Abstract
Diabetes mellitus is a significant worldwide health problem, with the incidence of type 2 diabetes increasing at alarming rates. Insulin resistance and dysregulated blood glucose control are established risk factors for microvascular complications and cardiovascular disease. Despite the recognition of diabetes as a major health issue and the availability of a growing number of medications designed to counteract its detrimental effects, real and perceived barriers remain that prevent patients from achieving optimal blood glucose control. The development and utilization of inhaled insulin as a novel insulin delivery system may positively influence patient treatment adherence and optimal glycemic control, potentially leading to a reduction in cardiovascular complications in patients with diabetes.
Introduction
Diabetes mellitus is a chronic disease resulting from an inability of the pancreas to produce enough of the regulatory hormone insulin and/or from ineffective systemic use of the insulin that it does produce (CitationWHO 2006). One result of insulin insufficiency is hyperglycemia, or elevated blood glucose (BG), a cardinal manifestation of uncontrolled diabetes that is indicative of a loss of normal metabolic homeostasis. Over time, hyperglycemia and its secondary effects negatively impact both macro- and microvascular targets, resulting variably in cardiovascular disease, stroke, blindness, renal failure, peripheral nerve damage, changes in the skin and joints, lower limb amputations, and premature death (CitationThe DCCT/EDIC Research Group 2000; CitationRoglic et al 2005; CitationYach et al 2006).
The WHO estimates that more than 180 million people worldwide have diabetes (CitationWHO 2006). This number is likely to more than double by 2030 (CitationWild et al 2004). In 2000, an estimated 2.9 million deaths worldwide were attributable to diabetes (CitationRoglic et al 2005; CitationWHO 2006). It is also estimated that 60% of all cases of diabetes can be directly attributed to obesity (CitationYach et al 2006). With a global rise in the incidence of obesity, the societal and economic impact of diabetes will only increase without effective intervention (CitationCenters for Disease Control and Prevention 2005; CitationYach et al 2006).
Glycosylated hemoglobin (HbA1c) is the most commonly used surrogate measure of average BG concentration over the life of the red blood cell (approximately 3 months). Subcutaneous administration of insulin is currently the primary mechanism for regulating HbA1c levels in patients who are severely insulin deficient (type 1 diabetes mellitus, T1DM) and in patients with insulin resistance and/or an insufficient insulin supply in whom lifestyle changes and/or oral anti-diabetic medications (OAMs) fail to elicit adequate metabolic response (type 2 diabetes mellitus, T2DM).
Initial insulin management of T2DM typically involves initiation of a single injection of long acting insulin (CitationAmerican Diabetes Association 2007). Although this treatment may allow nearly 60% of subjects to achieve target BG control with an HbA1c value below 7% (CitationRiddle et al 2003, Citation2006), this level of control is difficult to achieve in broad clinical practice using basal-only therapy, and additional strategies to intensify therapy may be needed.
Intensification of diabetes management often involves multiple (3 or more) daily injections of insulin and frequent BG monitoring (CitationDiabetes Control and Complications Trial Research Group 1993; CitationSchaumberg et al 2005). Although intensive management can prove effective in controlling HbA1c levels and reducing the risk of diabetes complications (CitationDiabetes Control and Complications Trial Research Group 1993), many patients with diabetes are reluctant to initiate or adhere to insulin therapy due to anxiety over multiple injections, inconvenience and social stigma (CitationHauber et al 2005; CitationWhite 2006).
The last 20 years has seen a revolution in research and development of alternative, injection-free insulin delivery methods. One of the most promising of these noninvasive candidates is inhaled insulin. In this article, we review the importance of insulin-mediated glycemic control in the prevention of diabetes-related cardiovascular disease, the development and current status of inhaled insulin as a novel drug delivery system, and the future potential of inhaled insulin as an effective therapy for reducing cardiovascular morbidity and mortality resulting from uncontrolled BG.
Overview of diabetes and cardiovascular disease
There is a strikingly high incidence of cardiovascular-related morbidity and mortality in individuals with diabetes; approximately 50% of all diabetes-related deaths are attributed to coronary artery disease (CitationJelesoff et al 1996). This finding is not surprising, considering the multiple mechanisms by which insulin and circulating glucose regulate normal cardiovascular function. Indeed, systemic changes related to chronic hyperglycemia directly contribute to the development of hypertension, atherosclerosis, thrombolytic events, and cardiomyopathy (CitationKing and Wakasaki 1999; CitationStenina 2005b; CitationWatala 2005; CitationYamagishi and Imaizumi 2005; CitationAhmed and Goldstein 2006; CitationRaman et al 2007).
Although much progress has been made in recent years, the mechanistic relationship between insulin resistance, hyperglycemia and the broad spectrum of diabetic complications remains an area of active research. Aspects of this field of study have been reviewed by many investigators (CitationKing and Wakasaki 1999; CitationSaltiel and Kahn 2001; CitationBrownlee 2005; CitationStenina 2005a) and will not be comprehensively reviewed here.
Diabetes can arise from a number of initiating conditions that collectively lead to common downstream outcomes (). At the cellular level, a variety of cell types develop altered sensitivity to insulin through little known mechanisms (CitationSaltiel and Kahn 2001; CitationBrownlee 2005; CitationYamagishi and Imaizumi 2005). Specifically, insulin docking with the insulin receptor on the target cell surface normally activates multiple cascades of intracellular signaling. In insulin resistance, one key pathway that includes the signaling kinases phosphatidylinositol-3-kinase and AKT fails to activate, disrupting normal intracellular insulin responsiveness and preventing key proteins involved in glucose uptake from being upregulated by insulin. As a result, cellular uptake of glucose is attenuated, circulating BG remains high, and tissues such as the endothelial lining of blood vessels passively accumulate glucose.
Figure 1 Summary of diabetes causes and progression. Diabetes represents a collection of disease processes involving progressive systemic loss of tissue sensitivity to insulin signaling and/or the loss of pancreatic β-cell number or function. Changes at the level of intracellular signaling include decreased expression of glucose transport proteins (GLUT4), decreased production of endothelial vasodilators (nitric oxide), increased intracellular oxidative stress (O2-), and release into the systemic circulation of pro-inflammatory (C-reactive protein, IL-6, TNFa) and coagulation (PAI-1, ICAM-1) mediators. These changes are both caused by and lead to decreased glucose absorption in several organs, increased levels of both circulating glucose (hyperglycemia) and non-esterified fatty acids (dyslipidemia), passive glucose diffusion into cells with unregulated glucose uptake, changes in vascular endothelial tone, and formation of atherosclerotic plaques. Glycosylation of intracellular and systemically circulating proteins, increased luminal fatty acid deposition, altered cellular metabolism, and increased clotting contribute to an increased risk for the microvascular and macrovascular risks associated with uncontrolled diabetes.
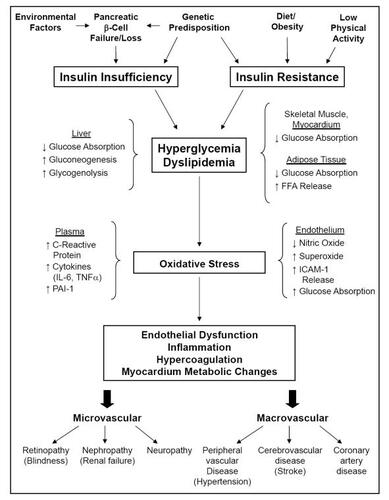
This rise in intracellular glucose increases glycolysis through the hexosamine metabolic pathway, damages cellular proteins and mitochondria through the accumulation of advanced glycation end products, and creates intracellular oxidative stress. The net effect of these changes is altered gene transcription and protein production, leading to a progressive impairment of normal cell function (CitationSaltiel and Kahn 2001; CitationBrownlee 2005; CitationYamagishi and Imaizumi 2005).
These progressive changes in normal cell and tissue function can lead to a spectrum of complications in patients with diabetes, including altered metabolism involving a shift from mixed glucose and fat use for energy production to the use of solely one or the other, altered vascular tone and responsiveness leading to hypertension, and altered production and/or secretion of proteins involved in vasorelaxation, inflammation, and coagulation (). As a result, patients with diabetes can develop vascular disorders that may result in blindness, kidney disease and renal failure, loss of limbs from poor peripheral circulation, and blood vessel-occluding plaques and blood clots, leading to heart attacks and stroke (CitationAmerican Diabetes Association 2007).
Support for both elevated BG and endothelial cell oxidative stress as contributors to these processes is mounting. Recent evidence that these factors are both interrelated and co-contributors to diabetes disease processes comes from reports that intensive glycemic control reduces circulating levels of markers of inflammation (CitationSchaumberg et al 2005) and normalizes endothelial function in patients with T1DM through the simultaneous control of hyperglycemia and oxidative stress (CitationCeriello et al 2007). Improved integrative approaches to diabetes therapy may come from these lines of investigation, but presently, achieving normal BG regulation remains a primary therapeutic goal in diabetes.
Importance of controlling HbA1c, fasting blood glucose and postprandial blood glucose
The central element in the development and progression of diabetes is the progressive loss of insulin production and/or insulin sensitivity, leading to dysregulation of systemic glucose utilization and increased BG concentrations. This rise in BG results in an increase in the covalent addition of glucose and its degradation products to both intracellular and circulating proteins (such as hemoglobin), which can contribute to altered vascular tone and atherosclerosis. Large clinical trials have demonstrated a direct correlation between HbA1c concentration and cardiovascular disease and mortality (CitationKhaw et al 2004). Fasting BG concentration and postprandial BG (PPBG) concentration are also used as indicators of effective short term BG regulation. Whereas fasting BG has long been used as a rapid indicator of insulin sufficiency, PPBG is thought to provide separate evidence of a person’s ability to adequately respond to a food challenge. Inadequate PPBG regulation occurs quite frequently in patients with T2DM, can occur even when metabolic control appears to be good, and may represent a separate risk factor for diabetic complications (CitationBonora et al 2006).
The role of therapeutic interventions that specifically target PPBG control is a matter of considerable debate (CitationCefalu 2007; CitationNathan 2007). There is general agreement, however, that any intervention that fails to achieve target HbA1c goals should be intensified, and, as discussed above, this will frequently require the addition of meal time insulin to a basal-only insulin regimen. Several studies have indicated that premixed insulin products (ie, products that contain both rapid-acting and basal insulin components) may allow patients to achieve superior HbA1c control compared to basal-only regimens (CitationMalone et al 2004, Citation2005, CitationRaskin et al 2005). Regardless of the type of therapy used, successful management of BG concentrations has been shown to decrease the risk of both the microvascular and macrovascular complications of T1DM and T2DM (CitationUK Prospective Diabetes Group 1991; CitationDiabetes Control and Complications Trial Research Group 1993; CitationWriting Team for the Diabetes Control and Complications Trial 2003; CitationNathan et al 2005).
For patients with pancreatic β-cell failure and insulin insufficiency, insulin therapy is critical for BG regulation and for patient survival. With T2DM, however, the need for insulin supplementation usually develops later in the disease process. There are a number of therapies other than insulin, including oral anti-hyperglycemic medications (OAMs), which are available for initial BG control in this population. These medications include older agents such as the sulfonylureas and metformin, used alone or in combination with cholesterol-lowering statins, and the insulin-sensitizing thiazolidinediones. One newer class of drugs consists of incretin mimetics (glucagon-like peptide 1 agonists or analogues) and incretin enhancers (dipeptidyl peptidase-4 [DPP-4] inhibitors). Incretins are gastric hormones that control postprandial hyperglycemia by slowing gastric emptying, enhancing glucose-mediated insulin release, and controlling elevated postprandial glucagon (CitationBlonde et al 2006; CitationDrucker and Nauck 2006). Drugs in this class that have received recent United States Food and Drug Administration and European Union approval include the incretin mimetic Byetta® (exenatide, Amylin Pharmaceuticals, San Diego, CA and Eli Lilly and Company, Indianapolis, IN) and the DPP-4 inhibitor Januvia™ (sitagliptin phosphate, Merck and Co., Whitehouse Station, NJ). This newer drug class shows consistent if moderate reductions in Hb1Ac levels in controlled clinical trials (CitationHeine et al 2005; CitationHerman et al 2005). Weight loss has also been reported with the use of Byetta (CitationBuse et al 2007).
Despite the availability of a growing number of treatment options for glycemic control in patients with T2DM, most patients taking OAMs do not achieve the recommended target of HbA1c below 7% (CitationThe ACE/ADA Task Force on Inpatient Diabetes 2006), let alone the more aggressive goals supported by other advisory bodies (CitationDiabetes Medical Guidelines Task Force 2002). This group of patients may require insulin treatment to supplement or replace OAMs; however, significant reluctance to initiating insulin therapy has been documented in both patients with T2DM and their clinicians. The reasons for this reluctance include the fear of needles with anticipated pain of daily injections, the inconvenience of injection and monitoring regimens, fear of hypoglycemia, perceptions of failure in disease progression, and concern about weight gain (CitationZambanini et al 1999; CitationKorytkowski 2002; CitationRichardson and Kerr 2003; CitationPeyrot et al 2005; CitationPeyrot et al 2006). Unfortunately, delays in initiating insulin therapy in patients with inadequate glycemic control can increase the risk of developing serious complications (CitationBrown et al 2004).
Overview of inhaled insulin delivery devices
In the search for alternative routes for systemic delivery of insulin, several possibilities have been explored, including nasal, buccal (oral cavity), and pulmonary routes. Of these options, the lung, with its highly vascularized and relatively permeable large alveolar surface area (~100 m2), currently presents the best option for an alternative route for insulin delivery. The idea of administering insulin through the lungs was first proposed in 1924 and inhaled delivery of locally targeted pharmacological therapies is used routinely (CitationPatton 2006). However, difficulties related to bioavailability, formulation and particle size, and the mechanics of deep lung protein delivery have, until recently, precluded routine insulin inhalation therapy. The impending worldwide epidemic rise in diabetes incidence has prompted a resurgence of interest in this area. Investigators have established several criteria for effective delivery of precise doses of proteins to the systemic circulation via the lung. These criteria include an optimal aerodynamic particle size, breath holding time, inspiratory flow rate, and adequate lung function (CitationHeinemann 2002; CitationPatton 2006). The ideal device should deliver a consistent insulin dose to the deep lung and be relatively easy and convenient to use. An insulin delivery system meeting these criteria will increase insulin acceptance by both patients and providers, thus facilitating earlier initiation of insulin therapy, better BG control, and better treatment outcomes.
There are a number of companies currently developing insulin inhalation systems and this rapidly developing field has been the subject of several recent reviews (CitationMastrandrea and Quattrin 2006; CitationMuchmore and Gates 2006; CitationPatton 2006; CitationRosenstock et al 2007a). The only inhaled insulin product that currently has marketing authorization is Exubera® (Pfizer/Nektar), but several others, including AERx® iDMS (Novo Nordisk), Technosphere® (MannKind) and AIR® Inhaled Insulin (Alkermes/Eli Lilly) are being studied in Phase 3 clinical trials. These devices use a variety of liquid and powder insulin preparations, unique delivery devices and unique technical methodologies ().
Table 1 Current insulin inhalation systems
Although there is some variability between these different insulin inhalation devices in time to peak BG-lowering activity and duration of activity, they share the common feature of rapid onset of activity (10–20 minutes) and relatively short duration (CitationPatton 2006). For most of these devices, the time to maximal insulin activity is similar to that of rapid-acting subcutaneous insulin lispro, making them suitable for preprandial insulin dosing to control postprandial spikes in BG concentration. Recent reports on the clinical evaluation of the efficacy of inhaled versus subcutaneous insulins are summarized for patients with T1DM in and for patients with T2DM in . Overall glycemic control was similar between inhaled insulin and subcutaneous insulin delivery in most of these studies, with similar safety profiles (discussed below). Importantly, the availability of inhaled insulin may promote acceptance of insulin therapy in patients with diabetes, which in turn may improve initiation of and compliance with insulin treatment (CitationFreemantle et al 2005, Citation2006).
Table 2 Summary of published efficacy and/or safety studies in patients with type 1 diabetes (T1DM)
Table 3 Summary of published efficacy and/or safety studies in patients with type 2 diabetes (T2DM)
Safety
Since diabetes is a chronic disease, one important concern in the development of inhaled insulin is whether it can be used safely over an extended period of time. The major areas of concern regarding the safety of long term use involve the incidence of low BG concentrations or hypoglycemia, increases in circulating anti-insulin antibodies, and changes in pulmonary function.
Hypoglycemia
In two recent clinical trials, hypoglycemia occurred less frequently but to a slightly higher degree of severity with inhaled insulin compared to subcutaneous insulin in patients with T1DM or T2DM (CitationHollander et al 2004; CitationQuattrin et al 2004; CitationRosenstock et al 2007a). A meta-analysis of 16 open-label clinical trials of inhaled insulin in more than 4000 patients was recently reported (CitationCeglia et al 2006). Results of this analysis showed that severe hypoglycemia was more likely to occur in patients with T2DM using inhaled insulin versus those using OAMs alone (risk ratio [RR]: 3.1; confidence interval [CI]: 1.0–9.1), but that this risk was no greater when compared with patients using subcutaneous insulin.
A number of studies have shown that fasting BG is lower during inhaled insulin treatment as compared to meal time insulin injection regimens (CitationHollander et al 2004; CitationQuattrin et al 2004; CitationGarg et al 2006; CitationSkyler et al 2007). Low fasting BG may increase the risk of nocturnal hypoglycemia, although only one publication to date specifically reports nocturnal hypoglycemia rates during inhaled insulin treatment (CitationGarg et al 2006). In this report, the rates for any hypoglycemia and severe hypoglycemia in patients with T1DM were similar between inhaled insulin and injected insulin groups. However, better fasting BG levels that were achieved with inhaled insulin were associated with an increased rate of nocturnal hypoglycemia. This effect was observed only among patients who used a rapid acting insulin analog during the injection phase of this crossover study; patients who used regular human insulin during the injection phase did not have this propensity (CitationGarg et al 2006). This observation suggests that behavioral guidance regarding insulin dosing, meals and snacks, and activity levels may be able to lower the risk of hypoglycemia. Studies using the AIR Insulin System to assess the efficacy of such behavioral modifications are underway.
Anti-insulin antibodies
Administration of exogenous insulin has been associated with an increased production of anti-insulin antibodies. These increases are common, especially in patients with T1DM. At similarly efficacious doses, inhaled insulin appears to trigger an enhanced production of anti-insulin antibodies compared with subcutaneous insulin. However, increased antibody levels have not been correlated with adverse events, or with relevant clinical correlates of HbA1c levels, insulin dose, hypoglycemia, or changes in pulmonary function (CitationCefalu et al 2005; CitationHeise et al 2005; CitationFineberg et al 2005; CitationSkyler et al 2005, Citation2007). The possible significance of increases in anti-insulin antibodies continues to be assessed in long term safety studies.
Pulmonary function
The potential impact of chronic deep-lung deposition of exogenous proteins on pulmonary function presents another potential safety concern. Cough is a common but transient side effect of initiating inhaled insulin therapy. Coughing episodes were generally described as mild and non-progressive and occurred at a similar frequency (~25%) to those observed in patients undergoing other forms of inhalation therapy (CitationOwens et al 2006). In the same meta-analysis described above, an increased risk of mild-to-moderate, non-progressive cough was identified in patients on inhaled insulin (CitationCeglia et al 2006).
However, in short term observational studies, no change or only small changes were observed between inhaled insulin and other treatment groups in pulmonary function tests (PFTs) (, ) (CitationWeiss et al 2003; CitationHermansen et al 2004; CitationRosenstock et al 2005; CitationCefalu et al 2006). When patients using inhaled insulin were followed for up to 2 years, changes in lung function from baseline were small when measured by forced expiratory volume in 1 second (FEV1, <1%) and carbon monoxide diffusing capacity (DLCO, <2%), the two most widely accepted indices of pulmonary function (CitationSkyler et al 2007). These changes occurred within the first 3 months of initiating treatment and were not progressive. Current recommendations for the only marketed inhaled insulin dictate that all patients initiating inhaled insulin therapy undergo baseline PFTs that include spirometry and assessment of FEV1 values. Inhaled insulin should not be used in patients whose FEV1 is below 70% of predicted values. It is also recommended that patients should be reassessed after 6 months and yearly thereafter, and that use of inhaled insulin should be discontinued if PFT values decrease by more than 20% (Exubera package insert).
Attributes and efficacy of the AIR Insulin System
The AIR Insulin System is comprised of three elements: AIR Inhaled Insulin, the AIR Insulin Inhaler, and the Directions for Use circular. AIR Inhaled Insulin is composed of a combination of rDNA native human insulin and an excipient based on a normal component of alveolar surfactant. AIR Inhaled Insulin is packaged as a dry powder in capsules designed to deliver doses approximately equivalent to either 2 U (0.9 mg capsule fill weight of insulin) or 6 U (2.6 mg capsule fill weight of insulin) of injected insulin. The two strengths provide dosing flexibility for individual patients. A higher dose formulation is currently undergoing clinical testing.
The AIR Insulin System incorporates unique features for pulmonary delivery of pharmaceutical products. The technology is based upon the inhalation of dry-powder aerosols composed of relatively large low-density particles. Individual particles contain both the active agent and an excipient dispersed throughout the particle. Even though the particles have a relatively large geometric size (median >5 μm), their low density (<0.4 g/mL) results in an effective aerodynamic particle with a diameter range affords access to the deep lung. Relative to standard inhalation aerosols, the larger and less dense AIR Insulin particles require less energy to disperse prior to delivery to the lungs. This relatively low agglomeration and high dispersability permit efficient delivery of both small and large drug doses to the deep lung in a single inhalation from a simple, breath-actuated inhaler (CitationEdwards et al 1997, Citation1998). Once deposited in the lung, the particles provide rapid and reliable uptake of protein therapeutics into the systemic circulation.
The AIR Insulin Inhaler is a small, hand-held, dry powder device that is capsule-based and breath-actuated (CitationRosenstock et al 2007a). It is designed such that the appropriate aerosol dose is delivered to the patient using the energy derived from a single inhalation of modest intensity. The inhaler is a passive device with moderate resistance to inspiratory flow, which invites patients to inhale at a moderate rate in order to optimize deep lung deposition. Delivery of a similar powder based on the AIR technology has been characterized in vivo by high and reproducible emitted doses (87%) and high lung deposition (51% of the total dose) independent of peak inspiratory flow rate across a broad range (12–86 L/min) (CitationDeLong et al 2005).
The AIR Insulin System is currently undergoing Phase III clinical testing, including long-term (24-month) safety and efficacy studies in patients with T1DM or T2DM, and additional safety evaluations in patients with comorbid lung disease and diabetes. Other studies that compare the efficacy and safety of AIR Insulin to monotherapy with insulin glargine are also underway. These studies, along with information on HbA1c from early phase clinical trials with AIR insulin, are described in separate reviews (CitationMuchmore and Gates 2006; CitationRosenstock et al 2007a).
Patient training, satisfaction and preference for inhaled insulin therapy
Some of the reported barriers to strict glycemic control in patients with diabetes include reluctance to initiate insulin therapy and poor patient adherence because of pain and fear of injection, inconvenience, and social stigma associated with injections. Satisfaction surveys assessing the flexibility and ease of use, pain, side effects, and social acceptance of inhaled insulin have been overwhelmingly favorable (CitationHollander et al 2004; CitationQuattrin et al 2004; CitationHayes et al 2007a). In patients with either T1DM or T2DM who have previously used subcutaneous insulin for diabetes management, 80% preferred inhaled insulin over conventional subcutaneous insulin for their meal time insulin therapy (CitationRosenstock et al 2004). In a recent report of self-directed versus intensive patient training for use of the AIR Insulin Inhaler, it was found that patients can be self-directed without detrimental effects on metabolic (CitationRosenstock et al 2007b) or patient-reported (CitationHayes et al 2007b) outcomes, including measures of vitality, diabetes-associated symptoms, fear of hypoglycemia, and insulin-delivery system satisfaction. These data support the AIR Insulin Inhaler as a patient-friendly insulin delivery method that should appeal to both clinicians and patients. Importantly, this sufficiency of patient-directed training should allow precious diabetes education resources to be deployed in other important aspects of diabetes care beyond teaching the mechanics of inhaled insulin administration.
Contraindications to the use of inhaled insulin
There are several patient populations for whom the use of inhaled insulin is not recommended. Women who are pregnant should not use it, and its use has not been approved for children or adolescents. Current tobacco smokers or patients who have smoked in the preceding six months are also not candidates for inhaled insulin. Smoking has been shown to increase the rate and extent of inhaled insulin absorption (CitationHimmelmann et al 2003; CitationBecker et al 2006; CitationPan et al 2007), while acute passive exposure to smoke decreases the rate and extent of absorption (CitationFDA Endocrinologic and Metabolic Drugs Committee 2005). Smoking cessation, nicotine replacement therapy, and acute smoking re-exposure are also associated with clinically significant alterations in inhaled insulin pharmacokinetics and glucodynamics (CitationPan et al 2007). Thus smokers or former smokers at risk of recidivism should not use inhaled insulin.
Patients with compromised lung function, such as those with asthma or chronic obstructive pulmonary disease (COPD), are also not candidates for Exubera, the inhaled insulin that is currently marketed, due to unpredictable absorption rates and possible problems with simultaneous use of bronchodilators (Exubera package insert). AIR Inhaled Insulin was recently reported to be well tolerated by patients with COPD, and to elicit time-exposure and time-action profiles similar to subcutaneous insulin lispro (Rave et al 2007). However, there was reduced insulin absorption and decreased metabolic effects when this population was compared with healthy subjects. Clinical evaluations of the use of inhaled insulins in these populations are ongoing (CitationRosenstock et al 2007a).
Summary and conclusions
Diabetes is a significant worldwide health problem. Insulin resistance and deregulated BG control are established risk factors for microvascular complications and cardiovascular disease, with risk reduced by adequate BG control and intensive diabetes therapy. Despite the availability of a variety of medications for BG regulation, most patients do not achieve optimal BG control. Inhaled insulin is a new, safe means to deliver insulin that may increase patient compliance with insulin therapy, helping them to achieve optimal glycemic control and possibly reducing their risk of developing cardiovascular complications. However, diabetes is a chronic illness requiring lifetime intervention. Thus, long term studies are still required in order to ensure the continued efficacy and safety of this new treatment for diabetes.
Disclosures
This work was sponsored by Eli Lilly and Company.
References
- AhmedIGoldsteinBJCardiovascular risk in the spectrum of type 2 diabetes mellitusMt Sinai J Med2006737596817008936
- American Diabetes AssociationStandards of medical care in diabetes – 2007Diabetes Care200730Suppl 1S4S4117192377
- BarnettAHDreyerMLangePAn open, randomized, parallel-group study to compare the efficacy and safety profile of inhaled human insulin (Exubera) with glibenclamide as adjunctive therapy in patients with type 2 diabetes poorly controlled on metforminDiabetes Care2006a2918182516873786
- BarnettAHDreyerMLangePAn open, randomized, parallel-group study to compare the efficacy and safety profile of inhaled human insulin (Exubera) with metformin as adjunctive therapy in patients with type 2 diabetes poorly controlled on a sulfonylureaDiabetes Care2006b291282716732009
- BarnettAHfor the Exubera Phase III Study GroupEfficacy and one-year pulmonary safety of inhaled insulin (Exubera®) as adjunctive therapy with metformin or glibenclamide in Type 2 diabetes patients poorly controlled on oral agent monotherapyDiabetes200453Suppl 2A107
- BeckerRHShaSFrickADThe effect of smoking cessation and subsequent resumption on absorption of inhaled insulinDiabetes Care2006292778216443873
- BlondeLRosenstockJTriplittCWhat are incretins, and how will they influence the management of type 2 diabetesJ Manag Care Pharm200612212
- BonoraECorraoGBagnardiVPrevalence and correlates of post-prandial hyperglycaemia in a large sample of patients with type 2 diabetes mellitusDiabetologia2006498465416532323
- BrownJBNicholsGAPerryAThe burden of treatment failure in type 2 diabetesDiabetes Care20042715354015220224
- BrownleeMThe pathobiology of diabetic complications: a unifying mechanismDiabetes20055416152515919781
- BuseJBKlonoffDCNielsenLLMetabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: An interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trialsClin Ther2007291395317379054
- CefaluWRosenstockJKliozeSSustained efficacy and tolerability of inhaled human insulin (Exubera®). Therapy over 2 years: patients with type 2 diabetesDiabetologia200649Suppl 13 Abstract 1004
- CefaluWTPoint: pulmonary inhalation of insulin: another “brick in the wall”Diabetes Care2007304394117259527
- CefaluWTSerdarevic-PeharMfor the Exubera Phase 3 Study GroupLong-term use of Exubera in type 2 diabetes: observations on glycemic control, pulmonary function and antibody formation [abstract]Proceedings of the American Diabetes Association 65th Annual Meeting200565 Abstract 356-OR
- CegliaLLauJPittasGMeta-Analysis: Efficacy and safety of inhaled insulin therapy in adults with diabetes mellitusAnn Intern Med20061456657517088580
- Centers for Disease Control and PreventionNational Diabetes Fact Sheet: General Information and National Estimate on Diabetes in the United States [online]2005 URL: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf
- CerielloAKumarSPiconiLSimultaneous control of hyperglycemia and oxidative stress normalizes endothelial function in type 1 diabetesDiabetes Care2007306495417327335
- DeFronzoRABergenstalRMCefaluWTEfficacy of inhaled insulin in patients with type 2 diabetes not controlled with diet and exercise: a 12-week, randomized, comparative trialDiabetes Care2005281922816043733
- DeLongMWrightJDawsonMDose delivery characteristics of the AIR pulmonary delivery system over a range of inspiratory flow ratesJ Aerosol Med200518452916379620
- Diabetes Control and Complications Trial Research GroupThe effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research GroupN Engl J Med1993329977868366922
- Diabetes Medical Guidelines Task ForceThe American Association of Clinical Endocrinologists Medical Guidelines for the Management of Diabetes Mellitus: The AACE System of Intensive Diabetes Self-Management – 2002 UpdateEndocr Pract20028Suppl 1408211939758
- DruckerDJNauckMAThe incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetesLancet2006368169670517098089
- DumasREnglandRDRieseRJExubera is well tolerated and achieves tight glycemic control in patients with type 1 diabetesProceedings of the American Diabetes Association 65th Annual Meeting200565 355-OR
- EdwardsDABen-JebriaALangerRRecent advances in pulmonary drug delivery using large, porous inhaled particlesJ Appl Physiol199885379859688708
- EdwardsDAHanesJCaponettiGLarge porous particles for pulmonary drug deliveryScience19972761868719188534
- FDA Endocrinologic and Metabolic Drugs CommitteeSummary Minutes [online]2005 URL: http://www.fda.gov/ohrms/dockets/ac/05/minutes/2005-4169M1.pdf
- FinebergSEKawabataTFinco-KentDAntibody response to inhaled insulin in patients with type 1 or type 2 diabetes. An analysis of initial phase II and III inhaled insulin (Exubera) trials and a two-year extension trialJ Clin Endocrinol Metab20059032879415741258
- FreemantleNBlondeLDuhotDAvailability of inhaled insulin promotes greater perceived acceptance of insulin therapy in patients with type 2 diabetesDiabetes Care200528427815677807
- FreemantleNBlondeLHobbsFDThe availability of inhaled human insulin may lead to a greater acceptance of insulin therapy in European type 2 diabetes patients failing on diet and/or oral agent therapy [abstract]Diabetologia200649Suppl 13 Abstract 1010
- GargSRosenstockJSilvermanBLEfficacy and safety of preprandial human insulin inhalation powder versus injectable insulin in patients with type 1 diabetesDiabetologia200649891916506054
- HauberABJohnsonFRSauriolLRisking health to avoid injections: preferences of Canadians with type 2 diabetesDiabetes Care2005282243516123499
- HayesRPMuchmoreDSchmitkeJEffect of inhaled insulin on patient-reported outcomes and treatment preference in patients with type 1 diabetesCurr Med Res Opin2007a234354217288697
- HayesRPNakanoMMuchmoreDEffect of standard (self-directed) training versus intensive training for Lilly/Alkermes human insulin inhalation powder delivery system on patient-reported outcomes and patient evaluation of the systemDiabetes Technol Ther2007b9899817316103
- HeineRJVan GaalLFJohnsDExenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trialAnn Intern Med20051435596916230722
- HeinemannLAlternative delivery routes: inhaled insulinDiabetes Nutr Metab2002154172212678460
- HeiseTBottSTusekCThe effect of insulin antibodies on the metabolic action of inhaled and subcutaneous insulin: a prospective randomized pharmacodynamic studyDiabetes Care2005282161916123484
- HermanGAStevensCVanDKPharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral dosesClin Pharmacol Ther2005786758816338283
- HermansenKRonnemaaTPetersenAHIntensive therapy with inhaled insulin via the AERx insulin diabetes management system: a 12-week proof-of-concept trial in patients with type 2 diabetesDiabetes Care200427162714693983
- HimmelmannAJendleJMellenAThe impact of smoking on inhaled insulinDiabetes Care2003266778212610021
- HollanderPABlondeLRoweREfficacy and safety of inhaled insulin (exubera) compared with subcutaneous insulin therapy in patients with type 2 diabetes: results of a 6-month, randomized, comparative trialDiabetes Care20042723566215451900
- JelesoffNEFeinglosMGrangerCBOutcomes of diabetic patients following acute myocardial infarction: a review of the major thrombolytic trialsCoron Artery Dis19967732438970764
- KhawKTWarehamNBinghamSAssociation of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in NorfolkAnn Intern Med20041414132015381514
- KingGLWakasakiHTheoretical mechanisms by which hyperglycemia and insulin resistance could cause cardiovascular diseases in diabetesDiabetes Care199922Suppl 3C31C710189560
- KorytkowskiMWhen oral agents fail; practical barriers to starting insulinInt J Obes200226S18S24
- MaloneJKKerrLFCampaigneBNCombined therapy with insulin lispro Mix 75/25 plus metformin or insulin glargine plus metformin: a 16-week, randomized, open-label, crossover study in patients with type 2 diabetes beginning insulin therapyClin Ther20042620344415823767
- MaloneJKBaiSCampaigneBNTwice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with Type 2 diabetesDiabet Med2005223748115787659
- MastrandreaLDQuattrinTClinical evaluation of inhaled insulinAdv Drug Deliv Rev20065810617517070613
- MuchmoreDBGatesJRInhaled insulin delivery – where are we now?Diabetes Obes Metab200686344217026487
- NathanDMCounterpoint: no time to inhale: arguments against inhaled insulin in 2007Diabetes Care200730442317259528
- NathanDMClearyPABacklundJYIntensive diabetes treatment and cardiovascular disease in patients with type 1 diabetesN Engl J Med200535326435316371630
- OwensDRGrimleyJKirkpatrickPInhaled human insulinNat Rev Drug Discov20065371216802444
- PanAXde la PenaAYeoKPEffects of smoking cessation, acute re-exposure, and nicotine replacement in smokers on AIR® Inhaled Insulin pharmacokinetics and glucodynamicsBr J Clin Pharmacol2007In Press
- PattonJPulmonary delivery of insulinCurr Med Res Opin200622Suppl 3511
- PeyrotMRubinRRLauritzenTResistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) studyDiabetes Care2005282673916249538
- PeyrotMRubinRRSiminerioLMPhysician and nurse use of psychosocial strategies in diabetes care: results of the cross-national Diabetes Attitudes, Wishes and Needs (DAWN) studyDiabetes Care20062912566216732005
- QuattrinTBelangerABohannonNJEfficacy and safety of inhaled insulin (Exubera) compared with subcutaneous insulin therapy in patients with type 1 diabetes: results of a 6-month, randomized, comparative trialDiabetes Care2004272622715504996
- RamanPKrukovetsIMarinicTEGlycosylation mediates upregulation of a potent antiangiogenic and proatherogenic protein, Thrombospondin-1, by glucose in vascular smooth muscle cellsJ Biol Chem200728257041417178709
- RaskinPAllenEHollanderPInitiating insulin therapy in type 2 Diabetes: a comparison of biphasic and basal insulin analogsDiabetes Care200528260515677776
- RaveKde la PenaATibaldiFSAIR® inhaled insulin in subjects with chronic obstructive pulmonary disease: pharmacokinetics, glucodynamics, safety, and tolerabilityDiabetes CareIn press
- RichardsonTKerrDSkin-related complications of insulin therapy: epidemiology and emerging management strategiesAm J Clin Dermatol20034661714507228
- RiddleMCThe Treat-to-Target Trial and related studiesEndocr Pract200612Suppl 171916627386
- RiddleMCRosenstockJGerichJThe treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patientsDiabetes Care2003263080614578243
- RoglicGUnwinNBennettPHThe burden of mortality attributable to diabetes: realistic estimates for the year 2000Diabetes Care2005282130516123478
- RosenstockJBaughmanRARivera-SchaubTA randomized, double-blind, placebo controlled study of the efficacy and safety of inhaled Technosphere® Insulin in patients with Type 2 diabetes (T2DM)Diabetes200554Suppl 1A357
- RosenstockJCappelleriJCBolinderBPatient satisfaction and glycemic control after 1 year with inhaled insulin (Exubera) in patients with type 1 or type 2 diabetesDiabetes Care20042713182315161782
- RosenstockJMuchmoreDSwansonDAIR® Inhaled Insulin System: A novel insulin delivery system for patients with diabetesExpert Rev Med Devices2007aIn press
- RosenstockJNakanoMSilvermanBLComparison of standard (self-directed) versus intensive patient training for the human insulin inhalation powder (HIIP) delivery system in patients with type 2 diabetes: efficacy, safety, and training measuresDiabetes Technol Ther2007b980817316102
- RosenstockJZinmanBMurphyLJInhaled insulin improves glycemic control when substituted for or added to oral combination therapy in type 2 diabetes: a randomized, controlled trialAnn Intern Med20051435495816230721
- SaltielARKahnCRInsulin signalling and the regulation of glucose and lipid metabolismNature200141479980611742412
- SchaumbergDAGlynnRJJenkinsAJEffect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the diabetes control and complications trialCirculation200511124465315867184
- SkylerJfor the Exubera Phase 2 Study GroupSustained long-term efficacy and safety of inhaled insulin during 4 years of continuous therapyDiabetes200453Suppl 2A115
- SkylerJSCefaluWTKouridesIAEfficacy of inhaled human insulin in type 1 diabetes mellitus: a randomised proof-of-concept studyLancet2001357331511210993
- SkylerJSJovanovicLKliozeSTwo-year safety and efficacy of inhaled human insulin (Exubera) in adult patients with Type 1 diabetesDiabetes Care2007305798517327324
- SkylerJSWeinstockRSRaskinPUse of inhaled insulin in a basal/bolus insulin regimen in type 1 diabetic subjects: a 6-month, randomized, comparative trialDiabetes Care2005281630515983312
- SteninaOIRegulation of vascular genes by glucoseCurr Pharm Des2005a1123678116022672
- SteninaOIVascular complications of diabetesCurr Pharm Des2005b112277816022667
- The ACE/ADA Task Force on Inpatient DiabetesAmerican College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control: A call to actionDiabetes Care20062919556216873812
- The DCCT/EDIC Research GroupRetinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research GroupN Engl J Med2000342381910666428
- UK Prospective Diabetes GroupUK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performanceDiabetologia199134877901778353
- WatalaCBlood platelet reactivity and its pharmacological modulation in (people with) diabetes mellitusCurr Pharm Des20051123316516022671
- WeissSRChengSLKouridesIAInhaled insulin provides improved glycemic control in patients with type 2 diabetes mellitus inadequately controlled with oral agents: a randomized controlled trialArch Intern Med200316322778214581245
- WhiteRDOptions for insulin delivery and overcoming physician and patient concernsJ Fam Pract2006551824
- WildSRoglicGGreenAGlobal prevalence of diabetes: estimates for the year 2000 and projections for 2030Diabetes Care20042710475315111519
- [WHO] World Health OrganizationDiabetes Fact Sheet [online]2006 URL: http://www.who.int/mediacentre/factsheets/fs312/en//
- Writing Team for the Diabetes Control and Complications TrialSustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) studyJAMA200329021596714570951
- YachDStucklerDBrownellKDEpidemiologic and economic consequences of the global epidemics of obesity and diabetesNat Med20061262616397571
- YamagishiSImaizumiTDiabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategyCurr Pharm Des20051122799916022668
- ZambaniniANewsonRBMaiseyMInjection related anxiety in insulin-treated diabetesDiabetes Res Clin Pract1999462394610624790