Abstract
Poor condition subarachnoid hemorrhage (SAH) patients present a high mortality and morbidity. In this study, we reviewed the acute interventional (surgical and endovascular) management of 109 SAH-poor condition patients, who were treated as early as logistically possible after confirming stable circulation parameters. Patients over the age of 70 years, without clinical response to painful stimulation were excluded. We recognized at least 3 different postinterventional therapeutic approaches: (1) Norm- or hypovolemic, normotensive hemodilution in 30 patients with space-occupying intracranial hematomas as well as in 31 cases with acute cerebro-spinal-fluid obstruction. (2) Normovolemic, hypertensive hemodilution after unilateral decompressive craniotomy in 23 surgical- and 2 endovascular-treated patients with focalized space occupying lesions and reduced cerebral perfusion. (3) Hypovolemic, normo-, or hypertensive hemodilution after bilateral decompressive craniotomy in 23 cases with massive brain-swelling. We observed a reduced mortality (21%). The overall late outcome was favorable in 56% and unfavorable in 23%. Selective aggressive treatment adapted to increase the cerebral perfusion, seems to be an effective therapy to improve the survival and outcome of several poor condition SAH-patients.
Keywords:
Introduction
The mortality rate among nonoperative treated poor condition subarachnoid hemorrhage (SAH) patients in some series reach 94% to 98% (CitationZubkov 1994). Improved outcome after early aneurysm surgery in poor grade patients has been attributed to the use of advanced microsurgical technique, anesthetic methods and the application of nimodipine (CitationSpetzger and Gilbasch 1994). However different results have been reported with this standard (CitationSaveland and Brandt 1994; CitationUngersboeck et al 1994; CitationHansen et al 1995; CitationDuke et al 1998; CitationHutchinson et al 2000; CitationLaidlaw and Kevin 2003; CitationKhandelwal et al 2005). In order to establish the possible benefit of acute individual managements on the patient’s outcome, we designed a retrospective clinical trial evaluating all poor condition SAH patients admitted to our department, who underwent acute surgical aneurysm occlusion following different decompression procedures.
Materials and methods
Medical records were retrospectively reviewed for all patients treated in our department in the past 13 years admitted in a poor clinical condition with a Hunt and Hess scale (H&H) grade IV and V. Those cases that underwent emergent (first 6 hours after bleeding) interventional treatment (endovascular or surgical) were analyzed and a follow-up for the survivors was obtained.
The following parameters were reviewed retrospectively in all selected patients: age and sex, clinical condition (assessed with the Hunt and Hess scale), sort of performed treatment, interventional procedure as well as intra-operative findings, and post-interventional management. Surgical indications were analyzed in order to clarify the real need for this kind of treatment. Outcome was early evaluated 6 months after event with the Glasgow Outcome Scale (GOS), where a GOS 1 is dead (CitationJennett et al 1975).
Decompressive surgery was performed by using a large bone flap reaching the floor of the middle cerebral fosse and a dura patch enlarging the decompressed surface in order to avoid brain prolapsed border damages. Aneurysm clipping after decompression was always possible even in patients with increased intracranial pressure avoiding the brain retraction-related secondary injuries. All patients had direct intracranial pressure (ICP), arterial blood pressure (ABP), and transcranial Doppler middle cerebral artery blood flow velocity (FV) monitored. ICP was measured with either implanted intraparenchymal microsensors (Camino Laboratories, San Diego, CA or Codman Group Inc., Munich, Germany) or an external ventricular drainage (Codman Group Inc); only periods with closed drainage were evaluated). Hypertensive therapy was performed with dopamine or phenylephrine to raise systolic values over 150 mmHg.
Cerebral autoregulation was evaluated with at least one of the following test. The transient hyperemic response test assessed the response of middle cerebral artery (MCA) blood FV after a brief (<5 seconds) compression of the ipsilateral common carotid artery. Normally, intact autoregulation is associated with vasodilatation during the period of carotid compression. The release of the carotid compression results in a transient hyperemic overshoot dilating the distal vascular bed. If autoregulation was lost, the response was absent. The pressure-reactivity index (PRx) (calculated as a moving correlation coefficient between 40 consecutive samples of values for ICP and ABP averaged over 5 seconds), was used to assess cerebrovascular reactivity to changes in ABP. A positive PRx signifies a positive association between ABP and ICP, indicating a nonreactive vascular bed, while a negative PRx is reflective of intact cerebral autoregulation, where ABP waves provoke inversely correlated waves in ICP.
To monitor hemodilution parameters, the hematocrit level, hemoglobin concentration, and red blood cell aggregation rate were daily measured. To assess the hemodynamic state and prevent pulmonary edema, a Swan-Ganz catheter was positioned via a subclavian approach to monitor the pulmonary artery pressure. Post-operative intensive care priorities after invasive ICP and hemodynamic monitoring in patients with damaged autoregulation included water restriction <1500 ml 24 hr, mannitol infusions 20% (0.25–0.5 g/kg every 4 to 6 h), vasopressor support (dopamine 2–20 μg × kg−1 × min−1) to maintain the cerebral perfusion pressure (CPP) ≥70 mmHg. Systemic administration of 23.5% hypertonic saline infusions were used for reversal of cerebral ischemia to normal perfusion in hypovolemic patients with reduced CPP. Low-molecular-weight dextran was not administered to avoid uncontrolled hypervolemic therapy, and the total fluid intake was kept below 3000 mL/d (normovolemic standard). Only Human albumin was employed in some patients. The combination of hypervolemia-induced hypertension and calcium channel blockers was not employed in patients with disturbed cerebral autoregulation to avoid secondary blood brain barrier damages and brain swelling impairments. Additional informations about cerebral perfusion and oxygenation were evaluated with different techniques like single photon emission computed tomography (SPECT), brain tissue oxygen pressure (PtiO2), and diffusion weighted magnetic resonance imaging (MRI). These studies were performed and periodically repeated in relationship with the magnitude of the diagnosed cerebral perfusion disorder, patient’s evolution and their chances to be transported.
Results
One hundred and nine poor grade SAH patients (61 H&H 4 and 48 H&H 5) were interventional (93 surgical- and 16 endovascular-treated). In this study, 12 patients over the age of 70 years as well as 8 cases without clinical response to painful stimulation were previously excluded. The bleeding sources were 38 anterior communicating artery aneurysms (AcoA), 41 MCA aneurysms, 19 internal carotid artery aneurysms (ICA), and 11 vertebrobasilar aneurysms (VB) in 49 males and 60 females, aged 11 to 70 years. Space-occupying intracerebral and subdural hematomas were removed in 28 cases and followed by the surgical occlusion of the aneurysm considered to be the source of the bleeding. Twenty four of these patients underwent initial decompressive craniotomy. Two patients with a mild mass occupying hematoma and severe cardiovascular accompanying disease were endovascular treated. Postoperative normotensive and norm- or hypovolemic hemodilution was employed in all patients with stabilized CPP and cerebral autoregulation. The hematocrit level of our patients undergoing normovolemic hemodilution therapy was 31% to 34%, which is a reasonable value on the basis of relative oxygen transport capacity. A summary of the patient’s clinical data, group’s characterization, postinterventional cerebral autoregulation findings and management with their respective outcomes is given in .
Ventriculostomy, angiography, and aneurysm clipping were performed in 22 cases and coiling in 9 patients with acute CSF obstruction. In patients with CSF obstruction and damaged cerebral autoregulation, only a postoperative normotensive hypovolemic hemodilution was administrated. Twenty four patients with initial CSF obstruction but without autoregulation disorders underwent a postoperative normotensive, normovolemic hemodilution. Patients with space occupying lesions due to vasospasms or developing massive cerebral infarction with concomitant edema (23 clipped and 2 coiled aneurysms), were treated with unilateral decompressive craniotomy and postoperative normovolemic hypertensive hemodilution. Patients with massive brain-swelling and computed tomography (CT)-scan absence of basal cisterns or ventricle as well as clinical herniation signs (23 cases), were managed with bilateral decompressive surgery. Aneurysm clipping had been previously performed in 20 and coiling in 3 of these cases. We observed a total reduced mortality (21%). The overall late outcome was favorable (GOS 4 to 5) in 56% and unfavorable in 23%. Space-occupying intracerebral and subdural hemorrhages were adequately removed and emergency aneurysm clipping performed with a reduced employment of brain retractors throughout an enlarged craniotomy (). Decompressive surgery was performed not only in cases with space-occupying intracerebral and subdural hematomas and increased ICP but also in those patients where CPP was reduced due to the development of massive secondary local or generalized brain swelling. Postoperatively, all these patients experienced immediate decreases in ICP to levels at or below 20 mmHg (presentation mean ICP, 35.2 mmHg; postoperative mean ICP, 15.2 mmHg) and postoperative CPP improvements from at least 22.5 mmHg. Patient’s age and general clinical condition as well as aneurysm morphology and its location played an important role by cases where a primary endovascular aneurysm treatment was decided. Intraoperative technical difficulties were documented in 24 (25%) cases. The described problems were as follow: (1) restricted surgical access to the area of the aneurysm in 15 cases. Only 2 of these patients required an additional resection of brain parenchyma for clip application; (2) excessive brain retraction in 5 cases as documented on postoperative CT-scans; (3) use of temporary clip application in 4 cases (3 of them located on the AcoA). Ventriculostomy, and aneurysm clipping in cases with acute CSF obstruction, mostly had a favorable outcome, even in cases with combined IC mass occupying bleeding (). However 4 of 9 endovascular-treated patients in this group had an unfavorable outcome and 3 died (). Osmotic therapy, mechanical hyperventilation, and even short-acting barbiturates failed to prevent herniation and brain infarct in 45 cases. Unilateral or bilateral decompressive craniotomy (DC) showed additional benefits for all these patients reducing ICP and increasing CPP. Quantitative SPECT analysis of 16 regions of interest (ROIs) and PtiO2 monitoring in many of these patients revealed an increase of the cerebral perfusion after DC. Adequate cerebral perfusion pressure was obtained in such cases even with hypovolemic normotensive hemodilution. Hypodense CT-scan regions in the radiological controls from 17 patients were not associated to clinical relevant neurological deficits.
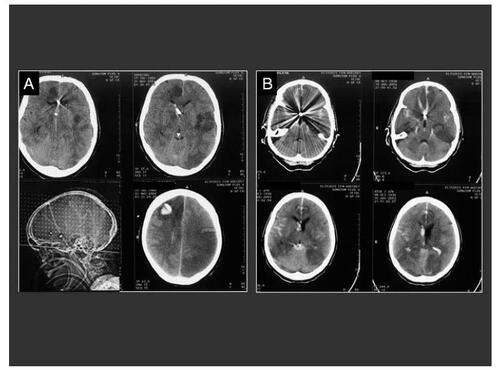
Figure 1 Upper sequence: SAH in a 48 year-old patient, H&H 4; (A) Preoperative CT-scan showing a right temporal mass occupying bleeding and secondary brain swelling; (B) Preoperative antero-posterior cerebral angiography of the right internal carotid artery demonstrating a multilobular giant aneurysm on the middle cerebral artery (white arrow) with vascular displacement; (C) Postoperative angiographic control showing a complete aneurysm occlusion and the borders of the decompressive craniotomy (black arrow); (D) Postoperative CT-scan demonstrating the clot-removal and the surgical decompression. Lower sequence: Fifty nine year-old woman with massive subdural and SAH and clinical herniation signs, H&H 5; A) Preoperative CT-scan demonstrating the right hemispheric subdural hemorrhage and the acute brain shift of the midline structures; (B) CT-angiography displaying the aneurysm (arrow) arising from the internal carotid artery; (C) Postoperative CT-scan demonstrating the brain reexpansion the surgical decompression.
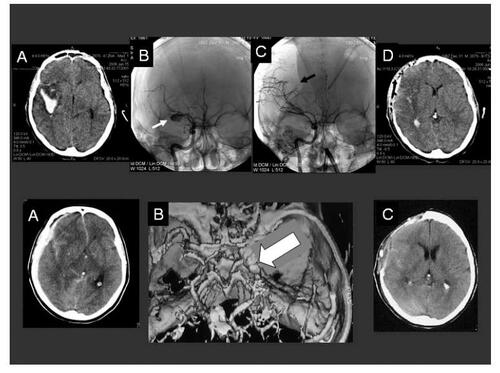
Figure 2 Eleven year-old child with H&H 5 SAH and clinical herniation signs; (A) Preoperative CT-scans demonstrating a left frontal mass occupying bleeding with intraventricular dissemination and acute hydrocephalus; (B) Postoperative CT-scans confirming the clot removal and the placement of the ventricular drainage. The borders of the decompressive craniotomy are well defined; (C) Preoperative cerebral angiography of the left internal carotid artery demonstrating the aneurysm (white arrow) and the caliber narrowing of the anterior cerebral artery (black arrow); (D) Postoperative, 10 hours later performed control angiography confirming the occlusion of the aneurysm and the improved cerebral perfusion.
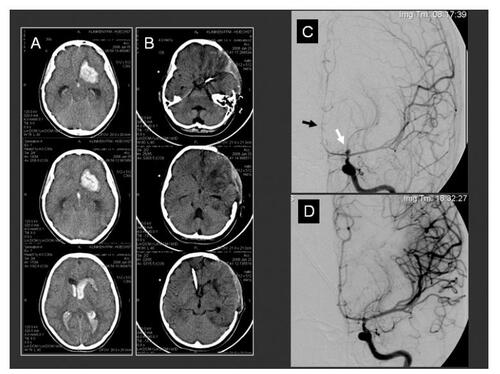
Figure 3 Two endovascular treated patients with delayed secondary ischemic lesions. These patients were both grade V at admission and with an acute CSF obstruction in CT-scans. Ventriculostomy, angiography and aneurysm coiling were in both patients performed. Multiple intracerebral hypodensities and the coils artifacts can be observed in both cases. (A) CT-scan control 5 days after aneurysm coiling and before decompressive procedure in this 44-years-old patient showing two coiled (AcoA and pericallosa) aneurysms. ICP values were obtained from the right side placed intraventricular catheter; (B) CT-scan control 4 days after aneurysm coiling (AcoA) and before decompressive procedure in a 63-years-old patient showing similar findings than patient A. Both cases underwent prolonged endovascular procedures.
Discussion
Patients with a poor clinical condition following aneurysmal SAH present an associated high mortality and morbidity (CitationBailes et al 1990). These patients have also a high ultra-early rebleeding rate, which indicates a need to urgently secure the ruptured aneurysm b and y performing surgery or coiling. On this particular point, in the last 7 years, several authors reached controversial conclusions after examining the benefits of endovascular and surgical techniques (CitationKremer et al 1999; CitationHutchinson et al 2000; CitationSmith et al 2002; CitationWeir et al 2003; CitationLaidlaw and Kevin 2003). Other authors have even proved the benefits of surgical clipping over endovascular technique in cases with severe subarachnoid hemorrhage (CitationKhandelwal et al 2005). The overall late outcome of surgically treated poor condition patients in our series was better than the outcome of endovascular-treated patients. A favorable outcome (GOS 4 to 5) was observed in 58% and unfavorable in 23% of our surgically treated patients. We observed also a reduced mortality in these patients of 19% compared with other surgical series from 22.2% (Ungersbock et al 1994). CitationWeir and colleagues (2003) reported sixteen patients (59%) dying within 30 days of SAH and only 8 patients (30%) had a good clinical outcome after endovascular treatment. Suzuki and colleagues (2006) reported thirty-nine patients (35.1%) with a favorable long-term clinical outcome. The overall mortality rate in the Suzuki patient’s series was 32.4%. Despite of the risk of bleeding with systemic full heparinization in patients endovascular treated, decompressive craniotomy seems have reduced the number of fatal outcomes in our cases. Four of 6 endovascular-treated patients survived after this treatment with severe neurological deficits.
Cerebral blood flow studies in SAH patients have shown that there is a close relationship between the severity of the clinical grade and the lowering of CBF (CitationRead et al 1983; CitationPickard et al 1985). Since autoregulation is impaired after SAH (CitationTenjin et al 1988), CBF becomes totally dependent on the CPP. Further increase of ICP causes significant CBF diminution (CitationSano et al 1987). Poor-grade patients require individual evaluation of their hemodynamic situation, before the previously damaged autoregulation gets overcharged with additional treatments. Vasoparalysis of vascular smooth muscle by blocking the calcium influx may increase cerebral blood volume (CBV), edema, and ICP (CitationGaab et al 1985). In our department, combined hypervolemic induced hypertension and calcium channel blockers were never employed in the period of damaged cerebral autoregulation or increased ICP (Carvi y Nievas et al 1999a). On the other side, despite the widespread use of hypertensive hypervolemic hemodilutional therapy during the past 20 years, there are no randomized, prospective, controlled clinical studies demonstrating that this management improves the short- or long-term neurologic outcome or survival after subarachnoid hemorrhage (Hansen et al 1999). Since outcome is not only determined by the initial hemorrhage but also by the subsequent development of intracranial hypertension (CitationLe Roux et al 1996), a decompressive craniotomy for these patients should be also indicated by intractable intracranial hypertension even many days after aneurysm occlusion. Despite hemispheric brain infarctions, the patients seem not to suffer from complete hemiplegia or are not permanently wheel chair bound. After decompressive craniotomy in cases of uncontrollable intracranial hypertension, brain tissue oxygen tension had also increased along with decreased ICP in the patients examined by several other authors (CitationUnterberg et al 1995). Most of the patients in poor condition show a tight brain, or will develop this within the following days, as a result of hypervolemic therapy-induced changes and damaged autoregulation (CitationShimoda et al 1993). Therefore, it is important to perform craniectomy already by the admission in many patients where brain swelling on the CT-scan can be correlated with clinical deterioration, even without signs of compartmental herniation or in cases of untreatable rising of the ICP. A broad primary craniotomy (CitationCarvi y Nievas 1999) in poor-grade patients, instead of an earlier advised pterional craniotomy to approach aneurysms of the anterior circulation, makes it frequently unnecessary to employ other drastic solutions for intractable brain swelling, such as a lobectomy or additional parenchymal resections (CitationLe Roux et al 1996). Patients with primary ischemic diffuse lesions after SAH and patients suffering from cardio-respiratory arrest, who were successfully resuscitated, should be candidates for bilateral decompressive craniotomy. These patients frequently are in Grade V at admission and have thick diffuse SAH, intraventricular or intracerebral hemorrhages (CitationShapiro 1996).
Several authors demonstrated that preoperative grade was not significantly correlated with the incidence or severity of delayed cerebral ischemia during any time interval (CitationSolomon et al 1991). Critical increased blood flow velocities after SAH without secondary neurological deficits do not indicate vasospasm, but hyperemia (CitationMeixensberger et al 1996). This hyperemia protects patients against rebleeding, but produces a diffuse ischemia. Actual management practices concentrate on efforts to reduce secondary worsening of the ischemic condition. Cerebral infarction with or without vasospasm is common in poor-grade patients. The presence of hypotension and ICH increases the risk of infarction three-times (CitationAdams et al 1989). The reduction of CBF progresses in many patients within 14 days after SAH. Impaired brain oxygenation is one of the main factors worsening the outcome of patients after SAH (CitationJödicke et al 2003). We observed 128 ischemic events in 30 of our patients monitored with an intracerebral implanted PtiO2 sensor. In 18 patients the events were multiple or diffuse and in 12 patients the events were focalized as confirmed with SPECT or MRI. The human brain has usually a critical PtiO2 between 15 and 20 mmHg, below which infarction may occur. As previously reported (CitationValadka et al 2002), PtiO2 values react linearly to regional and global cerebral blood flow changes and may significantly decrease with a combined brain pH reduction in relationship to the severity of the bleeding. In our department, early impaired brain oxygenation during angiography was identified in 5 of 10 poor conditions monitored patients. Three of them died days later as consequence of multiple bilateral cerebral infarctions. The alterations of PtiO2 values observed in these patients were not secondary to arterial oxygen pressure, mean arterial blood pressure, or ICP fluctuations (CitationCarvi y Nievas et al 2005). Taking into account that the results of brain PtiO2 measurement reflect the actual oxygen delivery to the neurons, the described findings should warn about the indiscriminate employment of prolonged endovascular procedures in patients with SAH in poor condition. The summation of these factors (poor condition and prolonged endovascular procedure), could perhaps explain the ischemic damage observed in our endovascular-treated patients illustrated in .
Arterial hypertension usually increases CPP values and normalizes tissue oxygenation in ischemic areas. Holding an elevated CPP (CitationOtsubo et al 1990; CitationOda et al 1996; CitationStocchetti et al 1998), minute ventilation adjusted to obtain levels of partial pressure of carbon dioxide in arterial blood between 30 and 35 mmHg, and the carefully use of mannitol and dexamethasone (Muizelaar et al 1983) are well known successful tools to reduce the effects of the ischemia until an adequate secondary decompressive craniotomy can be performed. Optional postoperative intensive care priorities include electroencephalograph (EEG)-controlled barbiturate therapy to block pathophysiological events leading to neuronal death (CitationShapiro et al 1996) and transluminal balloon angioplasty (CitationFirlik et al 1997) to treat severe vasospasm. Unfortunately, the region of the aneurysm clipping must be excluded from dilatation to avoid complications (CitationCarvi y Nievas et al 2007).
In summary, after recognition and treatment of the origins of the patient’s poor condition, additional factors associated with ischemic condition (increased ICP, decreased CPP, and damaged autoregulation) still need to be monitored. Selective aggressive treatment adapted to increase the cerebral perfusion in this study seems to be an effective therapy to improve the survival and outcome of several poor condition SAH-patients.
References
- AdamsHPKassellNFTornerJCUsefulness of computed tomography in predicting outcome after aneurysmal subarachnoid hemorrhage: a preliminary report of the cooperative aneurysm studyNeurology198935126374022373
- BailesJESpetzlerRFHadleyMNManagement morbidity and mortality of poor-grade aneurysm patientsJ Neurosurg199072559662319314
- Carvi y NievasMNPoor-grade subarachnoid hemorrhage patients: The use of nimodipine and other optional treatmentsNeurol Res1999216495210555185
- Carvi y NievasMNHaasEHöllerhageH-GSelective treatment in SAH grade IV and V. European Association of Neurological SocietiesEuropean Congress of Neurosurgery199911Monduzzi Editore. Publisher717
- Carvi y NievasMNToktamisSHaasEHyperacute measurement of brain-tissue oxygen, carbon dioxide, pH and ICP, before, during and after cerebral angiography in poor condition patients with aneurysmatic subarachnoid hemorrhageSurg Neurol200564362716182009
- Carvi y NievasMNHaasEHöllerhageH-GSevere intracranial bleedings during endovascular procedures. Outcome of surgically treated patientsNeurol Res200729819017427281
- DisneyLWeirBGraceMFactors influencing the outcome of aneurysms rupture in poor-grade patients: a prospective seriesNeurosurgery198823193173644
- DukeBJKindtGWBreezeREOutcome after urgent surgery for grade IV subarachnoid hemorrhageSurg Neurol199850169729701123
- FirlikADKaufmannAMJungreisCAEffect of transluminal angioplasty on cerebral blood flow in the management of symptomatic vasospasm following aneurismal subarachnoid hemorrhageJ Neurosurg19978683099126899
- GaabMRHaubitzTBrawanskyAAcute effects of nimodipine on the cerebral blood flow and intracranial pressureNeurochirurgie198528939
- HansenDHannemannLSpechtMCerebral vasospasm following aneurysmal subarachnoid hemorrhage. Therapeutic value of treatment with calcium antagonist, hypervolemic hemodilution and induced arterial hypertensionAnaesthesist199544219297785750
- HutchinsonPPowerDTripathiPOutcome from poor grade aneurismal subarachnoid haemorrhage – which poor grade subarachnoid haemorrhage patients benefit from aneurysm clipping?Br J Neurosurg2000142105910889881
- JaegerMSoehleMMeixensbergerJEffects of decompressive craniectomy on brain tissue oxygen in patients with intracranial hypertensionJ Neurol Neurosurg Psychiatry2003745131512640077
- JennettBBondMAssessment of outcome after severe brain injury: a practical scaleLancet19751480446957
- JödickeAHübnerFBökerD-KMonitoring of brain tissue oxygenation during aneurysm surgery: prediction of procedure-related ischemic eventsJ Neurosurg2003985152312650422
- KhandelwalPKatoYSanoHTreatment of ruptured intracranial aneurysms: our approachMinim Invasive Neurosurg200548325916432780
- KremerCGrodenCHansenHCOutcome after endovascular treatment of Hunt and Hess grade IV or V aneurysms. Comparison of anterior versus posterior circulationStroke19993026172210582987
- LaidlawJSiuKPoor-grade aneurysmal subarachnoid hemorrhage: Outcome after treatment with urgent surgeryNeurosurgery2003531612823868
- Le RouxPDElliotJPNewellDWPredicting outcome in poor-grade patients with subarachnoid hemorrhage: a retrospective review of 159 aggressively managed casesJ Neurosurg19968539498683281
- MeixensbergerJHamelbeckBDingsJCritical increase of blood flow velocities after subarachnoid haemorrhage: vasospasm versus hyperaemiaZentralbl Neurochir1996577058779272
- OdaSShimodaMSatoOEarly aneurysm surgery and dehydration therapy in patients with severe subarachnoid haemorrhage without ICHActa Neurochir199613810506
- OtsuboHTakemaeTInoueTNormovolemic induced hypertension therapy for cerebral vasospasm after subarachnoid hemorrageActa Neurochir19901031826
- PickardJDReadDHLovickAHAuerLMPreoperative assessment of cerebrovascular reactivity following subarachnoid hemorrhage – clinical correlationsTiming of aneurysm surgery1985NewYorkPublisher4751 Walter de Gruyter Berlin
- ReadDHLovickAHPickardJDA pre-operative test of cerebrovascular autoregulation following subarachnoid haemorrhageBrit J Anaesth198355918
- SanoKAsanoTTamuraATamuraSano AsanoAcute Ischemic Neurological DeficitsAcute Aneurysm Surgery. Pathophysiology and Management1987New YorkPublisher426 Springer-Verlag Wien
- SavelandHBrandtLWhich are the major determinants for outcome in aneurysmal subarachnoid hemorrhage? A prospective total management study from a strictly unselected seriesActa Neurol Scand199490245507839809
- ShapiroSManagement of subarachnoid hemorrhage patients who presented with respiratory arrest resuscitated with bystander CPRStroke199627178028841329
- ShimodaMOdaSTsuganeRIntracranial complications of hypervolemic therapy in patients with delayed ischaemic deficits attributed to vasospasmJ Neurosurg19937842398433144
- SmithERCarterBSOgilvyCSProposed use of prophylactic decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage patients presenting with associated large sylvian hematomasNeurosurgery2002511172412182408
- SolomonRAOnestiSTKlebanoffLRelationship between the timing of aneurysm surgery and the development of delayed cerebral ischemiaJ Neurosurg19917556612045919
- SpetzgerUGilbaschJMResults of early aneurysm surgery in poor grade patientsNeurol Res19941627307913525
- StocchetiNChieregatoADe MarchiMHigh cerebral perfusion pressure improves low values of local brain tissue O2 tension (PtiO2) in focal lesionsActa Neurochir1998711625
- TenjinHHirakawaKMizukawaNDysautoregulation in patients with ruptured aneurysms: cerebral blood flow measurements obtained during surgery by a temperature-controlled thermoelectrical methodNeurosurgery19882370593216967
- UngersboeckKBoecher-SchwarzHUlrichPAneurysm surgery of patients in poor grade condition. Indications and experienceNeurol Res1994163147913527
- UnterbergAKieningKSchneiderG-HMonitoring of cerebral oxygenation in severe head injury – jugular venous oxygen saturation vs brain tissue PO2 and near infrared spectroscopyJ Neurotrauma199512405
- ValadkaABHlatkyRFuruyaYBrain tissue PO2: correlation with cerebral blood flowActa Neurochir200281299301
- WeirRUMarcellusMLDoHMAneurysmal subarachnoid hemorrhage in patients with Hunt and Hess grade 4 or 5: treatment using the Guglielmi detachable coil systemAJNR Am J Neuroradiol2003245859012695185
- ZubkovYNTreatment of patients with intracranial arterial aneurysms in the hemorrhagic periodNeurol Res199416687913535