Abstract
Background:
The safety of once-daily (qd) dosing of valsartan in heart failure (HF) patients is not known.
Hypothesis:
This 10-week, double-blind trial examined the relative safety and efficacy of valsartan administered qd versus twice-daily (bid).
Methods:
HF patients (NYHA class II–III) receiving diuretics (87%), angiotensin-converting enzyme inhibitors (98%), beta-blockers (92%), aldosterone antagonists (25%), or digoxin (32%) were randomized to valsartan 40 mg bid (n = 60) or 80 mg qd (n = 55) and titrated to a maximum dose of 320 mg/day; doubling the dose every 2 weeks. Clinical and biochemical parameters were measured at Weeks 2, 4, 6, and 10.
Results:
The average dose of valsartan at the end of study was 245 mg in the bid group vs 256 mg in the qd group (P = NS). Similar proportions of patients tolerated qd vs bid dosing (bid 67% vs qd 68%). Outcome measures including reduction in blood pressure, incidence of hypotension, renal impairment, orthostatic dizziness or fatigue, changes in serum K+, creatinine, cystatin-C, and estimated glomerular filtration rate were similar between the 2 groups at all time-points. Brain natriuretic peptide levels decreased and plasma renin activity increased from baseline by the same amount in both groups at all time-points.
Conclusion:
Valsartan administered qd has a similar safety and tolerability profile with comparable 24-hour RAAS blockade, as assessed by increases in PRA, as bid dosing in patients with moderate to severe (NYHA class II–III) heart failure.
Introduction
The renin-angiotensin-aldosterone system (RAAS) is activated in patients with chronic heart failure (CHF) and its inhibition with beta-blockers and/or angiotensin-converting enzyme inhibitors (ACE-I) is associated with reductions in cardiovascular mortality and morbidity.Citation1–Citation6 Current CHF treatment guidelines recommend the use of angiotensin receptor blockers (ARBs) as reasonable alternatives to ACE-I or in patients on ACE-I who remain symptomatic.Citation7–Citation9 Two large randomized trials demonstrated a significant reduction in morbidity when an ARB was added in patients with New York Heart Association (NYHA) class II–IV CHF receiving prescribed therapies of ACE-I, diuretic, and beta-blocker.Citation10–Citation11 In the Valsartan Heart Failure Trial (Val-HeFT), valsartan was administered twice-daily (160 mg bid), despite being effective once daily (qd) in hypertension, to ensure sustained inhibition of the angiotensin II type 1 (AT1) receptor.Citation12,Citation13 Currently, valsartan is approved for use with bid dosing in the treatment of heart failure in patients who cannot use an ACE-I or as add-on therapy for patients already taking an ACE-I. We evaluated the tolerability, safety, and effectiveness of RAAS blockade after valsartan qd vs bid dosing in patients with stable heart failure (NYHA class II–III).
Methods
The Diovan Evaluation of Safety TwIce vs oNce dailY study in Heart Failure (DESTINY-HF) trial was a multicenter (34 sites), randomized, double-blind, active-controlled, parallel-group trial. Written informed consent was obtained from all patients.
Eligibility
Patients were recruited based on the inclusion criteria set originally for Val-HeFT.Citation12 Adult men and women with a history and clinical documentation of stable heart failure (NYHA class II–III) and left ventricular systolic dysfunction (ejection fraction <40%, measured within 3 months before screening) were eligible. Patients had to have been receiving stable doses of ACE-I and beta-blockers for at least 3 months and all other standard-care medication for heart failure (eg, diuretics, digoxin) for at least 2 weeks prior to screening. Investigators were instructed to keep each patient’s heart failure medication constant during the trial. Dose adjustments were not encouraged except for adverse events (eg, for reasons of hypotension, renal insufficiency, worsening CHF symptoms) during the study.
Exclusion criteria included standing blood pressure (BP) <95 mmHg; serum creatinine >2.5 mg/dL; serum potassium >4.8 mmol/L; and treatment with ARBs, including valsartan, within the 3 months prior to screening. In addition, pregnant, nursing women, and women of child-bearing potential who were not practicing effective contraceptive methods were excluded.
Study design
Qualifying patients entered a 10-week, double-blind treatment and were randomly assigned to 1 of the following parallel arms: (a) valsartan 80 mg qd for 2 weeks, titrated to a maximum of 320 mg qd, by doubling the dose every 2 weeks or (b) valsartan 40 mg bid for 2 weeks, titrated to a maximum of 320 mg administered as 160 mg bid, by doubling the dose every 2 weeks.
On the morning of any scheduled study clinic visit, patients were instructed not to take their study medication until after all study evaluations had been completed at the clinic. The reason (medical condition) why a patient could not be up-titrated at Weeks 2 and 4 was documented.
Outcome variables
The primary outcome variable was the percentage of patients who could tolerate the qd vs bid dosing regimen at Week 10. Inability to tolerate the dosing regimen was defined by any of the following: serum potassium ≥6.0 mEq/L, elevations in serum creatinine ≥2.5 mg/dL and increased by >50% from baseline, reduction in standing systolic blood pressure (SBP) to <90 mmHg, symptoms related to hypotension (eg, syncope, faintness, or orthostatic dizziness), or worsening of NYHA functional class.
The secondary end points were percentage of patients reaching the target total daily dose of 320 mg and NYHA classification status at Week 10. Change from baseline to Week 10 in SBP, diastolic blood pressure (DBP), serum potassium, estimated glomerular filtration rate (eGFR) based on serum creatinine and serum cystatin-C,Citation14 brain natriuretic peptide (BNP), and plasma renin activity (PRA) were other secondary variables measured.
Statistical analysis
The tolerability rate at Week 10 was calculated using both last-observation-carried-forward (LOCF) and observed-cases methods for the primary end point and categorical secondary end point (percentage of patients reaching target daily dose of 320 mg and NYHA classification status at Week 10). Cochran-Mantel-Haenszel chi-square test adjusting for pooled center and a 95% confidence interval (CI) was used to calculate the difference between the 2 treatment groups. For the continuous secondary variables, an analysis of covariance model with baseline measurement, pooled center, and treatment as covariates/factors was used. Mean differences, least squares mean differences, 95% CIs, and treatment P-value (significance level of 0.05) for the comparisons of the 2 treatment groups were reported. Because of the non-normal distribution of BNP and PRA data, non-parametric statistical analyses (Wilcoxon) were also performed.
Results
Of the 195 patients screened, 115 were randomized (55 qd group, 60 bid group). Twenty-nine patients (17 bid group, 12 qd group) withdrew prior to completion of the study, 25 for adverse events and 4 for withdrawal of consent.
There were no clinically relevant or statistically significant differences in the baseline characteristics of the 2 groups (). Overall, mean age was 65 years; 79% were male, 80% were white, and 57% were 65 years of age or older. All patients were either in NYHA class II (68%) or class III (32%). At randomization, 98% of patients were being treated with an ACE-I; 92% were receiving beta-blockers, 87% diuretics, 32% digoxin, and 25% spironolactone.
Table 1 Baseline characteristics of the patients according to treatment group
Dose of valsartan
The target dose of 320 mg daily was achieved by 67% of patients in the bid group vs 71% in the qd group by study end. The average dose of valsartan at study end was 245 mg and 256 mg in the bid and qd groups, respectively.
Safety
Valsartan was well tolerated with no differences observed between treatment groups. At Week 10, 35/45 (78%) patients in the bid group and 33/46 (72%) in the qd group were able to tolerate valsartan. The difference between treatment groups (6.0%) was not statistically significant (). At study end (LOCF criteria), 68% of patients (36/53) in the qd group tolerated the dosing regimen compared to 67% (38/57) in the bid group. There were no differences between the 2 dosing regimens for any of the individual tolerability criteria ().
Figure 1 Percentage of patients tolerating valsartan once-daily (qd) or twice-daily (bid) dosing regimen using observed cases. Using data from observed cases, the percentage of patients able to tolerate the dosing regimen at the end of the study was 78% for bid dosing and 72% for qd dosing (P = 0.56). Inability to tolerate the dosing regimen was defined as any of the following: serum potassium ≥6.0 mEq/L, elevations in serum creatinine ≥2.5 mg/dL and increased by >50% from baseline, reduction in standing SBP (<90 mmHg), symptoms related to hypotension (eg, syncope, faintness, or orthostatic dizziness), worsening of NYHA functional class (eg, fatigue or breathlessness) in patients with stable CHF (NYHA class II–III).
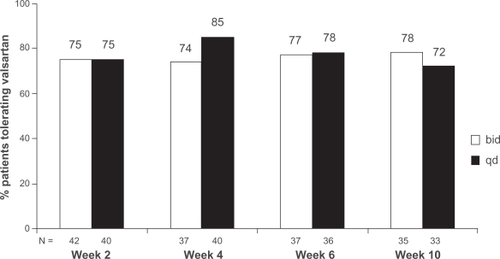
Table 2 Tolerability of valsartan once-daily versus twice-daily dosing during the study
The investigator-reported adverse events and serious adverse events are also listed in . There were no treatment-related differences in the incidence of adverse events. Examining adverse events specifically related to low BP (dizziness, hypotension, and syncope), they were found to be similar between the qd (n = 29, 53%) and bid groups (n = 26, 43%). Serious adverse events were reported in 7 patients in the bid group and 3 in the qd group. There were 25 (22%) patients who withdrew from the study due to adverse events (eg, dizziness, fatigue, worsened CHF, hypotension, diarrhea, and syncope): 14 (23%) in the bid group and 11 (20%) in the qd group.
Laboratory measures
No significant difference between the qd and bid groups was seen in change from baseline to study end (Week 10) in secondary outcome variables (). There was a small increase from baseline in serum potassium (0.08 mEq/L in the bid group vs 0.01 mEq/L in the qd group), with no significant difference between either treatment group. There was a significant reduction in eGFR (estimated using serum creatinine levels or cystatin-C); however, there was no difference between the 2 dosing regimens.
Table 3 Efficacy parameters: change from baseline at week 10/end of study
Efficacy: change in blood pressure
There was a small reduction in SBP and DBP in the qd group compared to the bid group, but no significant difference between the 2 dosing regimens ().
Efficacy: BNP and PRA
BNP levels decreased similarly after 2 weeks of treatment in both groups, but thereafter levels did not change significantly in either group from baseline (). PRA increased significantly in both groups, and there was no significant difference between the 2 treatment groups at any time-point ().
Table 4 Change from baseline in brain natriuretic peptide and plasma renin activity, by week
Efficacy: change in NYHA class from baseline
There were no significant changes in the NYHA class between the qd and bid groups. A total of 87% patients in both the qd and bid groups had no change in their NYHA class. No patient worsened to class IV in either treatment group. There were no statistically significant differences between treatment groups in the change in NYHA class from baseline at any study visit.
Discussion
We compared the use of valsartan in a bid dosing regimen, as used in Val-HeFT, to qd dosing in patients with stable heart failure (NYHA class II–III). Valsartan was well tolerated with a similar proportion of patients in both dosing groups achieving the target dose of 320 mg/d by study end. The mean daily dose of valsartan achieved in this study (245 mg in bid vs 256 mg in qd group) was similar to the 254-mg/d dose reported in Val-HeFT.Citation10 In addition, primary and secondary outcome variables were not different between the 2 dosing groups and were similar to those observed in Val-HeFT.Citation10
For the treatment of hypertension, all ARBs, including valsartan, are recommended in qd dosing. The qd indication for valsartan was based primarily on the 24-hour BP-lowering efficacy of 80–320 mg.Citation15,Citation16 When qd dosing of valsartan at 160 mg was compared with bid dosing of valsartan at 80 mg, a similar reduction in SBP and DBP was observed.Citation17 Despite evidence supporting qd use of valsartan in hypertension, no clinical studies have compared the tolerability of this strategy with bid dosing in heart failure; however, 2 trials using valsartan 160 mg qd have reported comparable efficacy and safety to either enalapril 20 mg/dCitation18 or lisinopril 20 mg/dCitation19 in this population.
Effect of valsartan on PRA and BNP levels
We have previously shown that the use of valsartan 160 mg bid in CHF patients receiving background ACE-I therapy is associated with an increase in PRA.Citation13 Similar findings have been reported in patients with chronic kidney disease.Citation20 This suggests inadequate RAAS blockade by ACE-I alone that could be related to the dose of background ACE-I use.Citation21 Although most patients in our study (98%) were receiving background ACE-I therapy, only 27% were receiving the target dose for their respective ACE-I. Furthermore, 43% (49/115) of patients were on an ACE-I dose below that recommended for treatment of CHF.Citation7,Citation22 Thus, the rise in PRA observed in both bid and qd valsartan dosing groups confirms additional blockade of the RAAS.
It is important to note that the PRA response in patients receiving an ACE-I or ARB can serve as an index of RAAS suppression. The similar increase in PRA between both dosing strategies in this study suggests similar effects of qd versus bid dosing of valsartan on the blockade of the RAAS over the 24-hour period. Previous studies have demonstrated that using valsartan 160–320 mg qd provides effective AT1 receptor blockade throughout the 24-hour period.Citation23,Citation24 Taken together, these findings and our PRA results suggest that valsartan 160–320 mg, given either once or twice a day, can achieve effective blockade of the RAAS throughout the 24-hour period. Moreover, a similar PRA response to qd and bid dosing of valsartan in this study suggests that both regimens had a similar level of treatment compliance.
In Val-HeFT, the addition of valsartan to heart failure patients resulted in significant reductions of BNP, close to what was reported herein. The Val-HeFT study reported reductions from 16–34 pg/mL in BNP during the course of study with average reductions of 21 pg/mL.Citation25 The similar BNP response reported for both dosing groups in our study suggests that valsartan has a similar effect on BNP, regardless of the dosing interval.
Conclusions
This study demonstrates that qd dosing with valsartan has a similar safety and tolerability profile to that of bid dosing in patients with NYHA class II–III heart failure. Additionally, treatment with qd-dosed valsartan results in similar 24-hour RAAS blockade to that seen following bid dosing, as assessed by increases in PRA.
Acknowledgements
The authors acknowledge Kanaka Sridharan, an employee at Novartis Pharmaceuticals Corporation, for editorial assistance in the manuscript preparation, J Howard from Oxford PharmaGenesis for styling the manuscript to journal requirements; and the diligent efforts of the study coordinators at the investigative sites.
Disclosures
This study was sponsored by: Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
Is Anand research support from Novartis Pharmaceuticals Corporation for the conduct of this trial; consultant and participant in the speaker bureau for Novartis. A Deswal: research support from Novartis Pharmaceuticals Corporation for the conduct of this trial. DJ Kereiakes: research support from Novartis Pharmaceuticals Corporation for the conduct of this trial; research/grant support from Amylin Pharmaceuticals, Biologics Delivery Systems Group, and Daiichi Sankyo, Inc; consultant and participant in the speaker bureau for Eli Lilly, Inc. D Purkayastha and DH Zappe: employees at Novartis Pharmaceuticals Corporation.
References
- The CONSENSUS Trial Study GroupEffects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS)N Engl J Med1987316142914352883575
- The SOLVD InvestigatorsEffect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failureN Engl J Med19913252933022057034
- PackerMBristowMRCohnJNfor the US Carvedilol Heart Failure Study GroupThe effect of carvedilol on morbidity and mortality in patients with chronic heart failureN Engl J Med1996334134913558614419
- CIBIS II Investigators and CommitteesThe Cardiac Insufficiency Bisoprolol Study (CIBIS II): a randomized trialLancet199935391310023943
- Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Study GroupEffect of metoprolol CR/XL in chronic heart failureLancet19993532001200710376614
- PackerMCoatsAJFowlerMBfor the Carvedilol Prospective Randomized Cumulative Survival Study GroupEffect of carvedilol on survival in severe chronic heart failureN Engl J Med20013441651165811386263
- HuntSAAbrahamWTChinMH2009Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung TransplantationCirculation2009119e391e47919324966
- European Society of CardiologyHeart Failure Association of the ESC (HFA)European Society of Intensive Care Medicine (ESICM)ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008Eur J Heart Fail20081093398918826876
- Heart Failure Society of AmericaExecutive summary: HFSA 2006 Comprehensive Heart Failure Practice GuidelineJ Card Fail200612103816500578
- CohnJNTognoniGfor the Valsartan Heart Failure Trial InvestigatorsA randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failureN Engl J Med20013451667167511759645
- McMurrayJJOstergrenJSwedbergKfor the CHARM Investigators and CommitteesEffects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trialLancet200336276777113678869
- CohnJNTognoniGGlazerRDRationale and design of the Valsartan Heart Failure Trial: a large multinational trial to assess the effects of valsartan, an angiotensin-receptor blocker, on morbidity and mortality in chronic congestive heart failureJ Card Fail1999515516010404355
- BaruchLAnandICohenISfor the Vasodilator Heart Failure Trial (V-HeFT) Study GroupAugmented short- and long-term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failureCirculation1999992658266410338459
- GrubbANymanUBjörkJSimple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for childrenClin Chem2005511420143115961546
- OparilSDykeSHarrisFThe efficacy and safety of valsartan compared with placebo in the treatment of patients with essential hypertensionClin Ther1996187978108930424
- PoolJOparilSHednerTDose-responsive antihypertensive efficacy of valsartan, a new angiotensin II-receptor blockerClin Ther199820110611149916605
- BlackHRGraffAShuteDValsartan, a new angiotensin II antagonist for the treatment of essential hypertension: efficacy, tolerability and safety compared to an angiotensin-converting enzyme inhibitor, lisinoprilJ Hum Hypertens1997114834899322828
- WillenheimerRHelmersCPantevEfor the Heart Failure Valsartan Exercise Capacity Evaluation Study GroupSafety and efficacy of valsartan versus enalapril in heart failure patientsInt J Cardiol20028526127012208593
- De TommasiEIacovielloMRomitoRComparison of the effect of valsartan and lisinopril on autonomic nervous system activity in chronic heart failureAm Heart J2003146854859
- WeinbergMSWeinbergAJZappeDHEffectively targeting the renin-angiotensin system in cardiovascular and renal disease: rationale for using angiotensin II receptor blockers in combination with angiotensin converting enzyme-inhibitorsJ Renin Angiotensin Aldosterone Syst2000121723311881029
- JacobsenPAndersenSJensenBRParvingHHAdditive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathyJ Am Soc Nephrol20031499299912660333
- McMurrayJCohen-SolalADietzRPractical recommendations for the use of ACE inhibitors, beta-blockers, aldosterone antagonists and angiotensin receptor blockers in heart failure: putting guidelines into practiceEur J Heart Fail2005771071216087129
- LatifFTandonSObelenieneRAngiotensin II type 1 receptor blockade with 80 and 160 mg valsartan in healthy, normotensive subjectsJ Card Fail2001726526811561228
- MaillardMPWürznerGNussbergerJCentenoCBurnierMBrunnerHRComparative angiotensin II receptor blockade in healthy volunteers: the importance of dosingClin Pharmacol Ther200271687611823759
- LatiniRMassonSAnandIfor the Valsartan Heart Failure Trial InvestigatorsEffects of valsartan on circulating brain natriuretic peptide and norepinephrine in symptomatic chronic heart failure: the Valsartan Heart Failure Trial (Val-HeFT)Circulation20021062454245812417542