Abstract
Endothelial activation and inflammation are important mediators of accelerated atherogenesis and consequent increased cardiovascular morbidity in obstructive sleep apnea (OSA). Repetitive episodes of hypoxia/reoxygenation associated with transient cessation of breathing during sleep in OSA resemble ischemia/reperfusion injury and may be the main culprit underlying endothelial dysfunction in OSA. Additional factors such as repetitive arousals resulting in sleep fragmentation and deprivation and individual genetic suseptibility to vascular manifestations of OSA contribute to impaired endothelial function in OSA. The present review focuses on possible mechanisms that underlie endothelial activation and inflammation in OSA.
Introduction
Obstructive sleep apnea (OSA), a condition that affects up to a quarter of the American adult population, remains largely unrecognized, and less than 5% of all OSA patients receive treatment (CitationYoung et al 1993, Citation1997). Raising awareness among medical practitioners, and cardiologists in particular, about the high prevalence of unrecognized OSA is of great importance considering that OSA is an independent and potentially reversible risk factor for hypertension, myocardial ischemia and stroke (CitationPeker et al 1999; CitationPeppard et al 2000; CitationYaggi et al 2005; CitationYumino et al 2007). Increased cardiovascular morbidity in patients with untreated OSA is attributed to accelerated atherosclerosis (CitationKato et al 2000; CitationIp et al 2004; CitationDrager et al 2005, Citation2007; CitationMinoguchi et al 2005; CitationSaletu et al 2006; CitationSavransky et al 2007). The initial stimulus that triggers accelerated atherogenesis has not been definitively established.
Figure 1 Intermediary mechanisms that mediate increased cardiovascular risk in obstructive sleep apnea (OSA). Repetitive hypoxia/reoxygenation associated with transient cessation of breathing while asleep promotes endothelial dysfunction in patients with OSA by increasing vascular inflammation, oxidative stress and apoptosis while reducing nitric oxide availability. Sleep fragmentation and deprivation resulting from repetitive arousals during sleep compound the effects of hypoxia/reoxygenation injury by independently promoting vascular inflammation. Genetic susceptibility may contribute to cardiovascular risk associated with OSA.
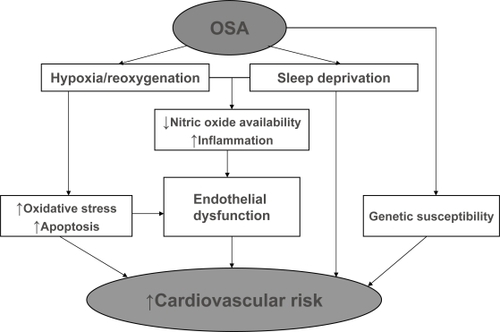
Impaired endothelial function plays an important role in the development of cardiovascular manifestations of OSA. Normal endothelium regulates vasomotor tone and preserves inflammatory and coagulation homeostasis (CitationAird 2007). These functions are altered in patients with OSA compared with healthy subjects (CitationCarlson et al 1996; CitationOhga et al 1999; CitationKato et al 2000; CitationDyugovskaya et al 2002, Citation2005; CitationIp et al 2004; Nieto et al 2004; CitationJelic et al 2008). Reduced numbers of endothelial progenitor cells and increased endothelial cell apoptosis in untreated OSA indicate impaired endothelial repair capacity (CitationEl Solh et al 2007, Citation2008; CitationJelic et al 2008; CitationMartin et al 2008). In addition, increased sympathetic nervous activity may also contribute to cardiovascular morbidity in OSA (CitationNarkiewicz and Somers 2003; CitationMaser et al 2008). However, the reversibility of autonomic alterations with OSA-specific therapy remains controversial (CitationNarkiewicz et al 1999; CitationEsler and Eikelis 2006; CitationMills et al 2006).
Repetitive hypoxia/reoxygenation associated with transient cessation of breathing during apneas and hypopneas is considered the main culprit for the impairment of endothelial function in OSA. Repetitive arousals resulting in sleep fragmentation and chronic sleep deprivation may also contribute to vascular dysfunction in OSA. Lastly, individual genetic susceptibility may play a role in development of cardiovascular manifestations of untreated OSA. The present review will focus on possible mechanisms of endothelial dysfunction in OSA.
Repetitive hypoxia/reoxygenation
Repetitive hypoxia/reoxygenation during transient cessation of breathing as occurs in OSA resembles ischemia/reperfusion injury. Although perfusion remains intact during obstructive events in OSA, alterations involving generation of reactive oxygen species, inflammation and reduced nitric oxide (NO) availability are similar to those observed in models of ischemia-reperfusion (CitationLiao et al 1995; CitationWang et al 1996; CitationKato et al 2000; CitationLaursen et al 2001; CitationDyugovskaya et al 2002; CitationTakemoto et al 2002; CitationKuzkaya et al 2003; CitationIp et al 2004; CitationMinoguchi et al 2005; CitationAntoniades et al 2006).
Endothelial nitric oxide availability
Indirect evidence such as impaired flow-mediated brachial arterial dilation and decreased circulating NO levels measured by serum nitrite/nitrate suggest reduced NO availability in OSA patients (CitationKato et al 2000; CitationIp et al 2000, Citation2004; CitationNoda et al 2007). Plasma levels of an endogenous inhibitor of endothelial nitric oxide synthase (eNOS) are increased and correlate inversely with flow-mediated dilation in patients with untreated OSA (CitationOhike et al 2005). Plasma levels of l-arginine, the substrate for NO production, increase after a single night of CPAP therapy in patients with OSA (CitationLavie et al 2003). Decreased eNOS activity and increased nitrotyrosine production, a byproduct of nitric oxide degradation, in freshly harvested venous endothelial cells provide direct evidence that nitric oxide bioavailability is reduced in OSA patients without overt cardiovascular disease (CitationJelic et al 2008).
Expression and activity of eNOS, a main source of basal endothelial NO, have been reported to be upregulated (CitationArnet et al 1996; CitationLe Cras et al 1998; CitationCoulet et al 2003; CitationShirai et al 2003), downregulated (CitationMcQuillan et al 1994; CitationLiao et al 1995; CitationPhelan and Faller 1996; CitationLaufs et al 1997; CitationToporsian et al 2000; CitationTakemoto et al 2002), or unchanged (CitationMurata et al 2001; Tahawi et al 2007) in various experimental models of hypoxia and repetitive hypoxia/reoxygenation. Contradictory reports of eNOS expression and activity appear to be due to temporal variations in experimental hypoxemic conditions, and differences in the species and vascular bed from which endothelial cells were derived. Long-term intermittent hypoxia that mimicked OSA, administered in 30-second cycles for 6–8 hours daily for 35 days, resulted in elevated diurnal resting mean arterial blood pressure in rats (CitationFletcher et al 1992; Tahawi et al 2007). Attenuated vasodilation in response to acetylcholine, a vasodilator that stimulates endothelial release of NO, and greater vasoconstriction with the NOS inhibitor NG-nitro-l-arginine methyl ester in rats exposed to long-term hypoxia/reoxygenation compared with controls, suggest decreased NO availability (Tahawi et al 2007). After prolonged hypoxia, eNOS activity is reduced (CitationKiss et al 1998; CitationTakemoto et al 2002), resulting in impaired vascular reactivity (CitationDanton et al 2002).
Several mechanisms of hypoxia-induced eNOS down-regulation have been proposed (CitationCoulet et al 2003; CitationTai et al 2004). On a transcriptional level, hypoxia-induced activation of hypoxia inducible factor-2 initially upregulates eNOS mRNA followed by a prolonged decrease in eNOS mRNA level (CitationCoulet et al 2003). On a post-transcriptional level, hypoxia destabilizes eNOS mRNA, in part via the Rho kinase pathway in human venous and pulmonary artery endothelial cells (CitationTakemoto et al 2002). Hypoxia increases arginase II activity in endothelial cells, which degrades l-arginine, an essential substrate for NO production by eNOS (CitationClarkson et al 2005). Oxidation of the eNOS cofactor tetrahydrobiopterin (BH4) by reactive oxygen species such as peroxynitrite appears to be an important mechanism linking oxidative stress to endothelial dysfunction (CitationKuzkaya et al 2003; CitationAntoniades et al 2006). Decreased BH4 availability promotes superoxide production by eNOS, an altered enzyme state labeled “uncoupling” (CitationVasquez-Vivar et al 1998; CitationXia et al 1998). Exposure to hypoxia for 24 hours leads to a time-dependent decrease in eNOS activator heat shock protein 90 that correlates with a decrease in eNOS activity in pulmonary artery endothelial cells (CitationGarcia-Cardena et al 1998; CitationSu and Block 2000). In contrast to short-term exposure to oxidative stress, prolonged oxidative stress as observed in untreated OSA reduces eNOS activity by suppressing its phosphorylation (CitationThomas et al 2002; CitationTanaka et al 2005). Reduced NO availability results in endothelial dysfunction and thereby increases the risk for vascular diseases in patients with OSA.
Endothelial and systemic inflammation
Elevated levels of plasma C-reactive protein (CitationShamsuzzaman et al 2002), leukocyte superoxide (CitationSchulz et al 2000; CitationDyugovskaya et al 2002) and soluble adhesion molecules (CitationOhga et al 1999) suggest the presence of chronic systemic inflammation in OSA patients. Upregulation of cyclooxygenase-2 (COX-2) and inducible NOS in venous endothelial cells harvested from patients with untreated OSA provides direct evidence of vascular inflammation in OSA (CitationJelic et al 2008).
Accumulation and adhesion of circulating leukocytes to the vascular endothelium lead to vessel inflammation and progression of atherosclerosis (CitationPrice and Loscalzo 1999; CitationAird 2007). Monocyte expression of the adhesion molecules CD15 and CD11c is increased in patients with OSA compared with controls matched for age and cardiovascular comorbidities, although not BMI (CitationDyugovskaya et al 2002). Enhanced oxidative stress and adhesion to cultured endothelial cells in monocytes collected in the morning from OSA patients suggest an adverse effect of OSA on diurnal vascular proinflammatory/antiinflammatory homeostasis (CitationDyugovskaya et al 2002). Lymphocytic production of interleukin-4 (IL-4), a proinflammatory cytokine, is greater, while production of IL-10, a potent antiinflammatory cytokine, is decreased in otherwise healthy patients with moderate to severe OSA, compared with subjects with an AHI <10/h (CitationDyugovskaya et al 2005).
Hypoxia/reoxygenation increases COX-2 gene and protein expression in endothelial cells in vivo and in vitro (CitationDomoki et al 1999; CitationLi et al 2003; CitationWu et al 2003). Although COX-2 is widely accepted as a proinflammatory agonist, its upregulation can be cardioprotective in ischemia-reperfusion injury (CitationBolli et al 2002). In addition, the use of COX-2 inhibitors may result in an increased incidence of cardiovascular events (CitationAntman et al 2005). COX-2 appears to have a dual role in inflammation: initially inducing the inflammatory process and later aiding its resolution (CitationGilroy et al 1999). Upregulation of COX-2 in OSA may result in increased oxidative stress caused by superoxide production and increased vasoconstrictor and/or inflammatory prostanoid production leading to increased platelet activation and endothelial dysfunction (CitationAntman et al 2005). Alternatively, induction of endothelial COX-2 in OSA patients may be a defense mechanism against repetitive hypoxia/reoxygenation.
Experimental models of repetitive hypoxia/reoxygenation that mimic OSA suggest that the proinflammatory transcription factor NF-κB is activated selectively over the hypoxia-inducible factor-1 (HIF-1) adaptive pathway in cultured endothelial cells, suggesting a maladaptive response to hypoxic stimulus in OSA (CitationRyan et al 2005; CitationGreenberg et al 2006). NF-κB upregulates several proinflammatory genes, including tumor necrosis factor-alpha (TNF-α) and IL-6 (CitationWilliams and Scharf 2007). Circulating levels of IL-6 and TNF-α are consistently elevated in patients with OSA, independently from central obesity (CitationAlam et al 2007). The initial sensing and signaling event for NF-κB activation remains unknown since it does not appear to be influenced by oxidative stress (CitationHayakawa et al 2003). In contrast, other investigators reported elevated levels of vascular endothelial growth factor (VEGF) and nocturnal erythropoietin, both mediated by the HIF-1 pathway, suggesting HIF-1 activation in patients with OSA (CitationLavie et al 2002; CitationWinnicki et al 2004). Since hypertension is associated with elevated VEGF concentrations, these discrepancies regarding activation of the adaptive HIF-1 pathway in OSA may be due to the coexistence of hypertension in some OSA patients (CitationValipour et al 2004). Alternatively, similar to COX-2 upregulation, VEGF upregulation may be an adaptive response to repetitive hypoxia/reoxygenation in OSA (CitationShweiki et al 1992; CitationForsythe et al 1996; CitationMarti et al 1998; CitationDor et al 2001).
Insummary,endothelialproinflammatory/antiinflammatory homeostasis is shifted toward vascular inflammation in patients with untreated OSA.
Endothelial oxidative stress
Recent studies have demonstrated increased lipid peroxidation and generation of reactive oxygen species (ROS) by blood cells in OSA (CitationBarcelo et al 2000; CitationChristou et al 2003a, Citation2003b; CitationLavie et al 2004; CitationJung et al 2005; CitationYamauchi et al 2005; CitationTan et al 2006). Vasoreactivity in OSA can be improved by antioxidants such as ascorbate and allopurinol, suggesting that oxidative stress contributes to endothelial dysfunction (CitationEl Solh et al 2006; CitationGrebe et al 2006). The reoxygenation/reperfusion phase of the hypoxia/reoxygenation cycle appears to promote production of ROS leading to oxidative stress in OSA (CitationDean and Wilcox 1993; CitationPrabhakar 2002; CitationLavie 2003). Short-term intermittent hypoxia enhances cardiac susceptibility to ischemia/reperfusion injury in mice whereas longer exposure does not, suggesting temporal variation in response to oxidative stress (CitationPark and Suzuki 2007). Repetitive episodes of hypoxia/reoxygenation increase production of reactive oxygen species in experimental models (CitationMcQuillan et al 1994; CitationLiao et al 1995). Exposure of lean rodents to repetitive hypoxia/reoxygenation increases lipid peroxidation and decreases tissue-scavenging mechanisms in both heart and brain tissues (CitationXu et al 2004; CitationChen et al 2005). Super-oxide rapidly scavenges NO, generating peroxynitrate, a toxic metabolite that nitrosylates tyrosine residues, forming nitrotyrosine, a marker of oxidative stress (CitationKnepler et al 2001). Levels of circulating free nitrotyrosine are similar in patients with OSA and healthy subjects (CitationSvatikova et al 2004). In contrast, expression of nitrotyrosine in endothelial cells harvested from otherwise healthy patients with OSA is greater than controls, suggesting enhanced endothelial oxidative stress (CitationJelic et al 2008). Endothelial expression of nitrotyrosine more closely reflects endothelial oxidative stress in OSA than levels of circulating free nitrotyrosine since the in vivo half-life of nitrotyrosine is short, and its volume of distribution is 20-fold greater than the plasma volume indicating its extensive distribution in the extravascular compartment (CitationTabrizi-Fard et al 1999). As endothelial oxidative stress increases and fewer cofactors are available for nitric oxide synthesis, eNOS preferentially promotes superoxide production, thereby perpetuating a vicious cycle of endothelial injury (CitationVasquez-Vivar et al 1998; CitationXia et al 1998; CitationLaursen et al 2001).
Repetitive hypoxia/reoxygenation promotes endothelial apoptosis by activating cell death receptors and mitochondria-dependent apoptotic pathways (CitationDhar-Mascareno et al 2005; CitationZhang et al 2005). Exposure of rat aortic rings to increasing concentrations of isolated endothelial apoptotic microparticles derived from cultured endothelial cells or patients with myocardial ischemia alters endothelium-dependent vasodilation and nitric oxide production while simultaneously increasing superoxide production (CitationBoulanger et al 2001; CitationBrodsky et al 2004). Furthermore, increased concentrations of endothelial apoptotic microparticles impair angiogenesis in vitro (CitationBrodsky et al 2004).
Thus, repetitive hypoxia/reoxygenation as observed in OSA adversely impacts endothelial function by promoting oxidative stress and inflammation, and reducing nitric oxide availability.
Sleep fragmentation and deprivation
Chronic sleep deprivation is associated with a 50% decline in endothelium-dependent vasodilation in healthy subjects suggesting reduced NO availability (CitationTakase et al 2004). Levels of pro-inflammatory markers such as C-reactive protein (CRP), IL-6, and TNF-α are elevated after partial and sustained sleep deprivation in healthy subjects (CitationMeier-Ewert et al 2004; CitationVgontzas et al 2004; CitationIrwin et al 2006; CitationHaack et al 2007). Levels of soluble TNF-α receptor 1 and IL-6 are elevated in healthy men after 4 days of sustained sleep deprivation, suggesting a role for sleep deprivation as a proinflammatory stimulus (CitationShearer et al 2001).
Sleep deprivation may alter coagulation homeostasis. Elevated levels of plasma D-dimer are associated with increased awake time after sleep onset in elderly subjects (CitationMausbach et al 2006; Citationvon Kanel et al 2006). Arousal index is correlated with plasma levels of von Willebrand’s factor, a mediator of platelet adhesion (Citationvon Kanel et al 2007). Wake after sleep onset time is correlated with levels of soluble tissue factor, an initiator of the coagulation cascade, after adjustment for AHI (Citationvon Kanel et al 2007).
Based on available evidence, sleep deprivation alone does not appear to promote oxidative stress. Sleep deprivation does not affect oxidant production, antioxidant enzyme activity, lipid peroxidation or protein oxidation in brain, liver, and skeletal muscle in a rat model (CitationGopalakrishnan et al 2004).
Chronic sleep deprivation is associated with greater prevalence of obesity that might itself increase cardiovascular risk in OSA. Sleep fragmentation, assessed with actigraphy, is associated with higher BMI and risk of obesity in community-dwelling elderly (Citationvan de Berg et al 2008). Acute partial sleep deprivation reduces levels of leptin, a hormone regulating satiety and energy homeostasis, elevates levels of ghrelin, a hormone associated with appetite, and increases subjective sensation of hunger, creating a weight-gaining phenotype in healthy subjects (CitationSpiegel et al 2004). Compared with longer sleep duration, self-reported sleep duration of ≤5 hours was associated with incident diabetes in 8992 subjects followed over 8–10 years (odds ratio = 1.5) (CitationGangwisch et al 2007). Self-reported reduced sleep duration in 70,000 participants in the Nurses Health Study was associated with increased cardiovascular risk over a 10-year period after adjustment for age, snoring and BMI (CitationAyas et al 2003). Acute sleep deprivation of healthy young men and women is associated with increased p-wave dispersion on electrocardiography, an electrophysiologic marker for the prediction of paroxysmal atrial fibrillation (CitationDilaveris et al 1998; CitationSari et al 2008). In addition, OSA itself has been associated with increased p-wave dispersion and incidence of atrial fibrillation (CitationGami et al 2004; CitationCan et al 2008). Sustained sleep deprivation for 36 h increased sympathetic and decreased parasympathetic activity as measured by heart rate and blood pressure variability (CitationZhong et al 2005). Sleep deprivation-induced elevated sympathetic activity may contribute to adverse cardiovascular outcomes in OSA.
In summary, chronic sleep deprivation associated with OSA may potentiate the adverse effects of hypoxia/reoxygenation on cardiovascular function.
Genetic contributions
Genetic susceptibility for cardiovascular manifestations of OSA may be mediated by gene polymorphisms associated with regulation of body weight, lipid metabolism, inflammatory response and autonomic vascular function (CitationKeavney et al 2000; CitationKadotani et al 2001; CitationPalmer et al 2003; CitationGottlieb et al 2004; CitationRiha et al 2005; Borgel et al 2006; CitationPopko et al 2007). OSA patients with and without coexistent coronary artery disease are more likely to have a family history of premature cardiovascular death than those without OSA, after adjustment for BMI (CitationGami et al 2007). In a murine model of OSA, genetic background determines both the pattern and magnitude of the chronotropic response to apnea (CitationIiyori et al 2005). Apolipoprotein E epsilon4 (ApoE epsilon4) is a well-known risk factor for cardiovascular disease (CitationSong et al 2004; CitationBennett et al 2007). The APOE epsilon4 allele is associated with increased risk of OSA, particularly in individuals under age 65 (CitationKadotani et al 2001; CitationGottlieb et al 2004).
Along with male gender, obesity is the strongest risk factor for the development of OSA (CitationPatel 2005). Obesity explains nearly 40% of the genetic heritability of OSA in a large cohort of 310 families with OSA (CitationPatel et al 2008). Levels of ghrelin, a hormone associated with appetite, decline to near normal levels following short-term CPAP therapy in obese OSA patients without concomitant change in body weight, suggesting that OSA itself promotes ghrelin production (CitationHarsch et al 2003).
The G-allele of a single nucleotide polymorphism in the pro-inflammatory IL-6 gene is associated with 6-fold increased odds of having OSA after adjustment for obesity (CitationLarkin et al 2008). Increased prevalence of the TNF-α (–308A) polymorphism, a genotype associated with increased production of the pro-inflammatory cytokine TNF-α, in OSA patients compared with population controls may reflect a propensity for enhanced inflammatory response to repetitive hypoxia/reoxygenation and sleep deprivation associated with OSA (CitationWilson et al 1997; CitationRiha et al 2005).
Reports of an association between angiotensin-converting enzyme (ACE) polymorphisms and OSA have been inconsistent. Treatment with an angiotensin II receptor blocker prevents elevation of blood pressure due to repetitive hypoxia/reoxygenation in an animal model of OSA (CitationFletcher et al 1999). The D allele and in particular the DD genotype of the ACE gene is associated with the development of hypertension and may confer an increased risk of cardiovascular disease (CitationO’Donnell et al 1998; CitationBengtsson et al 1999; CitationKeavney et al 2000). OSA increases the risk of hypertension, particularly in male carriers of the ACE gene D allele (CitationLin et al 2004; CitationBoström et al 2007). However, other investigators reported no significant correlation between AHI and ACE activity in OSA patients (CitationXiao et al 1999; Barcelo et al 2001).
Summary
Endothelial dysfunction and inflammation mediate the cardiovascular manifestations of OSA. Repetitive hypoxia/reoxygenation, as observed in OSA, adversely impacts endothelial function by promoting oxidative stress and inflammation, and reducing NO availability. Sleep fragmentation and deprivation may potentiate hypoxia/reoxygenation injury by independently promoting vascular inflammation and procoagulability. Genetic susceptibility for cardiovascular manifestations of OSA may be mediated by gene polymorphisms associated with regulation of body weight, lipid metabolism, inflammatory response and autonomic vascular function.
Disclosure
The authors report no conflicts of interest in this work.
References
- AirdWC2007Phenotypic heterogeneity of the endothelium: Representative vascular bedsCirc Res1001749017272819
- AlamILewisKStephensJW2007Obesity, metabolic syndrome and lseep apnea: all proinflammatory statesObes Rev81192717300278
- AntmanEMDeMetsDLoscalzoJ2005Cyclooxygenase inhibition and cardiovascular riskCirculation1127597016061757
- AntoniadesCShirodariaCWarrickN20065-methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase couplingCirculation114119320116940192
- ArnetUAMcMillanADinermanJL1996Regulation of endothelial nitric-oxide synthase during hypoxiaJ Biol Chem27115069738663208
- AyasNTWhiteDPMansonJE2003A prospective study of sleep duration and coronary heart disease in womenArch Intern Med163205912546611
- BarceloAElorzaMABarbeF1997Angiotensin-converting enzyme in patients with sleep apnea syndrome: plasma activity and gene polymorphismsEur Respir J177283211401071
- BarceloAMirallesCBarbeF2000Abnormal lipid peroxidation in patients with sleep apneaEur Respir J16644711106206
- BengtssonKOrho-MelanderMLindbladU1999Polymorphism in the angiotensin converting enzyme but not in the angiotensinogen gene is associated with hypertension and type 2 diabetes: the Skaraborg Hypertension and Diabetes ProjectJ Hypertens1715697510608470
- BennettAMDi AngelantonioEYeZ2007Association of apolipoprotein E genotypes with lipid levels and coronary riskJAMA29813001117878422
- BolliRShinmuraKTangXL2002Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioningCardiovasc Res555061912160947
- BoströmKBHednerJMelanderO2007Interaction between the angiotensin-converting enzyme gene insertion/deletion polymorphism and obstructive sleep apnoea as a mechanism for hypertensionJ Hypertens257798317351369
- BoulangerCMScoazecAEbrahimianT2001Circulating microparticles from patients with myocardial infarction cause endothelial dysfunctionCirculation10426495211723013
- BrodskySVZhangFNasjlettiA2004Endothelium-derived microparticles impair endothelial function in vitroAm J Physiol Heart Circ Physiol286H1910515072974
- CanIAytemirKDemirAU2008P-wave duration and dispersion in patients with obstructive sleep apneaInt J CardiolEpub ahead of print.
- CarlsonJRangemarkCHednerJ1996Attenuated endothelium-dependent vascular relaxation in patients with sleep apneaJ Hypertens14577848762200
- ChenLEinbinderEZhangQ2005Oxidative stress and left ventricular function with chronic intermittent hypoxia in ratsAm J Respir Crit Care Med1729152015976378
- ChristouKMarkoulisNMoulasAN2003aReactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patientsSleep Breath71051014569521
- ChristouKMoulasANPastakaC2003bAntioxidant capacity in obstructive sleep apnea patientsSleep Med42558
- ClarksonANLiuHRahmanR2005Clomethiazole: mechanisms underlying lasting neuroprotection following hypoxia-ischemiaFASEB J191036815809357
- CouletFNadaudSAgrapartM2003Identification of hypoxia-response element in the human endothelial nitric-oxide syn-thase gene promoterJ Biol Chem278462304012963737
- DantonGHPradoRTruettnerJ2002Endothelial nitric oxide synthase pathophysiology after nonocclusive common carotid artery thrombosis in ratsJ Cereb Blood Flow Metab22612911973434
- DeanRTWilcoxI1993Possible atherogenic effects of hypoxia during obstructive sleep apneaSleep16S15218178013
- Dhar-MascarenoMCárcamoJMGoldeDW2005Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin CFree Radic Biol Med3813112215855049
- DilaverisPEGialofosEFSiderisSK1998Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillationAm Heart J13573389588401
- DomokiFVeltkampRThrikawalaN1999Ischemia-reperfusion rapidly increases COX-2 expression in piglet cerebral arteriesAm J Physiol277H12071410484443
- DorYPoratRKeshetE2001Vascular endothelial growth factor and vascular adjustments to perturbations in oxygen homeostasisAm J Physiol280C136774
- DragerLFBortolottoLALorenziMC2005Early signs of atherosclerosis in obstructive sleep apneaAm J Respir Crit Care Med172613815901608
- DragerLFBortolottoLAFigueiredoAC2007Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apneaAm J Respir Crit Care Med1767061217556718
- DyugovskayaLLaviePLavieL2002Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patientsAm J Respir Crit Care Med1658596011934709
- DyugovskayaLLaviePLavieL2005Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apneaAnn NY Acad Sci10513405016126976
- El SolhAASalibaRBosinskiT2006Allopurinol improves endothelial function in sleep apnea: a randomized controlled studyEur Respir J27997100216707395
- El SolhAAAkinnusiMEBaddouraFH2007Endothelial cell apoptosis in obstructive sleep apnea. A link to endothelial dysfunctionAm J Respir Crit Care Med17511869117272785
- El SolhAAAkkinusiMEBerimIG2008Hemostatic implications of endothelial cell apoptosis in obstructive sleep apneaSleep BreathEpub ahead of print.
- EslerMEikelisN2006Is obstructive sleep apnea the cause of sympathetic nervous activation in human obesity?J Appl Physiol10011216357080
- FletcherECLesskeJQianW1992Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in ratsHypertension19555611592450
- FletcherCBaoGLiR1999Renin activity and blood pressure in response to chronic episodic hypoxiaHypertension343091410454459
- ForsytheJAJiangBHLyerNV1996Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1Mol Cell Biol164604138756616
- GamiASPressmanGCaplesSM2004Association of atrial fibrillation and obstructive sleep apneaCirculation110364715249509
- GamiASRaderSSvatikovaA2007Familial premature coronary artery disease mortality and obstructive sleep apneaChest1311182117218564
- GangwischJEHeymsfieldSBBoden-AlbalaB2007Sleep duration as a risk factor for diabetes incidence in a large US sampleSleep3016677318246976
- Garcia-CardenaGFanRShahV1998Dynamic activation of endothelial nitric oxide synthase by Hsp90Nature39282149580552
- GilroyDWColville-NashPRWillisD1999Inducible cyclooxygenase may have anti-inflammatory propertiesNat Med569870110371510
- GopalakrishnanAJiLLCirelliC2004Sleep deprivation and cellular responses to oxidative stressSleep27273514998234
- GottliebDJDeStefanoALFoleyDJ2004APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health StudyNeurology63664815326239
- GrebeMEiseleHJWeissmannN2006Antioxidant vitamin C improves endothelial function in obstructive sleep apneaAm J Respir Crit Care Med17389790116439717
- GreenbergHYeXWilsonD2006Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivoBiochem Biophys Res Commun343591616554025
- GuilleminaultCPartinenMHollmanK1995Familial aggregates in obstructive sleep apnea syndromeChest1071545517781344
- HaackMSanchezEMullingtonJM2007Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteersSleep3011455217910386
- HarschIAKonturekPCKoebnickC2003Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatmentEur Respir J2251712952256
- HayakawaMMiyashitaHSakamotoI2003Evidence that reactive oxygen species do not mediate NF-kappaB activationEMBO J2233566612839997
- IiyoriNShirahataMO’DonnellCP2005Genetic background affects cardiovascular responses to obstructive and simulated apneaPhysiol Genomics24657216249313
- IpMSMLamBChanLY2000Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressureAm J Respir Crit Care Med16221667111112132
- IpMSTseHFLamB2004Endothelial function in obstructive sleep apnea and response to treatmentAm J Respir Crit Care Med1693485314551167
- IrwinMWangMCampomayorCO2006Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammationArch Intern Med16617566216983055
- JelicSPadelettiMKawutSM2008Inflammation, oxidative stress and repair capacity of the vascular endothelium in obstructive sleep apneaCirculation1172270818413499
- JungHHHanHLeeJH2005Sleep apnea, coronary artery disease, and antioxidant status in hemodialysis patientsAm J Kidney Dis458758215861353
- KadotaniHKadotaniTYoungT2001Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adultsJAMA2852228889011401610
- KatoMRoberts-ThomsonPPhillipsBG2000Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apneaCirculation10226071011085964
- KeavneyBMcKenzieCParishS2000Large-scale test of hypothesised associations between the angiotensin converting-enzyme insertion/deletion polymorphism and myocardial infarction in about 5000 cases and 6000 controlsLancet3554344210841123
- KissHSchneebergerCTschugguelW1998Expression of endothelial (type III) nitric oxide synthase in cytotrophoblastic cell lines: regulation by hypoxia and inflammatory cytokinesPlacenta19603119859864
- KneplerJLJrTaherLNGuptaMP2001Peroxynitrite causes endothelial cell monolayer barrier dysfunctionAm J Physiol Cell Physiol281C10647511502585
- KuzkayaNWeissmannNHarrisonDG2003Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthaseJ Biol Chem278225465412692136
- LarkinEKPatelSRJennyNS2008A coding polymorphism for interleukin-6 (IL6) is associated with sleep apneaAm J Respir Crit Care Med177A213
- LaufsUFataVLLiaoJK1997Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthaseJ Biol Chem2723172599395516
- LaursenJBSomersMKurzS2001Endothelial regulation of vasomotion in ApoE deficient mice. Implications for interactions between peroxynitrite and tetrahydrobiopterinCirculation1031282811238274
- LavieLKraicziHHefetzA2002Plasma vascular endothelial growth factor in sleep apnea syndromeAm J Respir Crit Care Med1651624812070063
- LavieL2003Obstructive sleep apnea syndrome – An oxidative stress disorderSleep Med Rev7355112586529
- LavieLHefetzALuboshitzkyR2003Plasma levels of nitric oxide and L-arginine in sleep apnea patients: effects of nCPAP treatmentJ Mol Neurosci21576314500996
- LavieLVishnevskyALavieP2004Evidence for lipid peroxidation in obstructive sleep apneaSleep27123814998248
- Le CrasTDTylerRCHoranMP1998Effects of chronic hypoxia and altered hemodynamics on endothelial nitric oxide synthase expression in the adult rat lungJ Clin Invest1017958019466974
- LiRCRowBWGozalE2003Cyclooxygenase 2 and intermittent hypoxia-induced spatial deficits in the ratAm J Respir Crit Care Med1684697512773326
- LiaoJKZuluetaJJYuFS1995Regulation of bovine endothelial constitutive nitric oxide synthase by oxygenJ Clin Invest96266168675632
- LinLFinnLZhangJ2004Angiotensin-converting enzyme, sleep-disordered breathing, and hypertensionAm J Respir Crit Care Med17013495315447944
- MarinJMCarrizoSJVicenteE2005Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnea with or without treatment with continuous positive airway pressure: an observational studyLancet36510465315781100
- MartiHHRisauW1998Systemic hypoxia changes the organ specific distribution of vascular endothelial growth factor and its receptorsProc Natl Acad Sci U S A9515809149861052
- MartinKStanchinaMKouttabN2008Circulating endothelial cells and endothelial progenitor cells in obstructive sleep apneaLung1861455018401642
- MaserRELenhardMJRizzoAA2008Continuous positive airway pressure therapy improves cardiovascular autonomic function for persons with sleep-disordered breathingChest133869117951618
- MausbachBTAncoli-IsraelSvon KänelR2006Sleep disturbance, norepinephrine, and D-dimer are all related in elderly caregivers of people with Alzheimer diseaseSleep2913475217068989
- McQuillanLPLeungGKMarsdenPA1994Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanismsAm J Physiol267H192177526714
- Meier-EwertHKRidkerPMRifaiN2004Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular riskJ Am Coll Cardiol436788314975482
- MillsPJKennedyBPLoredoJS2006Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apneaJ Appl Physiol100343816357087
- MinoguchiKYokoeTTazakiT2005Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apneaAm J Respir Crit Care Med1726253016120716
- MurataTYamawakiHHoriM2001Hypoxia impairs endothelium-dependent relaxation in organ cultured pulmonary arteryEur J Pharmacol421455311408048
- NarkiewiczKKatoMPhillipsBG1999Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apneaCirculation1002332510587337
- NarkiewiczKSomersVK2003Sympathetic nerve activity in obstructive sleep apneaActa Physiol Scand1773859012609010
- NodaANakataSKoikeY2007Continuous positive airway pressure improves daytime baroreflex sensitivity and nitric oxide production in patients with moderate to severe obstructive sleep apnea syndromeHypertens Res306697617917313
- O’DonnellCJLindpaintnerKLarsonMG1998Evidence for association and genetic linkage of the angiotensin converting enzyme locus with hypertension and blood pressure in men but not in women in the Framingham Heart StudyCirculation971766729603529
- OhgaENagaseTTomitaT1999Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndromeJ Appl Physiol8710410409552
- OhikeYKozakiKIijimaK2005Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure – possible involvement of nitric oxide and asymmetric NG, NG-dimethylarginineCirc J69221615671617
- PalmerLJBuxbaumSGLarkinE2003A whole-genome scan for obstructive sleep apnea and obesityAm J Hum Genet7223405012506338
- ParkAMSuzukiYJ2007Effects of intermittent hypoxia on oxidative stress-induced myocardial damage in miceJ Appl Physiol10218061417272404
- PatelSR2005Shared genetic risk factors for obstructive sleep apnea and obesityJ Appl Physiol991600616160021
- PatelSRLarkinEKRedlineS2008Shared genetic basis for obstructive sleep apnea and adiposity measuresInt J Obes (Lond)3279580018209735
- PekerYKraicziHHednerJ1999An independent association between obstructive sleep apnoea and coronary artery diseaseEur Respir J131798410489848
- PeppardPEYoungTPaltaM2000Prospective study of the association between sleep-disordered breathing and hypertensionN Engl J Med34213788410805822
- PhelanMWFallerDV1996Hypoxia decreases constitutive nitric oxide synthase transcript and protein in cultured endothelial cellsJ Cell Physiol167469768655601
- PopkoKGorskaEWasikM2007Frequency of distribution of leptin receptor gene polymorphism in obstructive sleep apneaJ Physiol Pharmacol585516118204169
- PrabhakarNR2002Sleep apneas: an oxidative stress?Am J Respir Crit Care Med1658596011934709
- PriceDTLoscalzoJ1999Cellular adhesion molecules and atherogenesisAm J Med107859710403357
- RedlineSTostesonTTishlerPV1992Studies in the genetics of obstructive sleep apnea: familial aggregation of symptoms associated with sleep-related breathing disturbancesAm Rev Respir Dis14544041736754
- RedlineSTishlerPVTostesonTD1995The familial aggregation of obstructive sleep apneaAm J Respir Crit Care Med15168277881656
- RihaRLBranderPVennelleM2005Tumor necrosis factor-α (−308) gene polymorphism in obstructive sleep apnea-hypopnea syndromeEur Respir J26673816204600
- RyanSTaylorCTMcNicholasWT2005Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndromeCirculation1122660716246965
- SaletuMNosiskaDKapfhammerG2006Structural and serum surrogate markers of cerebrovascular disease in obstructive sleep apnea (OSA): association of mild OSA with early atherosclerosisJ Neurol2537465216511651
- SariIDavutogluVOzbalaB2008Acute sleep deprivation is associated with increased electrocardiographic p-wave dispersion in healthy young men and womenPacing Clin Electrophysiol314384218373762
- SavranskyVNanayakkaraALiJ2007Chronic intermittent hypoxia induces atherosclerosisAm J Respir Crit Care Med1751290717332479
- SchulzRMahmoudiSHattarK2000Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apneaAm J Respir Crit Care Med1625667010934088
- ShearerWTReubenJMMullingtonJM2001Soluble TNF-α receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflightJ Allergy Clin Immunol1071657011150007
- ShamsuzzamanASWinnickiMLanfranchiP2002Elevated C-reactive protein in patients with obstructive sleep apneaCirculation1052462412034649
- ShiraiMPearsonJTShimouchiA2003Changes in functional and histological distributions of nitric oxide synthase caused by chronic hypoxia in rat small pulmonary arteriesBr J Pharmacol13989991012839863
- ShweikiDItinASofferD1992Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesisNature35984351279431
- SongYStampferMJLiuS2004Meta-analysis: apolipoprotein E genotypes and risk for coronary heart diseaseAnn Intern Med1411374715262670
- SpiegelKTasaliEPenevPVan CauterE2004Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetiteAnn Intern Med1418465015583226
- StrohlKPSandersNAFeldmanNT1978Obstructive sleep apnea in family membersN Engl J Med29996973211413
- SuYBlockER2000Role of calpain in hypoxic inhibition of nitric oxide synthase activity in pulmonary endothelial cellsAm J Physiol Lung Cell Mol Physiol278L12041210835326
- SvatikovaAWolkRWangHH2004Circulating free nitrotyrosine in obstructive sleep apneaAm J Physiol Regul Integr Comp Physiol287R284715142836
- Tabrizi-FardMAMaurerTSFungHL1999In vivo disposition of 3-nitro-L-tyrosine in rats: implications on tracking systemic peroxynitrite exposureDrug Metab Dispos274293110232929
- TahawiZOrolinovaNJoshuaIG2001Altered vascular reactivity in arterioles of chronic intermittent hypoxic ratsJ Appl Physiol9020071311299297
- TaiSCRobbGBMarsdenPA2004Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vesselArterioscler Thromb Vasc Biol244051214656742
- TakemotoMSunJHirokiJ2002Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthaseCirculation106576212093770
- TakaseBAkimaTUehataA2004Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humansClin Cardiol27223715119699
- TanKCChowWSLamJC2006HDL dysfunction in obstructive sleep apneaAtherosclerosis1843778215975582
- TanakaTNakamuraHYodoiJ2005Redox regulation of the signaling pathways leading to eNOS phosphorylationFree Radic Biol Med3812314215808421
- ThomasSRChenKKeaneyJFJr2002Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinasedependent signaling pathwayJ Biol Chem27760172411744698
- ToporsianMGovindarajuKNagiM2000Downregulation of endothelial nitric oxide synthase in rat aorta after prolonged hypoxia in vivoCirc Res86671510747003
- ValipourALitschauerBMittermayerF2004Circulating plasma levels of vascular endothelial growth factor in patients with sleep disordered breathingResp Med9811806
- Van de BergJFNevenAKTulenJHM2008Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam StudyInt J Obes (Lond)3210839018414418
- Vasquez-VivarJKalyanaramanBMartasekP1998Superoxide generation by endothelial nitric oxide synthase: the influence of cofactorsProc Natl Acad Sci U S A95922059689061
- VgontzasANZoumakisEBixlerEO2004Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokinesJ Clin Endocrinol Metab8921192615126529
- von KänelRDimsdaleJEAncoli-IsraelS2006Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer’s diseaseJ Am Geriatr Soc54431716551309
- von KänelRLoredoJSAncoli-IsraelS2007Association between polysomnographic measures of disrupted sleep and prothrombotic factorsChest131733917356087
- WangPZweierJL1996Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. Evidence for peroxynitrite-mediated reperfusion injuryJ Biol Chem27129223308910581
- WilliamsAScharfSM2007Obstructive sleep apnea, cardiovascular disease, and inflammation – is NF-kappaB the key?Sleep Breath11697617380355
- WilsonAGSymonsJAMcDowellTL1997Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcription activationProc Natl Acad Sci U S A94319599096369
- WinnickiMShamsuzzamanALanfranchiP2004Erythropoietin and obstructive sleep apneaAm J Hypertens17783615363820
- WuGMannamAPWuJ2003Hypoxia induces myocyte-dependent COX-2 regulation in endothelial cells: role of VEGFAm J Physiol Heart Circ Physiol285H2420912881220
- XiaYTsaiALBerkaVZweierJL1998Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory processJ Biol Chem2732580489748253
- XiaoYHuangXQiuC1999Angiotensin 1-converting enzyme gene polymorphism in Chinese patients with obstructive sleep apnea syndromeChinese Med J1127014
- XuWChiLRowBw2004Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep apneaNeuroscience1263132315207349
- YaggiHKConcatoJKernanWN2005Obstructive sleep apnea as a risk factor for stroke and deathN Engl J Med35320344116282178
- YamauchiMNakanoHMaekawaJ2005Oxidative stress in obstructive sleep apneaChest1271674915888845
- YoungTPaltaMDempseyJ1993The occurrence of sleep-disordered breathing among middle-aged adultsN Engl J Med328123058464434
- YoungTEvansLFinnL1997Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and womenSleep2070569406321
- YuminoDTsurumiYTakagiA2007Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndromeAm J Cardiol99263017196456
- ZhangYZhangXParkTS2005Cerebral endothelial cell apoptosis after ischemia-reperfusion: role of PARP activation and AIF translocationJ Cereb Blood Flow Metab258687715729291
- ZhongXHiltonHJGatesGJ2005Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivationJ Appl Physiol9820243215718408