Abstract
The death rate of patients with ST-segment elevation myocardial infarction (STEMI) remains substantial. Fondaparinux is a synthetic selective Factor Xa inhibitor with a high efficacy and good safety, in terms of bleeding risk, in the prevention and treatment of venous thromboembolism, and in the treatment of non-ST elevation acute coronary syndromes (OASIS-5). The OASIS-6 trial was a randomized, double-blind trial comparing fondaparinux 2.5 mg once daily with standard therapy, either placebo or unfractionated heparin according to the indication, in 12092 patients with STEMI. At day 30, fondaparinux significantly reduced the occurrence of the primary efficacy outcome (death or recurrent myocardial infarction) by 14% (p=0.008). Consistent reductions in both death and recurrent MI were observed at 6-month follow-up. The benefits were significant in patients who received no reperfusion therapy or a thrombolytic agent, but not in patients undergoing primary percutaneous coronary interventions. There was a trend (p=0.13) towards fewer severe bleeds in the fondaparinux group (1.0% vs 1.3% in the control group). In conclusion, fondaparinux significantly reduced mortality without increasing severe bleeding in patients with STEMI. Overall, the data from the OASIS studies showed that fondaparinux 2.5 mg may represent a new anticoagulant standard in patients with acute coronary syndromes.
ST-segment elevation myocardial infarction (STEMI) is due to the occlusion of coronary arteries by a thrombus at the site of atherosclerotic plaque rupture (CitationTheroux and Fuster 1998). The aim of the treatments is to restore blood flow though the blocked coronary vessels, either pharmacologically with thrombolytic drugs or mechanically by percutaneous coronary intervention (PCI). This reperfusion therapy, central to the treatment of STEMI, is associated with the administration of adjunctive treatments designed to preclude the reocclusion of the coronary arteries. These treatments include antiplatelet agents (aspirin, clopidogrel, and/or antagonists of platelet glycoprotein IIb-IIIa), and anticoagulants (unfractionated heparin [UFH] or low-molecular-weight heparin) (Citationvan de Werf et al 2003; CitationAntman et al 2004). Nevertheless, despite the availability of these therapies, one third of STEMI patients die within 24 hours of the onset of STEMI (CitationAntman et al 2004), 8%–10% of patients die or suffer reinfarction during their hospitalization (CitationAntman et al 2004), and 6%–7% die within one month of discharge (Citationvan de Werf et al 2003). These results may be due to the limited antithrombotic efficacy of the primary treatments, but also to their effects on bleeding. Indeed, recent data showed that short-term bleeding events were associated with long-term mortality (CitationMoscucci et al 2003; CitationSpiess et al 2004; CitationRao et al 2005, Citation2006; CitationEikelboom et al 2006). For example, the in-hospital death rate was 22.8% in STEMI patients with major bleeding compared with 7.0% in those without major bleeding (CitationMoscucci et al 2003). Discontinuation of antithrombotic agents in the event of bleeding, or the deleterious effect of transfusion therapy, may play a role in these adverse outcomes. Consequently, the challenge for new antithrombotic strategies is to be more effective without increasing bleeding risk. This manuscript will focus on anticoagulants, and notably fondaparinux, which showed substantial benefit in a large phase III trial in patients with STEMI (CitationYusuf et al 2006b). Since fondaparinux is a new drug, we will also present data obtained in other medical and surgical settings with this anticoagulant.
Current recommendations for the use of anticoagulants in patients with STEMI
UFH is the anticoagulant drug currently recommended in patients with STEMI (Citationvan de Werf et al 2003; CitationAntman et al 2004), this drug showing a marginal benefit for preventing death in a meta-analysis of trials with or without UFH (CitationCollins et al 1997). However, the use of UFH is not recommended in all clinical situations. Its benefit depends mainly on the other therapeutic strategies used in combination with this drug. Thus, North American and European guidelines recommend the use of intravenous UFH in patients undergoing reperfusion therapy with fibrin-specific thrombolytic agents (Citationvan de Werf et al 2003; CitationAntman et al 2004). The dose is to be adjusted to maintain the activated partial thromboplastin time (aPTT) at 1.5–2.0 times the control value. The duration of treatment recommended is 48 hours; this duration may be adapted according to the clinical characteristics of the patient. On the other hand, the use of UFH in patients undergoing reperfusion therapy with a non-fibrin-specific thrombolytic drug is judged to be “reasonable” by the North American experts (CitationAntman et al 2004) and “optional” by the European experts (Citationvan de Werf et al 2003). Interestingly, since these recommendations were established, a meta-analysis of UFH trials in STEMI patients (including two trials using streptokinase, one alteplase, and one anistreplase) showed that intravenous UFH did not reduce death/reinfarction, while increasing bleeding (CitationEikelboom et al 2005). There was also a modest, non-significant excess of strokes in patients treated with UFH, which was largely accounted for by an increase in intracranial hemorrhages.
Because of their ease of administration, the predictability of their anticoagulant effect, and the good results obtained in patients with non-ST elevation acute coronary syndromes (CitationEikelboom et al 2000), low-molecular-weight heparins have been tested as a potentially valuable alternative to UFH in STEMI patients. Compared with placebo, low-molecular-weight heparins were shown to reduce reinfarction and death, but at the expense of an increased bleeding risk (CitationEikelboom et al 2005; CitationYusuf et al 2005). Compared with UFH, they reduced reinfarction but not death, according to a meta-analysis of various trials (CitationEikelboom et al 2005) and the more recent ExTRACT-TIMI 25 study (CitationAntman et al 2006); furthermore, they increased the bleeding risk. Thus, in the North American guidelines, low-molecular-weight heparins are considered as an alternative therapy to UFH in patients below 75 years of age (CitationAntman et al 2004). The European guidelines give no recommendations regarding these drugs, citing the need for additional studies (Citationvan de Werf et al 2003). Of note, these recommendations were issued before publication of the large ExTRACT-TIMI 25 study of enoxaparin. Another anticoagulant drug, bivalirudin, a specific thrombin inhibitor, similarly reduced reinfarction, but not death, and increased bleeding, compared with UFH (CitationWhite 2001).
Finally, in STEMI patients not undergoing reperfusion therapy and with no contraindication to anticoagulation, experts consider treatment with intravenous or subcutaneous UFH for at least 48 hours to be reasonable (CitationAntman et al 2004).
Fondaparinux, a new anticoagulant
Mechanism of action
Fondaparinux is the first drug of a new group of anticoagulant compounds, the synthetic inhibitors of activated factor X or factor Xa (CitationHerbert et al 1997). It is a single chemical entity (1728 Da) composed of 5 saccharides, designed specifically to bind strongly and exclusively to antithrombin. By binding to antithrombin, fondaparinux increases the ability of antithrombin to inactivate factor Xa by a factor of about 300 (CitationHerbert et al 1997). Although fondaparinux does not mediate the inhibition of coagulation factors other than factor Xa, it effectively inhibits thrombin generation (CitationBéguin et al 1989). This inhibition is observed even in the presence of platelets (CitationBéguin et al 1989), suggesting that fondaparinux may be effective for treating arterial as well as venous thrombotic disorders.
Pharmacokinetics and pharmacodynamics
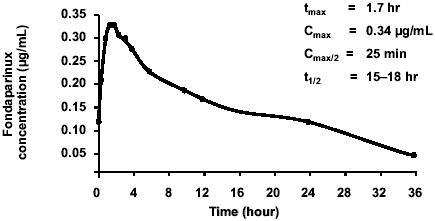
The main pharmacokinetic characteristics of fondaparinux after administration of a single subcutaneous dose of 2.5 mg, the approved thromboprophylactic dose, are shown in (CitationDonat et al 2002). On the whole, the subcutaneous absorption of fondaparinux is rapid, complete, and independent of the dose. Fondaparinux exerts very limited effects on routine hemostasis tests. Fondaparinux is almost completely excreted by the kidneys. The intra-subject and inter-subject variability of its pharmacokinetics is narrow, eliminating the need for dose adjustments and routine coagulation monitoring in the majority of patients. Furthermore, interaction between fondaparinux and several other commonly used drugs, including aspirin, is unlikely (CitationTurpie 2005).
Figure 1 Pharmacokinetics of fondaparinux administered at the subcutaneous dose of 2.5 mg. Drawn from data of CitationDonat et al (2002).
Prevention of venous thromboembolism
A single dose of fondaparinux, 2.5 mg once daily administered subcutaneously, was used in all trials in the prevention of venous thromboembolism, regardless of patient, surgical, or medical characteristics. This dose was selected on the basis of a phase II study in patients undergoing total hip replacement (CitationTurpie et al 2001). Compared with reference comparators, once-daily 2.5 mg fondaparinux was highly effective. In patients undergoing major orthopedic surgery, fondaparinux reduced the incidence of venous thromboembolism by more than 50% (p<0.001) compared with the low-molecular-weight heparin, enoxaparin (CitationBauer et al 2001; CitationEriksson et al 2001; CitationLassen et al 2002; CitationTurpie et al 2002a, Citation2002b). In patients undergoing high-risk abdominal surgery, once-daily 2.5 mg fondaparinux reduced the incidence of venous thromboembolism by 25.8% compared with dalteparin (p=0.14) (CitationAgnelli et al 2005). In acutely ill medical patients with restricted mobility, once-daily 2.5 mg fondaparinux reduced the incidence of venous thromboembolism by 46.7% compared with placebo (p=0.029) (CitationCohen et al 2006). In all these trials, the safety of fondaparinux in terms of bleeding risk, when used as recommended (notably with the first administration at least 6 hours after surgical closure), was comparable with that of the reference products (CitationTurpie 2005). In acutely ill medical patients, the incidence of major bleeding in both the fondaparinux and placebo groups was very low (0.2%) (CitationCohen et al 2006). Furthermore, no immuno-allergic thrombocytopenia was reported. Fondaparinux did not show any liver toxicity (CitationTurpie 2005).
Treatment of venous thromboembolism
The clinical benefit of fondaparinux in the treatment of venous thromboembolism was demonstrated in 2 randomized, non-inferiority trials (called MATISSE). In these trials, fondaparinux was at least as effective as enoxaparin or UFH in the treatment of patients with deep-vein thrombosis or pulmonary embolism, respectively (CitationBuller et al 2003, Citation2004). In this indication, the once-daily dose of fondaparinux was 7.5 mg (adjusted to 5 mg for patients with a body weight less than 50 kg and 10 mg for those with a body weight greater than 100 kg).
Treatment of patients with unstable angina or non-STEMI
The efficacy and safety of fondaparinux in the management of patients with non-STEMI coronary syndromes were investigated in the randomized, double-blind, non-inferiority OASIS-5 trial performed in 20 078 patients (CitationYusuf et al 2006a). Fondaparinux was administered subcutaneously at the once-daily dose of 2.5 mg. The reference comparator was enoxaparin administered at the approved subcutaneous dose of 1 mg/kg twice daily; the dose was reduced to 1 mg/kg once daily in patients with renal impairment as recommended. Both study treatments were administered for a mean of 5 days.
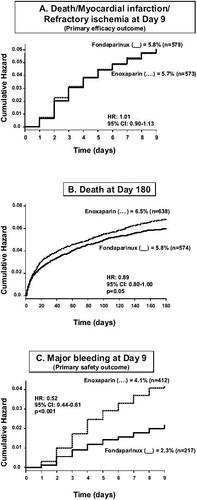
At day 9, fondaparinux was non-inferior to twice-daily enoxaparin, in terms of preventing death, myocardial infarction (MI), or refractory ischemia (5.9% in fondaparinux-treated patients vs 5.8% in enoxaparin-treated patients; hazard ratio: 1.01; 95% confidence interval: 0.90–1.13, below the prespecified boundary of 1.185; p=0.007 for non-inferiority) (). This result was consistent in all subgroups studied, including patients undergoing PCI. The rate of major bleeding was reduced by 48% (p<0.001) in the group of patients treated with fondaparinux (2.2% vs 4.1%). This result was observed in most subgroups, but the reduction in bleeding was greater in patients not undergoing revascularization procedures within 9 days following the index event (p<0.001). The reduction in major bleeding was also observed in patients with a creatinine clearance below 30 mL/min (2.4% vs 9.9%, p<0.001). Importantly, the lower rate of bleeding was associated with a significant reduction in the long-term risk of death, at one month by 17% (p=0.02), and at 6 months by 11% (p=0.05), confirming the relationship bleeding/long-term prognosis. Overall, fondaparinux presented a significantly (p<0.001) better benefit-to-risk ratio (defined as the composite of death, MI, refractory ischemia or major bleeding) up to 6 months after the index coronary event.
Figure 2 Main results of the OASIS-5 trial. Drawn from data of CitationYusuf et al (2006a).
Fondaparinux in STEMI
The benefit of fondaparinux in the management of patients with STEMI was evaluated in the recently completed OASIS-6 trial (CitationYusuf et al 2006b). The OASIS-6 trial was a phase III, multicenter, randomized, double-blind, controlled, parallel-group trial of fondaparinux vs standard therapy in patients with confirmed STEMI (). The aim of the trial was to demonstrate the superiority of fondaparinux vs standard therapy in terms of antithrombotic efficacy, with no increase in bleeding risk.
Figure 3 Study design of the OASIS-6 trial. Drawn from data of CitationYusuf et al (2006b).
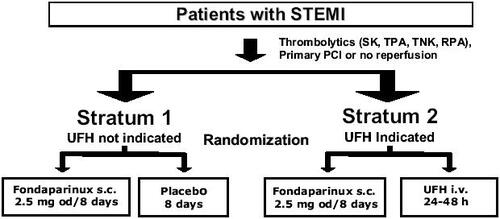
This was a very pragmatic trial performed in a broad range of patients with STEMI, ie, patients receiving either thrombolytic drugs, or primary PCI, or no revascularization treatment. Accordingly, randomization was stratified by indication for the use of UFH based on the investigator's judgment. Patients with no indication for UFH were enrolled in stratum 1, patients with indication for UFH being enrolled in stratum 2. Patients with an indication for UFH included patients in whom use of fibrin-specific thrombolytic drugs was intended, patients not eligible for thrombolytics but eligible for antithrombotics, and patients scheduled for primary PCI.
As in OASIS-5, the dose of fondaparinux was 2.5 mg once daily. This dosage regimen was chosen on the basis of data obtained in dose-ranging trials in patients with acute coronary syndromes, including patients with STEMI (CitationCoussement et al 2001; CitationSimoons et al 2004; CitationMehta et al 2005). Thus, patients in stratum 1 were assigned to receive either fondaparinux 2.5 mg or matching placebo once daily intravenously the first day, then subcutaneously on subsequent days for up to 8 days or hospital discharge if earlier. Patients in stratum 2 not scheduled for primary PCI were assigned to receive either fondaparinux 2.5 mg once daily (intravenously the first day, then subcutaneously on subsequent days) for up to 8 days or hospital discharge if earlier, or intravenous UFH for up to 48 hours as recommended. Patients in stratum 2 scheduled for primary PCI received single-bolus injections of either fondaparinux or UFH immediately before the procedure, the dose given depending on whether UFH and glycoprotein IIb/IIIa antagonists had been administered pre-randomization.
The primary efficacy outcome was the composite of death or recurrent MI up to day 30. The primary safety outcome was severe bleeding up to day 9. All deaths, reinfarction, and severe hemorrhage were centrally adjudicated using standardized definitions. The duration of follow-up was 180 days.
Initially, a sample size of 10 000 patients was planned, based on an event rate for death or reinfarction of about 8% at day 9. This sample size allowed detection of a relative risk reduction of 20% (two-sided alpha = 0.05), with a power of 90%. As the overall (blinded) event rate in the first 8000 randomized patients was lower than originally expected, the Steering Committee recommended two modifications: first, to ascertain the primary efficacy outcome at day 30 (instead of day 9); second, to increase the sample size to 12 000 patients. All analyses were performed according to the intention-to-treat principle.
A total of 12 092 patients were randomized. A thrombolytic drug was administered to 45% (n=5436) of patients, and primary PCI was performed in 31% (n=3789); 24% (n=2867) did not have any revascularization procedure. The thrombolytic drug was streptokinase in 73% of patients. After randomization, more than 95% of patients were treated with aspirin, and about 60% of patients received clopidogrel.
At day 30, fondaparinux significantly (p=0.008) reduced the occurrence of the primary efficacy outcome (death or recurrent MI) by 14%, from 11.2% in the control group to 9.7% (). Consistent reductions in both death and recurrent MI emerged at day 9 and were observed up to 6 months after the cardiac event. At 6 months, mortality was reduced (p=0.03) by 12% in the fondaparinux group (): this result mainly concerned cardiac deaths. The rate of stroke was comparable between the two groups (0.7% in the fondaparinux group and 0.9% in the control group). The effect of fondaparinux on the composite outcome of death or recurrent MI was not statistically heterogeneous between the two strata. It was consistent irrespective of gender, age, time from symptom onset to randomization, use of pre-randomization UFH, or type of thrombolytic agent administered. Significant benefits were observed in patients who received no reperfusion therapy or a thrombolytic agent, but not in patients undergoing primary PCI (). Although, as in OASIS-5 (CitationYusuf et al 2006a), a higher rate of guiding catheter thrombosis was observed with fondaparinux when PCI was performed without UFH, this was largely avoided if UFH was used before the procedure. Thus, in patients undergoing non-primary PCI in whom UFH was recommended before the procedure, there was no catheter thrombosis in either study group. There was a non-significant (p=0.13) trend towards fewer severe bleeds in the fondaparinux group (1.0%) compared with the control group (1.3%) ().
Figure 4 Primary efficacy results of the OASIS-6 trial. Drawn from data of CitationYusuf et al (2006b).
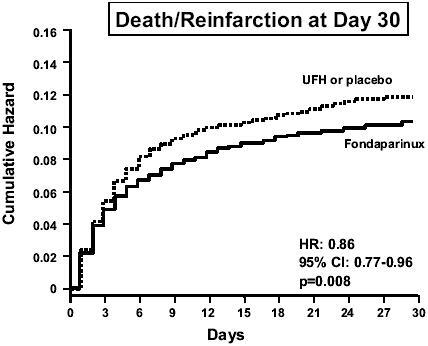
Figure 5 Mortality in the OASIS-6 trial. Drawn from data of CitationYusuf et al (2006b).
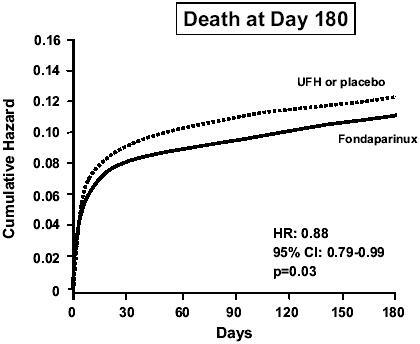
Figure 6 Results according to the type of reperfusion therapy in OASIS-6. Drawn from data of CitationYusuf et al (2006b).
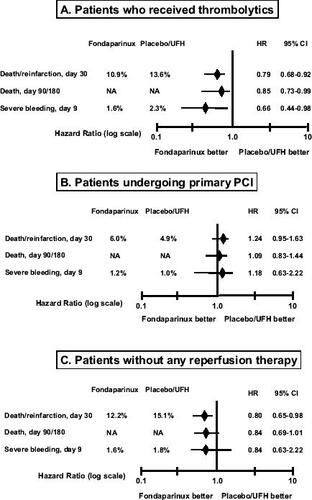
Table 1 Bleeding events in the OASIS-6 trial
Overall, throughout the study period, the balance of benefit and risk defined by the composite of death, recurrent MI, or severe bleeding was in favor of fondaparinux.
Place of fondaparinux compared with other anticoagulants in patients with STEMI
No trials similar to OASIS-6, encompassing such a broad range of clinical conditions, have been performed with other anticoagulant drugs. Thus, in order to better appraise the value of fondaparinux relative to other anticoagulant drugs, the data need to be analyzed according to various specific settings, notably the type of reperfusion administered to the patient.
In STEMI patients treated with a thrombolytic drug, fondaparinux significantly reduced death and reinfarction by 21% and severe bleeding by 34% as compared with standard treatment (). In the same context, low-molecular-weight heparins were shown to increase bleeding, either vs placebo (CitationYusuf et al 2005) or UFH (CitationASSENT 2001; CitationAntman et al 2006); furthermore, in the latter situation, low-molecular-weight heparins reduced recurrent myocardial infarction, but not death. Data suggesting an adverse effect of enoxaparin on bleeding were obtained even in trials in which dosing strategy was adjusted according to the patient's age and renal function (CitationAntman et al 2006). Similar results were found with bivalirudin vs UFH (CitationWhite 2001). Thus, fondaparinux could become the new standard anticoagulant drug in patients with STEMI treated with thrombolytic drugs. The benefit of fondaparinux in patients treated with fibrin-specific thrombolytic should nevertheless be confirmed in a larger sample of patients.
In STEMI patients treated with PCI, fondaparinux was no more effective or safe than standard treatment (). The specific role of low-molecular-weight heparins in the invasive management of STEMI has not yet been established. Indeed, most trials which have investigated the role of these drugs during PCI were performed in patients undergoing elective angioplasty or PCI for unstable angina or non-STEMI. Thus, in patients with STEMI undergoing primary PCI, UFH remains the standard therapy (CitationSilber et al 2005; CitationSmith et al 2006). The OASIS-6 data on fondaparinux are unlikely to prompt any change in this recommendation. However, the administration of fondaparinux after PCI may be valuable. In OASIS-6 patients undergoing primary PCI, there were fewer deaths or cases of reinfarction between 3 and 9 days after randomization in the fondaparinux group (1.4%) compared with the control group (2.0%) (CitationYusuf et al 2006b).
The group of STEMI patients not undergoing PCI or not receiving thrombolytic drugs is substantial. In OASIS-6, it represented 24% of the overall population. Likewise, registry data indicate that 25%–30% of eligible patients with STEMI never receive thrombolytic drugs and do not undergo early PCI (CitationEagle et al 2002). In this context, fondaparinux may represent a new reference anticoagulant drug, since it reduced the incidence of death/reinfarction without increasing severe bleeding (). In comparison, reviparin reduced death/reinfarction/stroke compared with placebo in patients enrolled in the CREATE study who did not receive any reperfusion treatment (21% of the overall population) (CitationYusuf et al 2005). Data on bleeding events in this subgroup were not given, but overall, reviparin increased the rate of major bleeding. In the TETAMI trial, enoxaparin was not superior to UFH in STEMI patients not eligible for reperfusion (CitationCohen et al 2003).
Conclusion
The OASIS-6 study showed that, unlike other anticoagulant agents, fondaparinux significantly reduced mortality among patients with STEMI, without increasing severe bleeding. These results were consistent vs either placebo or UFH, especially during long-term follow-up. The benefit of fondaparinux was marked in patients receiving thrombolytics, with a lower rate of major bleeding. Fondaparinux was also beneficial in patients undergoing no reperfusion therapy. However, fondaparinux showed no benefit in patients undergoing primary PCI.
Overall, the OASIS data (OASIS-5 and OASIS-6) obtained in about 32 000 patients with acute coronary syndromes showed that fondaparinux preserved or even improved the benefits of conventional anticoagulant drugs, while reducing bleeding (CitationYusuf et al 2006a, Citation2006b). Importantly, the safety data in terms of bleeding risk translated into improved long-term mortality and morbidity. These data were obtained with a single dosage regimen of 2.5 mg once daily. The absence of dose adjustments according to indication may also facilitate the use of this drug in routine practice and limit dosing errors associated with a risk of bleeding or death (CitationAlexander et al 2005).
References
- AgnelliGBergqvistDCohenATRandomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgeryBr J Surg20059212122016175516
- AlexanderKPChenAYRoeMTExcess dosing of antiplatelet and antithrombin agents in the treatment of non-ST-segment elevation acute coronary syndromesJAMA200529431081616380591
- AntmanEMAnbeDTArmstrongPWACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction)Circulation2004110e8229215339869
- AntmanEMMorrowDAMcCabeCHEnoxaparin versus unfractionated heparin with fibrinolysis for ST-elevation myocardial infarctionN Engl J Med200635414778816537665
- BauerKAErikssonBILassenMRFondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgeryN Engl J Med200134513051011794149
- BéguinSChoayJHemkerHCThe action of a synthetic pentasaccharide on thrombin generation in whole plasmaThromb Haemost1989613974012799755
- BullerHRDavidsonBLDecoususHSubcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolismN Engl J Med2003349169570214585937
- BullerHRDavidsonBLDecoususHFondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trialAnn Intern Med20041408677315172900
- CohenATDavidsonBLGallusASEfficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trialBMJ2006332325916439370
- CohenMGensiniGFMaritzFThe safety and efficacy of subcutaneous enoxaparin versus intravenous unfractionated heparin and tirofiban versus placebo in the treatment of acute ST-segment elevation myocardial infarction patients ineligible for reperfusion (TETAMI): a randomized trialJ Am Coll Cardiol20034213485614563573
- CollinsRPetoRBaigentCSleightPAspirin, heparin, and fibrinolytic therapy in suspected acute myocardial infarctionN Engl J Med1997336847609062095
- CoussementPKBassandJPConvensCA synthetic factor-Xa inhibitor (ORG31540/SR9017A) as an adjunct to fibrinolysis in acute myocardial infarction. The PENTALYSE studyEur Heart J20012217162411511121
- DonatFDuretJPSantoniAThe pharmacokinetics of fondaparinux sodium in healthy volunteersClin Pharmacokinet200241Suppl1912383039
- EagleKAGoodmanSGAvezumAPractice variation and missed opportunities for reperfusion in ST-segment-elevation myocardial infarction: findings from the Global Registry of Acute Coronary Events (GRACE)Lancet2002359373711844506
- EikelboomJWAnandSSMalmbergKUnfractionated heparin and low-molecular-weight heparin in acute coronary syndrome without ST elevation: a meta-analysisLancet200035519364210859038
- EikelboomJWMehtaSRAnandSSAdverse impact of bleeding on prognosis in patients with acute coronary syndromesCirculation20061147748216908769
- EikelboomJWQuinlanDJMehtaSRUnfractionated and low-molecular-weight heparin as adjuncts to thrombolysis in aspirin-treated patients with ST-elevation acute myocardial infarction: a meta-analysis of the randomized trialsCirculation200511238556716344381
- ErikssonBIBauerKALassenMRFondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgeryN Engl J Med2001345129830411794148
- HerbertJMPetitouMLormeauJCSR 90107/Org 31540, a novel anti-factor Xa antithrombotic agentCardiovasc Drug Rev199715126
- LassenMRBauerKAErikssonBIPostoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparisonLancet200235917152012049858
- MehtaSRStegPGGrangerCBArixtra Study in Percutaneous Coronary Intervention: A Randomized Evaluation (ASPIRE) Pilot TrialCirculation20051111390715781750
- MoscucciMFoxKACannonCPPredictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE)Eur Heart J20032418152314563340
- RaoSVO’GradyKPieperKSImpact of bleeding severity on clinical outcomes among patients with acute coronary syndromesAm J Cardiol2005961200616253582
- RaoSVO’GradyKPieperKSA comparison of the clinical impact of bleeding measured by two different classifications among patients with acute coronary syndromesJ Am Coll Cardiol2006478091616487850
- SilberSAlbertssonPAvilesFFGuidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of CardiologyEur Heart J2005268044715769784
- SimoonsMLBobbinkIWBolandJA dose-finding study of fondaparinux in patients with non-ST-segment elevation acute coronary syndromes; The Pentasaccharide in Unstable Angina (PENTUA) studyJ Am Coll Cardiol20044321839015193678
- SmithSCJrFeldmanTEHirshfeldJWJrACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update 2001 Guidelines for Percutaneous Coronary Intervention)Circulation2006113e16628616490830
- SpiessBDRoystonDLevyJHPlatelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomesTransfusion2004441143815265117
- [ASSENT] The Assessment of the Safety and Efficacy of New Thrombolytic Regimen (ASSENT)-3 InvestigatorsEfficacy and safety of tenecteplase in combination with enoxaparin, abciximab, or unfractionated heparin: the ASSENT-3 randomised trial in acute myocardial infarctionLancet20013586051311530146
- TherouxPFusterVAcute coronary syndromes: unstable angina and non-Q-wave myocardial infarctionCirculation19989711952069537346
- TurpieAGThe safety of fondaparinux for the prevention and treatment of venous thromboembolismExpert Opin Drug Saf200547072116011449
- TurpieAGBauerKAErikssonBIPostoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trialLancet2002a3591721612049860
- TurpieAGBauerKAErikssonBIFondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studiesArch Intern Med2002b16218334012196081
- TurpieAGGallusASHoekJAPentasaccharide InvestigatorsA synthetic pentasaccharide for the prevention of deep-vein thrombosis after total hip replacementN Engl J Med20013446192511228275
- van de WerfFArdissinoDBetriuAManagement of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of CardiologyEur Heart J200324286612559937
- WhiteHHirulog and Early Reperfusion or Occlusion (HERO)-2 Trial InvestigatorsThrombin-specific anticoagulation with bivalirudin versus heparin in patients receiving fibrinolytic therapy for acute myocardial infarction: the HERO-2 randomised trialLancet200135818556311741625
- YusufSMehtaSRChrolaviciusSComparison of fondaparinux and enoxaparin in acute coronary syndromesN Engl J Med2006a35414647616537663
- YusufSMehtaSRChrolaviciusSEffects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trialJAMA2006b29515193016537725
- YusufSMehtaSRXieCEffects of reviparin, a low-molecular-weight heparin, on mortality, reinfarction, and strokes in patients with acute myocardial infarction presenting with ST-segment elevationJAMA20052934273515671427