CVD (CitationRossouw 2005). This was based largely on epidemiological (observational) studies that demonstrated a beneficial effect of hormone replacement therapy on risk factors and outcomes for CVD (CitationStampfer et al 1991; CitationGrodstein et al 1996). However, the results of hormone replacement therapy clinical trials have had conflicting effects with respect to the risk for CVD. For instance, the Heart and Estrogen/Progestin Replacement Studies (HERS I and II) (CitationHulley et al 1998; CitationGrady et al 2002) and the Women’s Estrogen for Stroke Trial (WEST) (CitationViscoli et al 2001) did not show a reduction in the incidence of CVD. Moreover, the Women’s Health Initiative (WHI) terminated early the trial of estrogen plus progestin owing to increased risks of breast cancer and cardiovascular events (CitationRossouw et al 2002). Recently, CitationAlexandersen et al (2006) revealed that 2–3 years of hormone replacement therapy does not increase all-cause mortality and may lead to cardiovascular health benefits (including decreased severity of aortic calcification). Owing to the discrepancies in the field, it is apparent that hormone replacement therapy should be used cautiously considering the risk benefit ratio for each individual patient.
Priority: Cardiovascular disease versus osteoporosis
Physicians and health care professionals are often met with the challenge of assessing and managing the risk of individuals with varied medical conditions (such as multiple chronic diseases). In terms of screening and risk management osteoporosis is often considered to be of lower priority than cardiovascular disease.
While the incidence of CVD-related mortality and morbidity is shown to be greater than that for osteoporosis, the importance of preventing and treating osteoporosis cannot be discounted. As discussed previously, osteoporosis affects a significant proportion of society (in particular elderly women). Further, as the population ages, these numbers are expected to increase substantially (CitationTucci 2006). The economic and societal implications of osteoporosis are considerable owing to the significant morbidity, mortality, and health care costs associated with osteoporotic fractures (CitationSasser et al 2005; CitationMauck and Clarke 2006). According to CitationSasser et al (2005), in the United States alone, the average annual direct costs of osteoporosis per patient is $6,259 while the indirect cost associated with the disease is $4,039 demonstrating a significant financial burden. Therefore, overcoming the challenge of providing optimal health care while managing the costs associated with treatment is of great concern (especially as the population ages) (CitationMauck and Clarke 2006; CitationTucci 2006). CitationTucci (2006) suggests that in order to control costs, post-fracture care and identification of individuals with an increased risk of fracture may enable cost-effective management and treatment of osteoporosis. It is therefore necessary to ensure effective preventative measures and treatment protocols in order to reduce the number of individuals at risk of developing osteoporosis and experiencing an osteoporosis-related fracture (CitationTucci 2006).
Fortunately, exercise interventions can help address both CVD and osteoporosis concurrently, thereby limiting the need for clinicians to prioritize between conditions. In fact, it is likely that primary and secondary prevention through healthy lifestyle interventions may serve to decrease the physical, social, emotional and spiritual impairments to the individual as well as decrease the direct and indirect medical costs associated with the treatment of both conditions.
Physical activity and its role in the prevention of CVD and osteoporosis
Physical inactivity is a major risk factor for both osteoporosis and CVD (CitationWarburton et al 2006a, Citation2006b). There is also extensive literature indicating that physical activity is an effective primary and secondary preventive strategy against CVD, osteoporosis, and multiple other chronic diseases (including obesity, stroke, hypertension, type 2 diabetes, colon cancer, breast cancer, and several psychological disorders) (CitationWarburton et al 2006a).
Being fit or physically active has been shown to lead to a 30%–50% reduction in the risk of death from any cause and from CVD (CitationWarburton et al 2006a). Low maximal aerobic fitness is as important a risk factor for premature mortality as is overweight and obesity (CitationBlair and Brodney 1999). Several mechanisms may explain the reduced premature mortality rates and incidence of CVD in individuals who are habitually active (CitationWarburton et al 2006a, Citation2006b). For instance, regular aerobic exercise has been shown to improve body composition (via reduced abdominal adiposity and/or improved weight control) (CitationTremblay et al 1990; CitationSeidell et al 1991; CitationSlattery et al 1992; CitationMaiorana et al 2003), enhance lipid lipoprotein profiles (including increased HDL-cholesterol, reduced triglycerides, and decreased LDL-cholesterol) (CitationTaimela et al 1994; CitationHalle et al 1996; CitationBerg et al 1997), improve glucose homeostasis (CitationWallberg-Henriksson et al 1998; CitationKelley and Goodpaster 1999), decrease blood pressure (CitationAmerican College of Sports Medicine 1993; CitationWarburton et al 2006a), reduce systemic inflammation (CitationAdamopoulos et al 2001), enhance cardiac function CitationWarburton et al 1999, Citation2004a, Citation2004b), and improve endothelial function (CitationGokce et al 2002; CitationHambrecht et al 2003; CitationKobayashi et al 2003).
Particularly relevant to this discussion is the exercise-mediated changes in vascular health and endothelial function. Several studies have evaluated the effects of habitual exercise on endothelial function in elderly and patient populations. Cross-sectional investigations have revealed that active older adults have improved vascular health in comparison to sedentary individuals (CitationTaddei et al 2000; CitationMcKechnie et al 2001; CitationGaletta et al 2006a). Moreover, habitual physical activity appears to attenuate the age-associated decline in endothelial function (CitationTaddei et al 2000; CitationGaletta et al 2006a, Citation2006b). Research trials have also revealed the ability of exercise interventions to improve vascular health and endothelial function in asymptomatic and symptomatic populations (CitationHambrecht et al 1998, Citation2000, Citation2003; CitationGokce et al 2002; CitationKobayashi et al 2003) (with the beneficial adaptations being particularly apparent in clinical populations (CitationGreen et al 2004)).
A direct relationship exists between lifetime physical activity and bone health. In particular, weight-bearing exercise appears to have a great effect on bone mineral density (CitationWarburton et al 2001a, Citation2001b, Citation2006a). For instance, adults performing repetitive, high-intensity, weight-bearing exercise consistently have greater BMD than sedentary individuals (CitationBassey and Ramsdale 1994; CitationHeinonen et al 1995; CitationLohman et al 1995; CitationWarburton et al 2001a, Citation2001b). Prospective studies in post-menopausal women using weight bearing activity have often reported an increase or maintenance of total body, hip, and lumbar spine bone mineral density (CitationHatori et al 1993; CitationWelsh and Rutherford 1996; CitationKohrt et al 1997). In fact, resistance training often results in site-specific and load-dependent improvements in BMD (CitationSmidt et al 1992; CitationKerr et al 1996; CitationKohrt et al 1997; CitationWarburton et al 2001a, Citation2001b). A meta-analysis of randomized controlled trials reported that exercise training interventions result in the prevention or reversal of approximately 1% of bone loss per year in the lumbar spine and femoral neck in both pre- and post-menopausal women (CitationWolff et al 1999). Moreover, exercise training has been shown to reduce the risk and number of falls (CitationTinetti et al 1994; CitationWolf et al 1996; CitationShaw and Snow 1998; CitationCarter et al 2001a, Citation2001b; CitationLiu-Ambrose et al 2004a) and fractures (CitationStevens et al 1997; CitationJoakimsen et al 1998; CitationGregg et al 2000; CitationKujala et al 2000). Preliminary evidence also indicates that routine physical activity is effective in improving bone density in older women with low bone mineral density (CitationLiu-Ambrose et al 2004b).
It is clear that regular physical activity prolongs one’s lifespan (CitationLee et al 1997), but it also delays greatly the onset of chronic disease and/or disability. Therefore, physical activity is an effective means to increase the number of years that a person lives in a healthy state, thereby minimizing the years spent in a dependent state. If disability does occur it is generally for a short period of time at the end of life (CitationPowell and Blair 1994). This is particularly important with the ever-increasing aging population.
Summary
It is clear that both CVD and osteoporosis are major health burdens affecting millions of people globally. A growing body of research supports a direct association between CVD and osteoporosis providing an explanation for why (in part) individuals often exhibit both chronic conditions. There are numerous factors that may account for this relationship including risk factors that are common to both debilitating chronic conditions, and varied pathophysiological mechanisms. Both chronic conditions share similar modifiable risk factors (including physical inactivity) and such effective treatment strategies can be developed to address both diseases.
There appear to be distinct differences between (and within) sexes across the lifespan with respect to each chronic condition that need to be considered when treating the individual patient. It is important to have an understanding of the effects of varied primary and secondary preventative strategies on multiple chronic conditions. Preventative strategies should not only target individual risk factors for chronic disease, but also address the overall health-related quality of life of the individual. We advocate a holistic approach to the prevention and treatment of these chronic debilitating diseases; an approach that considers the social, emotional, spiritual and physical well-being of the individual.
When designing an intervention (such as a physical activity intervention) to address both chronic conditions, clinicians must be able to balance the risk of each condition. Clinicians must be aware that there is a potential for the risk reduction in one disease to predispose the individual to an increased risk for the other condition. With this knowledge, clinicians will be able to develop effective interventions that attenuate the risk while addressing the specific limitations of each chronic condition.
Although CVD takes a higher toll on society in terms of premature mortality, morbidity rates, and health care costs, the importance of the prevention/treatment of osteoporosis (particularly in elderly women) can not be overlooked. Habitual physical activity is an effective primary and secondary preventative strategy for both chronic conditions across the lifespan. Training-induced adaptations in endothelial function appear to be particularly important for the concurrent reductions in the risk of CVD and osteoporosis.
References
- AdamiSBragaVZamboniMRelationship between lipids and bone mass in 2 cohorts of healthy women and menCalcif Tissue Int2004741364214668965
- AdamopoulosSParissisJKroupisCPhysical training reduces peripheral markers of inflammation in patients with chronic heart failureEur Heart J200122791711350112
- AguirreJButteryLO’ShaughnessyMEndothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activityAm J Pathol20011582475711141498
- AlexandersenPTankoLBBaggerYZThe long-term impact of 2–3 years of hormone replacement therapy on cardiovascular mortality and atherosclerosis in healthy womenClimacteric200691081816698657
- American College of Sports MedicinePosition stand: Physical activity, physical fitness, and hypertensionMed Sci Sports Exerc199325ix8231750
- American Heart Association2006Dallas, TexasAmerican Heart Association
- AndersonTJNitric oxide, atherosclerosis and the clinical relevance of endothelial dysfunctionHeart Fail Rev20038718612652161
- AoyagiKRossPDOrloffJLow bone density is not associated with aortic calcificationCalcif Tissue Int20016920411685429
- ArmourKEArmourKJGallagherMEDefective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthaseEndocrinology2001a142760611159848
- ArmourKEVanTHRJGrabowskiPSEvidence for a pathogenic role of nitric oxide in inflammation-induced osteoporosisJ Bone Miner Res19991421374210620073
- ArmourKJArmourKEvan’t HofRJActivation of the inducible nitric oxide synthase pathway contributes to inflammation-induced osteoporosis by suppressing bone formation and causing osteoblast apoptosisArthritis Rheum2001b442790611762939
- BaggerYZRasmussenHBAlexandersenPLinks between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per seOsteoporos Int2007185051217109061
- BaggerYZTankoLBAlexandersenPRadiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hipJ Intern Med200625959860516704561
- BaldiniVMastropasquaMFrancucciCMCardiovascular disease and osteoporosisJ Endocrinol Invest200528697216550727
- BarengoltsEIBermanMKukrejaSCOsteoporosis and coronary atherosclerosis in asymptomatic postmenopausal womenCalcif Tissue Int199862209139501953
- BasseyEJRamsdaleSJIncrease in femoral bone density in young women following high-impact exerciseOsteoporos Int199447258003843
- BerensonGSSrinivasanSRBaoWAssociation between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart StudyN Engl J Med1998338165069614255
- BergAHalleMFranzIPhysical activity and lipoprotein metabolism: epidemiological evidence and clinical trialsEur J Med Res19972259649182653
- BlairSNBrodneySEffects of physical inactivity and obesity on morbidity and mortality: current evidence and research issuesMed Sci Sports Exerc199931S6466210593541
- BlakeGJRidkerPMNovel clinical markers of vascular wall inflammationCirc Res2001897637111679405
- BocchiLOrsoCAPassarelloFAtherosclerosis of the microcirculation in the femoral head: based on a study by optical and electron microscopy of femoral heads removed at operationItal J Orthop Traumatol198511365703910613
- BocchiLOrsoCAPassarelloFAtherosclerosis of the vessels in the ligamentum teres. Optical and electron microscopy findings in elderly patients with femoral neck fracturesItal J Orthop Traumatol19871336593452610
- BraithwaiteRSColNFWongJBEstimating hip fracture morbidity, mortality and costsJ Am Geriatr Soc2003513647012588580
- BrownJPJosseRG2002 clinical practice guidelines for the diagnosis and management of osteoporosis in CanadaCmaj2002167S13412427685
- BrownerWSLuiLYCummingsSRAssociations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly womenJ Clin Endocrinol Metab200186631711158021
- BrownerWSSeeleyDGVogtTMNon-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research GroupLancet199133835581677708
- BucayNSarosiIDunstanCROsteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcificationGenes Dev199812126089573043
- Canadian Multicentre Osteoporosis StudyCanadian Multicentre Osteoporosis Study2006
- CarterNDKannusPKhanKMExercise in the prevention of falls in older people: a systematic literature review examining the rationale and the evidenceSports Med2001a314273811394562
- CarterNDKhanKMPetitMAResults of a 10 week community based strength and balance training programme to reduce fall risk factors: a randomised controlled trial in 65–75 year old women with osteoporosisBr J Sports Med2001b353485111579072
- CelermajerDSSorensenKEBullCEndothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interactionJ Am Coll Cardiol1994241468747930277
- CelermajerDSSorensenKEGoochVMNon-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosisLancet1992340111151359209
- CenterJRNguyenTVSchneiderDMortality after all major types of osteoporotic fracture in men and women: an observational studyLancet19993538788210093980
- ChrischillesEAButlerCDDavisCSA model of lifetime osteoporosis impactArch Intern Med19911512026321929691
- Cohen-SolalMEGrauletAMDenneMAPeripheral monocyte culture supernatants of menopausal women can induce bone resorption: involvement of cytokinesJ Clin Endocrinol Metab1993771648538263153
- De LaetCKanisJAOdenABody mass index as a predictor of fracture risk: a meta-analysisOsteoporos Int2005161330815928804
- DohertyTMAsotraKFitzpatrickLACalcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroadsProc Natl Acad Sci U S A200310011201614500910
- DohertyTMUzuiHFitzpatrickLARationale for the role of osteoclast-like cells in arterial calcificationFaseb J2002165778211919160
- EakerEDChesebroJHSacksFMCardiovascular disease in womenCirculation199388199920098403349
- EnsrudKEFullmanRLBarrett-ConnorEVoluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men studyJ Clin Endocrinol Metab2005901998200415671096
- EnsrudKENevittMCYunisCCorrelates of impaired function in older womenJ Am Geriatr Soc19944248198176141
- FioreCEPennisiPCutuliVML-arginine prevents bone loss and bone collagen breakdown in cyclosporin A-treated ratsEur J Pharmacol2000408323611090650
- FoxSWChowJWNitric oxide synthase expression in bone cellsBone199823169662123
- FreedmanDSDietzWHSrinivasanSRThe relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart StudyPediatrics199910311758210353925
- GalettaFFranzoniFPlantingaYAmbulatory blood pressure monitoring and endothelium-dependent vasodilation in the elderly athletesBiomed Pharmacother2006a60443716904861
- GalettaFFranzoniFVirdisAEndothelium-dependent vasodilation and carotid artery wall remodeling in athletes and sedentary subjectsAtherosclerosis2006b1861849216102774
- GokceNVitaJABaderDSEffect of exercise on upper and lower extremity endothelial function in patients with coronary artery diseaseAm J Cardiol200290124712106840
- GottliebSGoldbourtUBoykoVMortality trends in men and women with acute myocardial infarction in coronary care units in Israel. A comparison between 1981–1983 and 1992–1994. For the SPRINT and the Israeli Thrombolytic Survey GroupsEur Heart J2000212849510653676
- GradyDHerringtonDBittnerVCardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II)Jama2002288495712090862
- GreenDJMaioranaAO’DriscollGEffect of exercise training on endothelium-derived nitric oxide function in humansJ Physiol200456112515375191
- GreenlandPReicher-ReissHGoldbourtUIn-hospital and 1-year mortality in 1,524 women after myocardial infarction. Comparison with 4,315 menCirculation199183484911991367
- GreggEWPereiraMACaspersenCJPhysical activity, falls, and fractures among older adults: a review of the epidemiologic evidenceJ Am Geriatr Soc2000488839310968291
- GreyEBratteliCGlasserSPReduced small artery but not large artery elasticity is an independent risk marker for cardiovascular eventsAm J Hypertens200316265912670741
- GrodsteinFStampferMJMansonJEPostmenopausal estrogen and progestin use and the risk of cardiovascular diseaseN Engl J Med1996335453618672166
- HakAEPolsHAvan HemertAMProgression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal studyArterioscler Thromb Vasc Biol20002019263110938013
- HalleMBergAvon SteinTLipoprotein(a) in endurance athletes, power athletes, and sedentary controlsMed Sci Sports Exerc19962896268871904
- HambrechtRAdamsVErbsSRegular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthaseCirculation20031073152812810615
- HambrechtRFiehnEWeiglCRegular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failureCirculation1998982709159851957
- HambrechtRWolfAGielenSEffect of exercise on coronary endothelial function in patients with coronary artery diseaseN Engl J Med20003424546010675425
- HamermanDOsteoporosis and atherosclerosis: biological linkages and the emergence of dual-purpose therapiesQjm2005984678415955801
- HanleyDAJosseRGPrevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. 1. IntroductionCmaj199615592138837540
- HatoriMHasegawaAAdachiHThe effects of walking at the anaerobic threshold level on vertebral bone loss in postmenopausal womenCalcif Tissue Int19935241148369985
- Health CanadaStatistical report on the health of Canadians, 1997 [online]1999 Accessed on September 4th, 2007 URL: http://www.statcan.ca/english/freepub/82-570-XIE/82-570-XIE1997001.pdf
- Health CanadaMinister’s Message: Heart Month 2005 [online]2005 Accessed on September 4th, 2007. URL: http://www.hc-sc.gc.ca/ahc-asc/minist/health-sante/messages/2005_OZ_e.html
- Heart and Stroke Foundation of Canada2000Ottawa, ONHeart and Stroke Foundation of Canada
- HeinonenAOjaPKannusPBone mineral density in female athletes representing sports with different loading characteristics of the skeletonBone1995171972038541131
- HeinrichJAssmannGFibrinogen and cardiovascular riskJ Cardiovasc Risk199521972057584794
- Hermann-ArnhofKMKastenbauerTPubligTInitially elevated osteoprotegerin serum levels may predict a perioperative myocardial lesion in patients undergoing coronary artery bypass graftingCrit Care Med200634768016374159
- HulleySGradyDBushTRandomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research GroupJama1998280605139718051
- JamalSABrownerWSBauerDCIntermittent use of nitrates increases bone mineral density: the study of osteoporotic fracturesJ Bone Miner Res199813175599797485
- JoakimsenRMFonneboVMagnusJHThe Tromso Study: physical activity and the incidence of fractures in a middle-aged populationJ Bone Miner Res1998131149579661079
- JohnellOThe socioeconomic burden of fractures: today and in the 21st centuryAm J Med199710320S25S discussion, 25S–26S9302894
- JorgensenLJoakimsenOMathiesenEBCarotid plaque echogenicity and risk of nonvertebral fractures in women: a longitudinal population-based studyCalcif Tissue Int2006792071317048067
- KadoDMBrownerWSBlackwellTRate of bone loss is associated with mortality in older women: a prospective studyJ Bone Miner Res20001519748011028450
- KannusPHaapasaloHSankeloMEffect of starting age of physical activity on bone mass in the dominant arm of tennis and squash playersAnn Intern Med199512327317762910
- KatzmarzykPTGledhillNShephardRJThe economic burden of physical inactivity in CanadaCmaj200016314354011192648
- KelleyDEGoodpasterBHEffects of physical activity on insulin action and glucose tolerance in obesityMed Sci Sports Exerc199931S6192310593537
- KerrDMortonADickIExercise effects on bone mass in postmenopausal women are site-specific and load-dependentJ Bone Miner Res199611218258822346
- KhanKMcKayHAHaapasaloHDoes childhood and adolescence provide a unique opportunity for exercise to strengthen the skeletonJ Sci Med Sport200031506411104307
- KiechlSSchettGWenningGOsteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular diseaseCirculation200410921758015117849
- KielDPKauppilaLICupplesLABone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart StudyCalcif Tissue Int200168271611683533
- Klein-NulendJHelfrichMHSterckJGNitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependentBiochem Biophys Res Commun1998250108149735341
- KobayashiNTsuruyaYIwasawaTExercise training in patients with chronic heart failure improves endothelial function predominantly in the trained extremitiesCirc J2003675051012808267
- KohrtWMEhsaniAABirgeSJJrEffects of exercise involving predominantly either joint-reaction or ground-reaction forces on bone mineral density in older womenJ Bone Miner Res1997121253619258756
- KujalaUMKaprioJKannusPPhysical activity and osteoporotic hip fracture risk in menArch Intern Med2000160705810724057
- LarocheMMoulinierLLegerPBone mineral decrease in the leg with unilateral chronic occlusive arterial diseaseClin Exp Rheumatol200321103612673899
- LarocheMPouillesJMRibotCComparison of the bone mineral content of the lower limbs in men with ischaemic atherosclerotic diseaseClin Rheumatol19941361147697964
- LeeIMPaffenbargerRSJrHennekensCHPhysical activity, physical fitness and longevityAging (Milano)199792119177581
- LeesonCPWhincupPHCookDGFlow-mediated dilation in 9- to 11-year-old children: the influence of intrauterine and childhood factorsCirculation199796223389337195
- Liu-AmbroseTKhanKMEngJJResistance and agility training reduce fall risk in women aged 75 to 85 with low bone mass: a 6-month randomized, controlled trialJ Am Geriatr Soc2004a526576515086643
- Liu-AmbroseTYKhanKMEngJJBoth resistance and agility training increase cortical bone density in 75- to 85-year-old women with low bone mass: a 6-month randomized controlled trialJ Clin Densitom2004b7390815618599
- LohmanTGoingSPamenterREffects of resistance training on regional and total bone mineral density in premenopausal women: a randomized prospective studyJ Bone Miner Res1995101015247484276
- MacdonaldHMNewSACampbellMKInfluence of weight and weight change on bone loss in perimenopausal and early postmenopausal Scottish womenOsteoporos Int2005161637115185065
- MagnusJHBroussardDLRelationship between bone mineral density and myocardial infarction in US adultsOsteoporos Int20051620536216249840
- MaioranaAO’DriscollGTaylorRExercise and the nitric oxide vasodilator systemSports Med20033310133514599231
- MauckKFClarkeBLDiagnosis, screening, prevention, and treatment of osteoporosisMayo Clin Proc2006816627216706264
- McGavockJMandicSLewanczukRCardiovascular adaptations to exercise training in postmenopausal women with type 2 diabetes mellitusCardiovasc Diabetol20043315023235
- McGillHCJrMcMahanCAHerderickEEOrigin of atherosclerosis in childhood and adolescenceAm J Clin Nutr2000721307S1315S11063473
- McKayHJPCAhamedYBC Ministry of Health Services Victoria, BC2004
- McKayHAMacLeanLPetitM“Bounce at the Bell”: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal childrenBr J Sports Med200539521616046335
- McKechnieRRubenfireMMoscaLAssociation between self-reported physical activity and vascular reactivity in postmenopausal womenAtherosclerosis20011594839011730830
- MeltonLJ3rdChrischillesEACooperCPerspective. How many women have osteoporosisJ Bone Miner Res199271005101414493
- MinHMoronySSarosiIOsteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesisJ Exp Med20001924637410952716
- NabhanAFA randomized clinical trial of the effects of isosorbide mononitrate on bone formation and resorption in post-menopausal women: a pilot studyHum Reprod2006211320416410328
- National Osteoporosis Foundation2007Washington, DCNational Institute of Health
- Osteoporosis CanadaOsteoporosis Canada, Toronto, Ontario2007
- PalaciosSBorregoRSFortezaAThe importance of preventive health care in post-menopausal womenMaturitas200552Suppl 1S536016129574
- ParhamiFBasseriBHwangJHigh-density lipoprotein regulates calcification of vascular cellsCirc Res200291570612364384
- ParhamiFGarfinkelADemerLLRole of lipids in osteoporosisArterioscler Thromb Vasc Biol2000202346811073836
- ParhamiFJacksonSMTintutYAtherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cellsJ Bone Miner Res19991420677810620066
- ParhamiFMorrowADBalucanJLipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patientsArterioscler Thromb Vasc Biol19971768079108780
- ParhamiFTintutYBeamerWGAtherogenic high-fat diet reduces bone mineralization in miceJ Bone Miner Res200116182811149483
- PowellKEBlairSNThe public health burdens of sedentary living habits: theoretical but realistic estimatesMed Sci Sports Exerc19942685167934758
- Public Health Agency of Canada2002Ottawa, OntarioPublic Health Agency of Canada102
- RajzbaumGBezieYPostmenopausal osteoporosis and atheromaJoint Bone Spine200673661617064947
- ReidCDyckLMcKayHA1999VancouverBritish Columbia Centre of Excellence for Women’s Health249
- RomneyJSLewanczukRZVascular compliance is reduced in the early stages of type 1 diabetesDiabetes Care2001242102611723091
- RossouwJECoronary heart disease in menopausal women: implications of primary and secondary prevention trials of hormonesMaturitas200551516315883110
- RossouwJEAndersonGLPrenticeRLRisks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trialJama20022883213312117397
- RubinMRSilverbergSJVascular calcification and osteoporosis – the nature of the nexusJ Clin Endocrinol Metab2004894243515356015
- SamuelsAPerryMJGibsonRLRole of endothelial nitric oxide synthase in estrogen-induced osteogenesisBone20012924911472887
- SasserACRousculpMDBirnbaumHGEconomic burden of osteoporosis, breast cancer, and cardiovascular disease among postmenopausal women in an employed populationWomens Health Issues2005159710815894195
- SchulzEArfaiKLiuXAortic calcification and the risk of osteoporosis and fracturesJ Clin Endocrinol Metab20048942465315356016
- SchwartzAVSellmeyerDEStrotmeyerESDiabetes and bone loss at the hip in older black and white adultsJ Bone Miner Res20052059660315765178
- SeidellJCCigoliniMDeslypereJPBody fat distribution in relation to physical activity and smoking habits in 38-year-old European men. The European Fat Distribution StudyAm J Epidemiol1991133257652000843
- ShawJMSnowCMWeighted vest exercise improves indices of fall risk in older womenJ Gerontol A Biol Sci Med Sci199853M5389467434
- SinnottBSyedISevrukovACoronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with agingCalcif Tissue Int20067819520216604285
- SlatteryMLMcDonaldABildDEAssociations of body fat and its distribution with dietary intake, physical activity, alcohol, and smoking in blacks and whitesAm J Clin Nutr19925594391570801
- SmidtGLLinSYO’DwyerKDThe effect of high-intensity trunk exercise on bone mineral density of postmenopausal womenSpine19921728051566165
- SorensenKEKristensenIBCelermajerDSAtherosclerosis in the human brachial arteryJ Am Coll Cardiol199729318229014983
- StampferMJColditzGAWillettWCPostmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health studyN Engl J Med1991325756621870648
- Statistics Canada1999aOttawa, OntarioStatistics Canada
- Statistics Canada1999bOttawa OntarioStatistics Canada
- StevensJAPowellKESmithSMPhysical activity, functional limitations, and the risk of fall-related fractures in community-dwelling elderlyAnn Epidemiol1997754619034407
- StevensonJCHormone replacement therapy: review, update, and remaining questions after the Women’s Health Initiative StudyCurr Osteoporos Rep2004212616036077
- TaddeiSGalettaFVirdisAPhysical activity prevents age-related impairment in nitric oxide availability in elderly athletesCirculation2000101289690110869260
- TaimelaSViikariJSPorkkaKVLipoprotein (a) levels in children and young adults: the influence of physical activity. The Cardiovascular Risk in Young Finns StudyActa Paediatr1994831258637734865
- TankoLBBaggerYZChristiansenCLow bone mineral density in the hip as a marker of advanced atherosclerosis in elderly womenCalcif Tissue Int200373152014506949
- TankoLBChristiansenCCoxDARelationship between osteoporosis and cardiovascular disease in postmenopausal womenJ Bone Miner Res20052019122016234963
- TinettiMEBakerDIMcAvayGA multifactorial intervention to reduce the risk of falling among elderly people living in the communityN Engl J Med199433182178078528
- TintutYMoronySDemerLLHyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivoArterioscler Thromb Vasc Biol200424e61014670933
- TremblayADespresJPLeblancCEffect of intensity of physical activity on body fatness and fat distributionAm J Clin Nutr19905115372305702
- TroianoRPFlegalKMKuczmarskiRJOverweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991Arch Pediatr Adolesc Med19951491085917550810
- TucciJRImportance of early diagnosis and treatment of osteoporosis to prevent fracturesAm J Manag Care200612S1819016686587
- UyamaOYoshimotoYYamamotoYBone changes and carotid atherosclerosis in postmenopausal womenStroke199728173029303016
- ViscoliCMBrassLMKernanWNA clinical trial of estrogen-replacement therapy after ischemic strokeN Engl J Med20013451243911680444
- VogtMTCauleyJAKullerLHBone mineral density and blood flow to the lower extremities: the study of osteoporotic fracturesJ Bone Miner Res1997a1228399041062
- VogtMTSan ValentinRForrestKYBone mineral density and aortic calcification: the Study of Osteoporotic FracturesJ Am Geriatr Soc1997b4514059033510
- von der ReckePHansenMAHassagerCThe association between low bone mass at the menopause and cardiovascular mortalityAm J Med1999106273810190374
- Wallberg-HenrikssonHRinconJZierathJRExercise in the management of non-insulin-dependent diabetes mellitusSports Med19982525359458525
- WarburtonDEGledhillNQuinneyAThe effects of changes in musculoskeletal fitness on healthCan J Appl Physiol2001a2616121611312416
- WarburtonDEGledhillNQuinneyAMusculoskeletal fitness and healthCan J Appl Physiol2001b262173711312417
- WarburtonDEHaykowskyMJQuinneyHABlood volume expansion and cardiorespiratory function: effects of training modalityMed Sci Sports Exerc2004a36991100015179169
- WarburtonDENicolCWBredinSSHealth benefits of physical activity: the evidenceCmaj2006a174801916534088
- WarburtonDENicolCWBredinSSPrescribing exercise as preventive therapyCmaj2006b1749617416567757
- WarburtonDESheelAWHodgesANEffects of upper extremity exercise training on peak aerobic and anaerobic fitness in patients after transplantationAm J Cardiol2004b939394315050506
- WarburtonDERGledhillNJamnikVInduced hypervolemia, cardiac function, VO2max and performance of elite cyclistsMed Sci Sports Exerc19993180080810378906
- WelshLRutherfordOMHip bone mineral density is improved by high-impact aerobic exercise in postmenopausal women and men over 50 yearsEur J Appl Physiol Occup Physiol19967451178971492
- WhitneyCWarburtonDEFrohlichJAre cardiovascular disease and osteoporosis directly linkedSports Med20043477980715462612
- WilsonEWielgoszAThe changing face of heart disease and stroke in Canada – release of the fifth report from the Canadian Heart and Stroke Surveillance systemCan J Cardiol1999151075910523471
- WittemanJCGrobbeeDEKokFJIncreased risk of atherosclerosis in women after the menopauseBmj198929864242496790
- WolfSLBarnhartHXKutnerNGReducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. lanta FICSIT Group. Frailty and Injuries: Cooperative Studies of Intervention TechniquesJ Am Geriatr Soc199644489978617895
- WolffIvan CroonenborgJJKemperHCThe effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal womenOsteoporos Int1999911210367023
- World Health Organization2001World Health Organization
- World Health Organization2003GenevaWorld Health Organization149
- World Health Organization2006World Health Organization
- WrightVJOsteoporosis in menJ Am Acad Orthop Surg2006143475316757674
- YamaguchiTSugimotoTYanoSPlasma lipids and osteoporosis in postmenopausal womenEndocr J200249211712081241
- YanoKTsudaEWashidaNImmunological characterization of circulating osteoprotegerin/osteoclastogenesis inhibitory factor: increased serum concentrations in postmenopausal women with osteoporosisJ Bone Miner Res1999145182710234572