Abstract
Microvascular complications characterized by retinopathy, nephropathy, and neuropathy are highly prevalent among diabetics. Glycemic control has long been the mainstay for preventing progression of these complications; however, such control is not easily achieved. Currently, alternative adjunctive approaches to treating and preventing microvascular damage are being undertaken by targeting the molecular pathogenesis of diabetic complications. This review summarizes the specific pathogenic mechanisms of microvascular complications for which clinical therapies have been developed, including the polyol pathway, advanced glycation end products, protein kinase c, vascular epithelium growth factor, and the superoxide pathway. The review further focuses on therapies for these targets that are currently available or are undergoing late-stage clinical trials.
Introduction
Diabetes is estimated to have affected 171 million people worldwide in 2006 and is projected to affect 366 million by 2030 (CitationThe World Health Organization 2007). It was the 5th leading cause of death in 2000, and diabetic microvascular complications account for a significant portion of the morbidity and mortality (CitationRoglic et al 2005). Diabetic retinopathy, nephropathy, and neuropathy are the leading causes of blindness, end-stage renal disease, and amputations in the US (CitationAmerican Diabetes Association 2007). Although type 1 and type 2 diabetes originate from different pathogenetic causes, there is a significant association between hyperglycemia and the diabetic microvascular complications in both type 1 and type 2 diabetes (CitationThe Diabetes Control and Complications Trial Research Group 1993; CitationUK Prospective Diabetes Study (UKPDS) Group 1998). In the past decade, large, long-term prospective trials involving treatment and control of hyperglycemia in type 1 and 2 diabetics have shown microvascular related morbidity can be significantly reduced but not entirely prevented through long-term glycemic and blood pressure control (CitationThe Diabetes Control and Complications Trial Research Group 1993; CitationUK Prospective Diabetes Study (UKPDS) Group. 1998). Newer strategies to ameliorate or prevent microvascular complications involve a molecular understanding of diabetic complications.
Overview
The pathologic mechanisms underlying the susceptibility of retinal capillary endothelial, renal glomeruli mesangial, and neural tissues to chronic hyperglycemia has been studied over the past century. With the 1966 discovery of the polyol pathway, a number of pathogenic mechanisms have been described including advanced glycation end products (AGEPs) , protein kinase C (PKC-β), the hexosamine pathway, and the unifying mechanism of superoxide production (CitationBrownlee 2005). The activity of inflammatory and angionenic regulating cytokines, such as nuclear factor (NF)-κB, tumor growth factor (TGF)-β, vascular endothelial growth factor (VEGF), connective tissue growth factor, and platelet derived growth factor, within the these mechanisms are well described. Ultimately, reactive oxygen species (ROS) are the end products of these various pathways.
With increasing understanding of diabetic pathophysiology, the number of potential therapeutic targets for diabetic complications has multiplied as well. Within these targets lies the possibility of independently improving microvascular outcomes in addition to enhancing glycemic and blood pressure control. While all aspects of diabetic pathophysiology are being targeted in experimental studies, the polyol pathway, AGEs, PKC-β, VEGF, and ROS have promising therapies developed against them that are now being clinically tested or are already approved for use. The following is a review of the clinical data supporting these emerging therapies.
Aldose reductase inhibitors and the polyol pathway
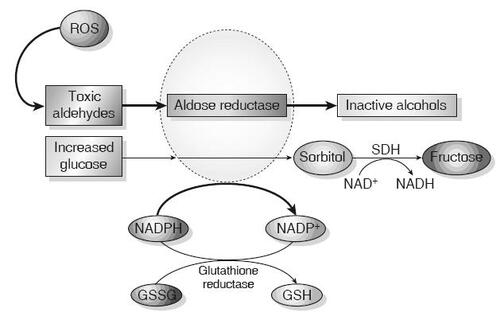
The polyol pathway reduces toxic aldehydes generated by ROS to inactive alcohols () (CitationBrownlee 2001; CitationSheetz and King 2002). Aldose reductase (AR), via the consumption of NADPH, is responsible for the initial and rate-limiting step in the process. Glucose can be reduced to sorbitol, and eventually fructose, through this pathway, but AR has a low affinity for glucose at normal concentrations. Elevated intracellular glucose can increase AR activity, resulting in significantly decreased NADPH. NADPH is also required for glutathione reductase activity, which reduces glutathione (GSH)–a major mechanism for reducing intracellular oxidative stress (CitationLee and Chung 1999). Decreased NADPH and resulting decreased GSH reductase activity ultimately increases oxidative stress and activates pathways that increase cellular damage.
Figure 1 Aldose reductase and the polyol pathway. Aldose reductase reduces aldehydes generated by ROS to inactive alcohols, and glucose is converted to sorbitol, using NADPH as a co-factor. For cells in which aldose reductase activity is sufficient to deplete reduced GSH, oxidative stress is augmented. Sorbitol dehydrogenase (SDH) oxidizes sorbitol to fructose using NAD+ as a co-factor (CitationBrownlee 2001) (Adapted by permission from Macmillan Publishers Ltd: Nature, Vol. 414, 2001).
Aldose reductase inhibition (ARI) is ostensibly an ideal target for reducing the deleterious effects associated with polyol pathway activation. However, clinical trials with ARIs have shown lack of efficacy or adverse effects. In the 1980’s, sorbinil became the first ARI to undergo clinical trials after promising preclinical results. Results from several studies on neuropathy were mixed, but the majority suggested a lack of significant effects (CitationJaspan et al 1986; CitationMartyn et al 1987; CitationGuy et al 1988; CitationSorbinil Retinopathy Trial Research Group 1993). Sorbinil was evaluated for treating retinopathy and nephropathy in the early 1990’s but again showed a lack of efficacy (CitationSorbinil Retinopathy Trial Research Group 1990; CitationSorbinil Retinopathy Trial Research Group 1993). Hypersensitivity reactions, occurring at increased doses, further limited the agent’s effectiveness.
Subsequent clinical evaluation of ARIs such as tolrestat or lidorestat were halted due to toxicities before their efficacy could be definitively evaluated (CitationFoppiano and Lombardo 1997). Others such as ponalrestat and zopolrestat were ineffective despite having more favorable side effect profiles (CitationSundkvist et al 1992). Zenarestat improved nerve conduction velocity and nerve morphology in a rigorous, year-long randomized, placebo-controlled trial (CitationGreene et al 1999). However, further Phase 3 studies were eventually halted due to significant creatinine elevations in study participants (CitationBrown et al 2004).
Epalrestat was the first successful ARI to be developed and was approved for use in Japan in 1992 for treatment of diabetic peripheral neuropathy. Randomized, double blinded, placebo-controlled trials of 12 and 24 weeks in diabetics with mild neuropathy suggested improved nerve conduction, vibration thresholds, and symptoms (CitationGoto et al 1995: CitationUchida et al 1995). Improvement in peripheral neuropathy and minimal side effects were observed in a prospective observational study of more than 5,000 diabetics treated over 3–12 months (CitationHotta et al 1996). Smaller long-term studies have also suggested the utility of epalrestat in ameliorating autonomic neuropathies related to cardiac function and gastric and esophageal motility (CitationIkeda et al 1999; CitationNakayama et al 2001; CitationOkamoto et al 2003; CitationKinekawa et al 2005). Epalrestat’s effects on nephropathy were evaluated via a placebo-controlled, randomized trial over 5 years. Thirty-five type 2 diabetics with baseline microalbuminuria were studied, and microalbuminuria was found to be unchanged in the treatment group, but significantly increased in the placebo arm suggesting a benefit in treating nephropathy (CitationIso et al 2001). Recently, a 3-year randomized controlled trial involving 594 diabetics with mild to moderate neuropathy again showed that epalrestat was effective in improving median nerve conduction, vibration perception threshold, and symptoms without severe adverse effects (CitationHotta et al 2006). The study also showed a statistical difference in improvement and progression of retinopathy, although not microalbuminuria, between control and treatment groups. Cardiac autonomic effects were positive but not significant.
Two new ARIs, fidarestat and ranirestat, have more recently been evaluated in safety and efficacy studies in a randomized, double-blinded, placebo-controlled trial in the US and Japan in which 279 diabetics were studied (CitationHotta et al 2001). Improvement in peripheral neuropathy, as evaluated by electrophysiological nerve studies and symptom reports, was statistically significant compared to placebo with no difference in adverse effects between study and placebo groups. No data were reported regarding retinopathy or nephropathy. In 2004, Phase 2 trials were halted despite the positive results due to corporate restructuring of the trial sponsor. Whether evaluation of fidarestat will be resumed is unclear.
Ranirestat effectively penetrates peripheral nerves and has shown encouraging effects on peripheral neuropathy at both 5 mg and 20 mg doses in a 12-week, double-blinded, placebo-controlled trial (CitationBril and Buchanan 2004). A 48-week extension trial involving 86 of the participants continued the evaluation of nerve conduction studies, vibration perception thresholds, and neuropathic symptoms as assessed by the Toronto clinical neuropathy score (CitationBril and Buchanan 2006). The placebo arm participants were given low-dose ranirestat (5 mg) and the other treatment groups continued with their prior doses of 5 and 20 mg. Further declines in nerve conduction were prevented and a statistically significant improvement was observed in nerve conduction and symptoms by the study’s end. Although the extension was not sufficiently powered or placebo controlled, the results are encouraging and must be validated in future trials. Phase 3 trials for ranirestat were completed in the US in December 2006, and publication of results are currently pending.
Inhibitors of AGEPs
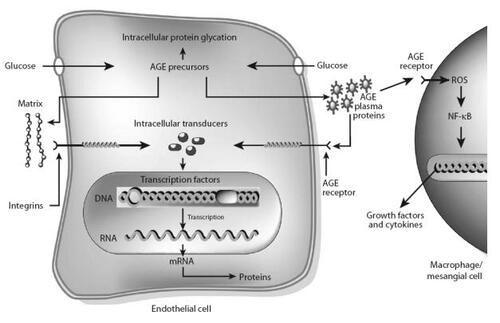
AGEPs are a heterogeneous group of modified proteins, lipids, and nucleic acids implicated in the aging process and diabetes. In intracellular hyperglycemia, these products are formed primarily through nonenzymatic reactions (Maillard reactions) between amino groups and glucose or highly reactive glucose derivatives known as dicarbonyls () (CitationBrownlee 2001). Hyperglycemia may also drive AGEP formation through polyol pathway-derived intermediates and oxidative stress (CitationHamada et al 1996). AGEPs alter intracellular and extracellular proteins and their functions (CitationBrownlee 2001; CitationGoldin et al 2006; CitationHuebschmann et al 2006). Receptors for AGEPs (RAGE) may also bind AGEPs causing the NF-κB-mediated activation of various cytokines, pro-coagualtory and pro-inflammatory factors, and increased vascular permeability (CitationBrownlee 2001; CitationGoldin et al 2006; CitationHuebschmann et al 2006).
Figure 2 Mechanisms by which intracellular production of advanced glycation end-product (AGE) precursors damages vascular cells. Covalent modification of intracellular proteins by dicarbonyl AGE precursors alters several cellular functions. Modification of extracellular matrix proteins causes abnormal interactions with other matrix proteins and with integrins. Modification of plasma proteins by AGE precursors creates ligands that bind to AGE receptors, inducing changes in gene expression in endothelial cells, mesangial cells and macrophages (CitationBrownlee 2001) (Adapted by permission from Macmillan Publishers Ltd: Nature, Vol. 414, 2001).
Studies in diabetic populations show AGEPs correlate with the development and severity of retinopathy, neuropathy, and nephropathy as well as macrovascular complications (CitationMonnier et al 2005). RAGE also exists in a soluble state (sRAGE) within the circulation and is thought to clear AGEP from the circulation, thereby preventing the binding of cellular targets (CitationKatakami et al 2005). Type 2 diabetics have lower sRAGE levels compared to nondiabetics, suggesting a downregulation of sRAGE in hyperglycemia. Strategies to prevent AGEP effects, aside from glycemic control, include decreasing exogenous AGEP intake, inhibiting AGEP formation, disrupting AGEP cross-links, enhancing removal of AGEPs, and reducing oxidative stress.
Approximately 10% of exogenous AGEPs and their precursors consumed through diet are absorbed through the intestinal epithelium (CitationGoldberg et al 2004). Foods with high AGEP content include meat, meat substitutes, high-fat foods, and foods cooked for prolonged periods or cooked at high temperatures (broiling, roasting, or frying). Conversely, fruits, vegetables, complex carbohydrates, lower temperature cooking methods, shorter cooking duration, or methods employing higher water contents (ie, boiling) are associated with lower AGEP levels. Clinical studies in human diabetics are limited but do show increased circulating AGEP levels and markers of inflammation associated with increased dietary intake (CitationGoldberg et al 2004; CitationUribarri et al 2005). Exogenous AGEPs are also increased through tobacco smoking – smoking cessation reduces exogenous AGEPs (CitationCerami et al 1997).
Aminoguanidine, the first targeted AGEP therapy, is a hydrazine derivative that prevents AGEP formation by binding reactive carbonyl intermediates (CitationThornalley 2003). Aminoguanidine’s effects on nephropathy and retinopathy in 690 type 1 diabetics were evaluated in a randomized, double-blinded, placebo-controlled trial. Progression of glomerular filtration rate (GFR) decline, proteinuria, and retinopathy was significantly improved, although 3 patients given high-dose aminoguanidine developed glomerulonephritis (CitationBolton et al 2004). A similar follow-up study was halted due to safety concerns and an apparent lack of efficacy (CitationFreedman et al 1999).
A newer agent, alagebrium chloride (ALT-711) cleaves AGEP and protein cross-links thereby facilitating AGEP clearance. Two clinical trials with mixed diabetic and non-diabetic populations with atherosclerosis and heart failure reveals that Alt-711 can improve vascular and left ventricular compliance with adverse effects similar to placebo (CitationKass et al 2001; CitationLittle et al 2005). In animal studies, ALT-711 was beneficial in treating diabetic renal complications (CitationForbes et al 2003); however, no clinical studies to date have evaluated its effects on the microvascular system.
The effects of two B vitamins on AGEPs have also been clinically studied in humans. The highly bioavailable thiamine derivative, benfotiamine, was studied in 13 diabetics who consumed an AGEP-rich meal (CitationStirban et al 2006). Postprandial AGEP levels and markers of endothelial dysfunction were significantly decreased after bentofamine was administered for 3 days. Benfotiamine has also been shown to improve the symptoms of diabetic polyneuropathy in 2 studies in which AGEPs were not measured (CitationWinkler et al 1999; CitationHaupt et al 2005). A combination of bentofamine and pyroxidine (as well as vitamin B12) have been studied in both diabetic and alcoholic neuropathy and both improved symptoms and nerve conduction velocity, although AGEP effects were not specifically evaluated (CitationStracke et al 1996; CitationWoelk et al 1998). Pyridoxamine is proposed to improve AGEP-related complications through dicarbonyl clearance, prevention of oxidative damage, and prevention of AGEP formation (CitationVoziyan and Hudson 2005). A Phase II randomized, double-blinded, placebo-controlled trial with 128 diabetics with nephropathy, most of whom were on ACE or ARB therapy, revealed significantly decreased rates of creatinine elevations and albuminuria that was especially marked among those with more advance disease (CitationWilliams et al 2003). Adverse events did not include neurotoxicities and were similar to placebo (CitationWilliams 2006).
In additional studies, AGEPs have been evaluated in diabetes, hypertension, and lipid modulation. Epalrestat has been shown to reduce serum AGEPs in diabetics after 2–3 months of use (CitationNakamura et al 2003; CitationHamada et al 2000). AGEP modulation by metformin was compared to insulin, sulfonyureas, or insulin plus sulfonyureas in type 2 diabetics with similar glycemic control and no renal impairment (CitationBeisswenger et al 1999). Metformin was superior in reducing reactive dicarbonyl precursors compared to insulin or sulfonyureas in any combination independent of glycemic control. In type 1 diabetics, perindopril has been shown to increase soluble RAGE, an endogenous RAGE clearance mechanism, and decrease low molecular weight AGEPs over 2 years when compared to nifedipine (CitationForbes et al 2005). In contrast, the ARB, irbesartan, did not affect AGEPs in a separate clinical evaluation (CitationPersson et al 2006). Simvastatin treatment and adherence to an American Heart Association diet for 4 months also has been shown to decrease cellular RAGE in carotid plaques of type 2 diabetics independent of glycemic control versus dietary modifications alone (CitationCuccurullo et al 2006). None of these studies specifically evaluated microvascular indices and further clinical trials are needed to confirm potential outcome benefits.
Diacylglycerol PKC inhibition
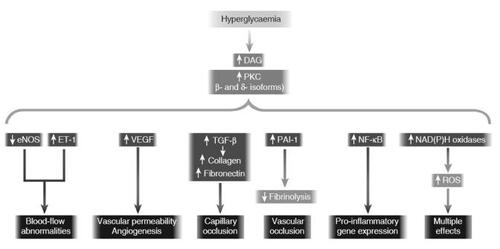
The PKC family consists of a group of 12 seronine/threonine kinases involved in intracellular signaling related to a variety of vascular, cardiac, immunologic, and other systemic functions (CitationMellor and Parker 1998; CitationSheetz and King 2002). Diacylglycerol (DAG) is an upstream activator in the majority of PKC isoforms (CitationKoya and King 1998; CitationInoguchi et al 1992). De novo DAG formation increases with elevated intracellular glucose with a resultant increase of primarily PKC-β1/2 and PKC-δ isoform activity () (CitationKoya and King 1998). PKC may also be activated by growth factors, and hyperglycemia-induced superoxide and AGE formation (CitationKoya and King 1998; CitationSheetz and King 2002).
Figure 3 Consequences of hyperglycemia-induced activation of protein kinase C (PKC). Hyperglycemia increases diacylglycerol (DAG) content, which activates PKC, primarily the b- and d-isoforms. Activation of PKC has a number of pathogenic consequences by affecting expression of endothelial nitric oxide synthetase (eNOS), endothelin-1 (ET-1), VEGF, TGF-β, and plasminogen activator inhibitor-1 (PAI-1), and by activating NF-κB and NAD(P)H oxidases (CitationBrownlee 2001) (Adapted by permission from Macmillan Publishers Ltd: Nature, Vol. 414, 2001).
PKC-β1 and 2 are chiefly responsible the deleterious effects on retinal, neural, and renal tissues (CitationInoguchi et al 1992; CitationShiba et al 1993; CitationCraven et al 1990). These isoforms impair retinal and renal blood flow, and increase capillary leakage (CitationFeke et al 1994). PKC-induced increased extracellular matrix production and upregulation of various inflammatory cytokines further damage the macro and microvascular systems (CitationCraven et al 1997).
PKC412, while not exclusively a PKC inhibitor, was the first PKC inhibitory agent to undergo clinical evaluation in a randomized, double-blinded, placebo-controlled trial (CitationCampochiaro et al 2004). While effective in treating diabetic macular edema, further studies of PCK412 were abandoned due to hepatotoxicity. Ruboxistaurin is a selective PKC-β inhibitor that has been shown to improve retinal circulation parameters and decrease diabetic macular edema retinal leakage without significant adverse effects (CitationStrom et al 2005; CitationAiello et al 2006a). In clinical trials to control progression of retinopathy, ruboxistaurin’s results are mixed. In a randomized, double blinded placebo-controlled study (PKC-DRS) of 192 diabetics with moderate to severe nonproliferative retinopathy treated with various doses of ruboxistaurin, retinopathic progression did not decrease over a period of up to 4 years, although moderate vision loss was significantly decreased in the high-dose (32 mg) treatment group (CitationThe PKC-DRS Study Group 2005). In a subgroup with macular edema, additional vision loss was prevented in the high-dose treatment group versus placebo, and adverse effects were similar to placebo. In the follow up study (PKC-DRS 2), 685 diabetics with macular edema for 36 months were assessed for the prevention of sustained vision loss as the primary end point. As in the prior studies, ruboxistaurin (32 mg) prevented progression of sustained moderate visual loss with a relative risk reduction of 45% versus placebo (CitationAiello et al 2006b). Also, significant prevention of macular edema progression and a decreased need for initial photocoagulation was observed in the treatment group; although, retinopathic progression was not affected.
A recent randomized, double-blinded, placebo-controlled trial of 123 diabetics with albuminuria who were taking ACE or ARB therapy indicated that ruboxistaurin reduces albuminuria:creatinine ratios versus placebo (CitationTuttle et al 2005). GFR was also preserved relative to baseline in the treatment group, but this study was not of sufficient statistical power to compare GFR trends between treatment and placebo groups. The effect of ruboxistaurin on diabetic peripheral neuropathy (DPN) has also been evaluated in a 1-year randomized, double-blinded, placebo-controlled trial of 205 diabetics (CitationVinik et al 2005). While participants with symptomatic DPN showed significant improvement of symptoms, only a subgroup with less severe baseline features showed significant improvement of their vibration detection threshold and symptoms. Ruboxustaurin is currently pending FDA approval for the treatment of diabetic macular edema.
VEGF inhibitors
VEGF is a glycoprotein whose production is increased in hyperglycemia, primarily through the PKC pathway. VEGF mediates its effects on the retina through the receptor tyrosine kinases VEGFR-1 and VEGFR-2 (CitationShen et al 1993; CitationFerrara 2004). In turn, angiogenesis is stimulated and capillary permeability and leakage are increased (CitationShen et al 1993; CitationDvorak et al 1995; CitationFerrara 2004). Vitreous VEGF levels reflect the severity of neovascularization in diabetic retinopathy and decline with photocoagulation (CitationAiello et al 1994; CitationFunatsu et al 2006). Experimental data suggests there are similar elevations in renal tissue although the pathogenesis is less well established (CitationHohenstein et al 2006).
Systemic therapies against VEGF are impractical as VEGF is vital in processes such as angiogenesis in the myocardium and wound healing (Citationvan Wijngaarden et al 2005). Intraocular injections of VEGF inhibitors represent a method of targeted therapy that would avoid the adverse systemic effects. Three drugs are currently being studied in clinical settings: pegaptanib, ranizumab, and bevacizumab.
Pegaptanib sodium is an anti-VEGF aptamer which binds VEGF and prevents it from interacting with its receptors (CitationGragoudas et al 2004). Two concurrent, prospective, double-blinded, randomized, sham-controlled trials in patients with non-diabetic age related macular edema suggest that have intraocular injections at 6-week intervals over the course of 48 weeks slow visual loss (CitationGragoudas et al 2004). In diabetics, a similarly designed study over 36 weeks showed that pegaptanib improved visual acuity outcomes, preserved central retinal thickness, and required less photocoagulation therapy in 172 participants with baseline diabetic macular edema (CitationCunningham Jr et al 2005). Adverse events were similar among treatment and control groups, although 8% of all participants experienced a severe adverse event, and 16 of the participants also had baseline retinal neovascularization and were subsequently followed for neovascular progression in a separate study (CitationAdamis et al 2006). Pegaptanib led to regression in 62% of treated eyes versus 0% of the control group.
Ranizumab and bevacizumab are recombinant humanized monoclonal antibody fragments and full-length antibodies, respectively (CitationSteinbrook 2006). Both agents are derived from the same mouse monoclonal antibody precursor and show high affinity for VEGF, neutralizing its effects by binding to VEGF and preventing VEGFR interaction. Bevacizumab was originally designed for intravenous injection as a chemotherapeutic against metastatic colorectal cancer, but it has been adapted for off-label use as an intraocular injection similar to ranizumab (CitationHurwitz et al 2004; Rosenfeld 2006a). Several case series and studies have also suggested bevacizumab to be safe and effective in decreasing retinal neovascularization and macular edema in diabetics (CitationAvery et al 2006; CitationHaritoglou et al 2006; CitationJorge et al 2006; CitationMason et al 2006; CitationOshima et al 2006; CitationSpaide and Fisher 2006). Two large prospective randomized studies have recently been completed in which ranizumab was assessed for treatment of nondiabetic, age-related macular edema (CitationBrown and Kaiser 2006; Rosenfeld et al 2006b). The studies show ranizumab to be superior in improving visual acuity and regression of neovascularization with a low incidence of serious adverse events over 1 year versus either sham injection or verteporfin photodynamic therapy. In a 2-year pilot study in 10 diabetics with clinically significant macular edema, similar findings of improved visual acuity and central retinal thickness were observed, and safety was not a concern (CitationChun et al 2006).
The new VEGF inhibitors show promise in targeting diabetic retinopathy, but are cost-prohibitive (CitationSteinbrook 2006), and no trials have indicated that one is superior to the others. Additionally, systemic effects have been noted in the intraocular preparations (CitationJorge et al 2006). Bevacizumab, in particular, has been investigated in this regard due to its longer half life (CitationSteinbrook 2006). Finally, the question of whether prolonged inhibition of VEGF may actually further retinal deterioration remains to be answered (CitationCsaky 2003).
Antioxidant therapy and ROS
ROS are end products of pathogenic pathways as well as the hexosamine pathway and they are the cause of diabetic microvascular injury. ROS have long been targeted in therapeutic trials for diabetic microvascular complications. A detailed review of antioxidants is beyond the scope of this paper, but clinical studies of diabetics who were supplemented with traditional antioxidants (vitamins C and E) indicated that markers of oxidative stress were alleviated microvascular complications were not prevented or improved (CitationLonn et al 2002; CitationScott and King 2004; CitationLiu et al 2006).
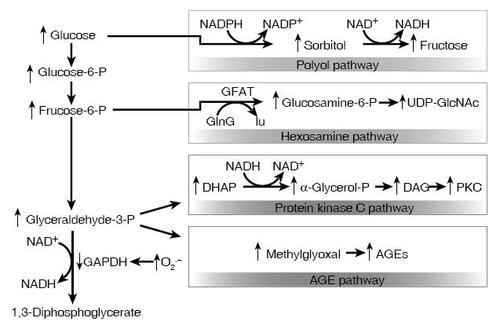
Our understanding of the roles of oxygen free radicals in diabetic complications has recently evolved with the description of the super oxide pathway (CitationBrownlee 2001). In hyperglycemia, experimental endothelial cells have increased flux of glucose through glycolysis, pyruvate decarboxylation, and the citric acid cycle resulting in mitochondrial electron transport chain overload (CitationNishikawa et al 2000). In turn, overloaded mitochondria produce excessive superoxide anions which ultimately lead to decreases in nitric oxide, DNA damage, AGE formation, and activation of the polyol, PKC, and hexosamine pathways as well as activation of poly (ADP-ribose) polymerase () (CitationDu et al 2000; CitationNishikawa et al 2000; CitationBrownlee 2001).
Figure 4 Potential mechanism by which hyperglycemia-induced mitochondrial superoxide overproduction activates four pathways of hyperglycemic damage. Excess superoxide partially inhibits the glycolytic enzyme GAPDH, thereby diverting upstream metabolites from glycolysis into pathways of glucose overutilization. This results in increased flux of dihydroxyacetone phosphate (DHAP) to DAG, an activator of PKC, and of triose phosphates to methylglyoxal, the main intracellular AGE precursor. Increased flux of fructose-6-phosphate to UDP-N-acetylglucosamine increases modification of proteins by O-linked N-acetylglucosamine (GlcNAc) and increased glucose flux through the polyol pathway consumes NADPH and depletes GSH (CitationBrownlee 2001) (Adapted by permission from Macmillan Publishers Ltd: Nature, Vol. 414, 2001).
The realization that ROS both contribute to induction of major pathways of diabetic complications and comprise the pathway’s end products may explain why traditional antioxidants have failed. Vitamin E and others antioxidants act primarily to non-enzymatically scavenge certain end-product ROS thereby limiting their effects to only a portion of the damaging end-product (CitationDu et al 2000). Currently used agents for diabetic microvascular control, including thiazolinediones, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and statins are believed to derive some of their benefit from modulating superoxides (CitationCeriello 2003). To improve the effect of antioxidant therapy, compounds are being studied that specifically act against superoxide and prevent induction of the various pathogenic mechanisms.
α-lipoic acid is one such compound that has received the most attention in clinical trials, which indicated that it can reduce markers of oxidation in poorly controlled diabetics and in patients with metabolic syndrome (CitationBorcea et al 1999; CitationSola et al 2005). A meta analysis of 4 randomized, double-blinded, placebo-controlled, 3-week trials of 1,258 diabetics showed that 600 mg of α-lipoic acid significantly improved measures of DPN (CitationZiegler et al 2004). More recently, a randomized, double-blinded, placebo-controlled trial of 182 type 1 and 2 diabetics were treated with α-lipoic acid (600–1800 mg) over 5 weeks was conducted to evaluated the effects on DPN (CitationZiegler et al 2006). All treated groups had significantly improved pain and neuropathy as assessed by a total symptom score (TSS), a neuropathy impairment score, and a neuropathy and symptoms and change score (NCS). Nerve conduction studies and symptoms of numbness and parathesia did not significantly improve and dose-related nausea, vomiting, and vertigo were noted with treatment. No clinical data exist regarding treatment of diabetic retinopathy, although at least one study assessed its effects on nephropathy. A prospective non-randomized, open, placebo-controlled study of 84 type 1 and 2 diabetics was conducted to evaluate the effect of α-lipoic acid over 18 months (CitationMorcos et al 2001). Urinary albuminuria was increased in the placebo group but unchanged in the treatment group.
Conclusion
Greater understanding of diabetic pathophysiology is yielding promising results by guiding the development of these new targeted therapies. Agents such as epalrestat, ruboxistaurin, ranizumab, and α-lipoic acid have been well studied and are now or soon to be available in certain countries. The newer ARI and AGE therapies require further large-scale trials to verify their efficacy, but the initial results are encouraging. While not covered here, other notable new therapies that do not necessarily target specific diabetic pathophysiologic mechanisms such as glycosaminoglycan sulodexide for nephropathy, intraocular steroids for retinopathy, or monochromatic near-infrared treatment for neuropathy are also showing benefit (CitationSolini et al 1997; CitationGambaro et al 2002; CitationSimpson et al 2004; CitationSutter et al 2004; CitationAchour et al 2005; CitationClifft et al 2005; CitationGillies et al 2006). These new therapies are likely to represent a significant change in the management of diabetic retinopathy, nephropathy, and neuropathy beyond glycemic and blood pressure control.
References
- AchourAKacemMOne year course of oral sulodexide in the management of diabetic nephropathyJ Nephrol2005185687416299683
- AdamisAPAltaweelMChanges in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individualsOphthalmology200611323816343627
- AielloLPVascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disordersN Engl J Med1994331148077526212
- AielloLPClermontAInhibition of PKC beta by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patientsInvest Ophthalmol Vis Sci200647869216384948
- AielloLPDavisMDEffect of ruboxistaurin on visual loss in patients with diabetic retinopathyOphthalmology200611322213016989901
- American Diabetes AssociationAll about diabetes [online]2007 Accessed 25 February 2007. URL: http://www.diabetes.org/aboutdiabetes.jsp
- AveryRLPearlmanJIntravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathyOphthalmology2006113e11517011951
- BeisswengerPJHowellSKMetformin reduces systemic methylglyoxal levels in type 2 diabetesDiabetes1999481982029892243
- BoltonWKCattranDCRandomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathyAm J Nephrol200424324014685005
- BorceaVNourooz-ZadehJAlpha-Lipoic acid decreases oxidative stress even in diabetic patients with poor glycemic control and albuminuriaFree Radic Biol Med199926149550010401614
- BrilVBuchananRAAldose reductase inhibition by AS-3201 in sural nerve from patients with diabetic sensorimotor polyneuropathyDiabetes Care20042723697515451902
- BrilVBuchananRALong-term effects of ranirestat (AS-3201) on peripheral nerve function in patients with diabetic sensorimotor polyneuropathyDiabetes Care200629687216373898
- BrownMJBirdSJNatural progression of diabetic peripheral neuropathy in the Zenarestat study populationDiabetes Care2004271153915111537
- BrownDMKaiserPRanibizumab versus verteporfin for neovascular age-related macular degenerationN Engl J Med200635514324417021319
- BrownleeMBiochemistry and molecular cell biology of diabetic complicationsNature20014148132011742414
- BrownleeMThe pathobiology of diabetic complications: a unifying mechanismDiabetes20055416152515919781
- CampochiaroPAC99-PKC412-003 Study GroupReduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412Invest Ophthalmol Vis Sci2004459223114985312
- CeramiCFoundsHTobacco smoke is a source of toxic reactive glycation productsProc Natl Acad Sci U S A19979413915209391127
- CerielloANew insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapyDiabetes Care20032615899612716823
- ChunDWHeierJSA pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edemaOphthalmology200611317061217011952
- ClifftJKKasserRJThe effect of monochromatic infrared energy on sensation in patients with diabetic peripheral neuropathy: a double-blind, placebo-controlled studyDiabetes Care200528289690016306551
- CravenPADavidsonCMIncrease in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipidsDiabetes199039667742347431
- CravenPAStuderRKNitric oxide inhibition of transforming growth factor-beta and collagen synthesis in mesangial cellsDiabetes199746671819075810
- CsakyKAnti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration: promises and pitfallsOphthalmology20031108798112750083
- CuccurulloCLezziASuppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetesArterioscler Thromb Vasc Biol20062627162317038636
- CunninghamETJrA phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edemaOphthalmology200511217475716154196
- The Diabetes Control and Complications Trial Research GroupThe effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitusN Engl J Med1993329977868366922
- DuXLEdelsteinDHyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylationProc Natl Acad Sci USA20009712222611050244
- DvorakHFBrownLFVascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesisAm J Pathol19951461029397538264
- FekeGTBuzneySMRetinal circulatory abnormalities in type 1 diabetesInvest Ophthalmol Vis Sci1994352968758206714
- FerraraNVascular endothelial growth factor: basic science and clinical progressEndocr Rev20042558161115294883
- FoppianoMLombardoGWorldwide pharmacovigilance systems and tolrestat withdrawalLancet19973493994009033472
- ForbesJMThallasVThe breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetesFASEB J2003171762412958202
- ForbesJMThorpeSRModulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathyJ Am Soc Nephrol20051623637215930093
- FreedmanBIWuerthJPDesign and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II)Control Clin Trials19992049351010503809
- FunatsuHYamashitaHVitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor are related to diabetic macular edemaOphthalmology200611329430116406543
- GambaroGKinalskaIOral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the Di.N.A.S. randomized trialJ Am Soc Nephrol20021316152512039991
- GilliesMCSutterFKIntravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trialOphthalmology20061131533816828501
- GoldbergTCaiWAdvanced glycoxidation end products in commonly consumed foodsJ Am Diet Assoc200410412879115281050
- GoldinABeckmanJAAdvanced glycation end products: sparking the development of diabetic vascular injuryCirculation200611459760516894049
- GotoYHottaNEffects of an aldose reductase inhibitor, epalrestat, on diabetic neuropathy. Clinical benefit and indication for the drug assessed from the results of a placebo-controlled double-blind studyBiomed Pharmacother199549269777579007
- GragoudasESAdamisAPPegaptanib for neovascular age-related macular degenerationN Engl J Med200435128051615625332
- GreeneDABrownMBEffect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Zenarestat Study GroupNeurology1999535809110449124
- GuyRJGilbeySGDiabetic neuropathy in the upper limb and the effect of twelve months sorbinil treatmentDiabetologia19884214203290018
- HamadaYArakiNRapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathwayBiochem Biophys Res Commun1996228539438920948
- HamadaYNakamuraJEpalrestat, an aldose reductase inhibitor, reduces the levels of Nepsilon-(carboxymethyl)lysine protein adducts and their precursors in erythrocytes from diabetic patientsDiabetes Care20002315394411023149
- HaritoglouCKookDIntravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edemaRetina200626999100517151486
- HauptELedermannHBenfotiamine in the treatment of diabetic polyneuropathy - a three-week randomized, controlled pilot study (BEDIP study)Int J Clin Pharmacol Ther20054371715726875
- HohensteinBHausknechtBLocal VEGF activity but not VEGF expression is tightly regulated during diabetic nephropathy in manKidney Int20066916546116541023
- HottaNSakamotoNClinical investigation of epalrestat, an aldose reductase inhibitor, on diabetic neuropathy in Japan: multicenter study. Diabetic Neuropathy Study Group in JapanJ Diabetes Complications199610168728807467
- HottaNToyotaTClinical efficacy of fidarestat, a novel aldose reductase inhibitor, for diabetic peripheral neuropathy: a 52-week multicenter placebo-controlled double-blind parallel group studyDiabetes Care20012417768211574441
- HottaNAkanumaYLong-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative Aldose Reductase Inhibitor-Diabetes Complications TrialDiabetes Care20062915384416801576
- HuebschmannAGRegensteinerJGDiabetes and advanced glycoxidation end productsDiabetes Care20062914203216732039
- HurwitzHFehrenbacherLBevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancerN Engl J Med200435023354215175435
- IkedaTIwataKLong-term effect of epalrestat on cardiac autonomic neuropathy in subjects with non-insulin dependent diabetes mellitusDiabetes Res Clin Pract199943193810369429
- InoguchiTBattanRPreferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantationProc Natl Acad Sci USA19928911059631438315
- IsoKTadaHLong-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in Type 2 diabetic patientsDiabetes Complications2001152414
- JaspanJBTowleVLClinical studies with an aldose reductase inhibitor in the autonomic and somatic neuropathies of diabetesMetabolism19863583923083212
- JorgeRCostaRAIntravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study)Retina20062610061317151487
- KassDAShapiroEPImproved arterial compliance by a novel advanced glycation end-product crosslink breakerCirculation200110414647011571237
- KatakamiNMatsuhisaMDecreased endogenous secretory advanced glycation end product receptor in type 1 diabetic patients: its possible association with diabetic vascular complicationsDiabetes Care20052827162116249545
- KinekawaFKuboFEffect of an aldose reductase inhibitor on esophageal dysfunction in diabetic patientsHepatogastroenterology200552471415816460
- KoyaDKingGLProtein kinase C activation and the development of diabetic complicationsDiabetes199847859669604860
- LeeAYChungSSContributions of polyol pathway to oxidative stress in diabetic cataractFASEB J1999123309872926
- LeonardDRFarooqiMHRestoration of sensation, reduced pain, and improved balance in subjects with diabetic peripheral neuropathy: a double-blind, randomized, placebo-controlled study with monochromatic near-infrared treatmentDiabetes Care2004271687214693984
- LittleWCZileMRThe effect of alagebrium chloride (ALT-711), a novel glucose cross-link breaker, in the treatment of elderly patients with diastolic heart failureJ Card Fail200511191515812746
- LiuSLeeIMVitamin E and risk of type 2 diabetes in the women’s health study randomized controlled trialDiabetes20065528566217003353
- LonnEYusufSHoogwerfBEffects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudyDiabetes Care20022519192712401733
- MartynCNReidWSix-month treatment with sorbinil in asymptomatic diabetic neuropathy. Failure to improve abnormal nerve functionDiabetes198736987903111914
- MasonJO3rdNixonPAIntravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathyAm J Ophthalmol2006142685817011869
- MellorHParkerPJThe extended protein kinase C superfamilyBiochem J19981281929601053
- MorcosMBorceaVEffect of alpha-lipoic acid on the progression of endothelial cell damage and albuminuria in patients with diabetes mellitus: an exploratory studyDiabetes Res Clin Pract2001521758311323087
- MonnierVMSellDRGlycation products as markers and predictors of the progression of diabetic complicationsAnn N Y Acad Sci200510435678116037280
- NakamuraNYamazakiKEffects of eparlestat on plasma levels of advanced glycation end products in patients with type 2 diabetesIn Vivo2003171778012792982
- NakayamaMNakamuraJAldose reductase inhibition ameliorates pupillary light reflex and F-wave latency in patients with mild diabetic neuropathyDiabetes Care2001241093811375376
- NishikawaTEdelsteinDNormalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damageNature20004047879010783895
- OkamotoHNomuraMEffects of epalrestat, an aldose reductase inhibitor, on diabetic neuropathy and gastroparesisIntern Med2003426556412924487
- OshimaYSakaguchiHRegression of iris neovascularization after intravitreal injection of bevacizumab in patients with proliferative diabetic retinopathyAm J Ophthalmol2006142155816815267
- PerssonFRossingPIrbesartan treatment reduces biomarkers of inflammatory activity in patients with type 2 diabetes and microalbuminuria: an IRMA 2 substudyDiabetes2006553550517130503
- RoglicGUnwinNThe burden of mortality attributable to diabetes: realistic estimates for the year 2000Diabetes Care200592130516123478
- RosenfeldPJIntravitreal avastin: the low cost alternative to lucentis?Am J Ophthalmol2006142141316815262
- RosenfeldPJBrownDRanibizumab for Neovascular Age-Related Macular DegenerationN Engl J Med200635514193117021318
- ScottJAKingGLOxidative stress and antioxidant treatment in diabetesAnn N Y Acad Sci200410312041315753146
- SheetzMJKingGLMolecular understanding of hyperglycemia’s adverse effects for diabetic complicationsJAMA200228825798812444865
- ShenHClaussMCharacterization of vascular permeability factor/vascular endothelial growth factor receptors on mononuclear phagocytesBlood1993812767738490183
- ShibaTInoguchiTCorrelation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulationAm J Physiol1993265E783938238505
- SolaSMirMQIrbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) studyCirculation2005111343815655130
- SoliniAVergnaniLGlycosaminoglycans delay the progression of nephropathy in NIDDMDiabetes Care199720819239135948
- Sorbinil Retinopathy Trial Research GroupRandomized trial of sorbinil, an aldose reductase inhibitor, in diabetic retinopathyArch Ophthalmol19901081234442119168
- Sorbinil Retinopathy Trial Research GroupThe sorbinil retinopathy trial: neuropathy resultsNeurology199343114198170559
- SpaideRFFisherYLIntravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhageRetina200626275816508426
- SteinbrookRThe price of sight - ranibizumab, bevacizumab, and the treatment of macular degenerationN Engl J Med200635514091217021315
- StirbanANegreanMBenfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetesDiabetes Care20062920647116936154
- StrackeHLindemannAA benfotiamine-vitamin B combination in treatment of diabetic polyneuropathyExp Clin Endocrinol Diabetes1996104311168886748
- StromCSanderBEffect of ruboxistaurin on blood-retinal barrier permeability in relation to severity of leakage in diabetic macular edemaInvest Ophthalmol Vis Sci2005463855816186374
- SundkvistGArmstrongFMPeripheral and autonomic nerve function in 259 diabetic patients with peripheral neuropathy treated with ponalrestat (an aldose reductase inhibitor) or placebo for 18 months. United Kingdom/Scandinavian Ponalrestat TrialJ Diabetes Complications19926123301611136
- SutterFKSimpsonJMIntravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trialOphthalmology20041112044915522370
- The PKC-DRS Study GroupThe effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trialDiabetes20055421889715983221
- ThornalleyPJUse of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproductsArch Biochem Biophys2003419314014568006
- TuttleKRBakrisGLThe effect of ruboxistaurin on nephropathy in type 2 diabetesDiabetes Care20052826869016249540
- UchidaKKigoshiTNakanoSEffect of 24 weeks of treatment with epalrestat, an aldose reductase inhibitor, on peripheral neuropathy in patients with non-insulin-dependent diabetes mellitusClin Ther19951746067585850
- UK Prospective Diabetes Study (UKPDS) GroupIntensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33)Lancet1998352837539742976
- UribarriJCaiWDiet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjectsAnn N Y Acad Sci20051043461616037267
- van WijngaardenPCosterDJInhibitors of ocular neovascularization: promises and potential problemsJAMA200529315091315784876
- VinikAIBrilVTreatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trialClin Ther20052711648016199243
- VoziyanPAHudsonBGPyridoxamine: the many virtues of a maillard reaction inhibitorAnn N Y Acad Sci200510438071616037308
- WilliamsMBoltonWDegenhardtTA phase 2 clinical trial of pyridoxamine (pyridorin) in type 2 and type 2 diabetic patients with overt nephropathy (PYR-206) [abstract]J Am Soc Nephro2003147A
- WilliamsMENew potential agents in treating diabetic kidney disease: the fourth actDrugs20066622879817181372
- WinklerGPalBEffectiveness of different benfotiamine dosage regimens in the treatment of painful diabetic neuropathyArzneimittelforschung199949220410219465
- WoelkHLehrlSBenfotiamine in treatment of alcoholic polyneuropathy: an 8-week randomized controlled study (BAP I Study)Alcohol Alcohol19983363189872352
- The World Health OrganizationDiabetes Programme: facts and figures [online]2007 Accessed 25 February 2007. URL: http://www.who.int/diabetes/facts/en
- ZieglerDNowakHTreatment of symptomatic diabetic polyneuropathy with the antioxidant a-lipoic acid: a meta-analysisDiabetic Med2004211142114984445
- ZieglerDAmetovAOral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy. The SYDNEY 2 TrialDiabetes Care20062923657017065669