Abstract
Gadofosveset (Vasovist®, Bayer Schering Pharma AG, Berlin/Germany) is the first intravascular contrast agent approved for use with magnetic resonance angiography in the European Union, Switzerland, Turkey, Canada, and Australia. Gadofosveset reversibly binds to albumin providing extended intravascular enhancement compared wth existing extracellular magnetic resonance contrast agents. Prior to approval, gadofosveset underwent extensive testing to evaluate the safety and efficacy of the drug; the clinical trials show that gadofosveset-enhanced magnetic resonance angiography (MRA) is safe and well tolerated in patients with vascular disease and effective for the detection of vascular stenosis and aneurysms gadofosveset has the potential to open new horizons in diagnostic MRA by increasing the spatial resolution and the robustness of MRA examinations and facilitating the examination of multiple vascular beds.
Introduction
Atherosclerosis is a generalized disease and contributes to cardiac death, stroke, limb loss, and a range of other illnesses. Disease in the major arteries, including the infra-renal abdominal aorta, internal iliac arteries, renal arteries, and peripheral vasculature remains a major cause of morbidity and mortality. For example, the prevalence of disease in the infra-renal abdominal aorta ranges from <3% in patients <60 years old to 20% in patients ≥75 years (CitationCriqui et al 1985; CitationVogt et al 1992), and the incidence increases with increasing age. As the average age of the population increases, the burden of vascular disease is expected to increase.
Until recently, conventional X-ray angiography (XRA) requiring arterial catheterization and the use of substantial volumes of iodinated contrast agent was the clinical standard practice when a detailed image of the vasculature was required; however, less invasive imaging techniques using X-ray computed tomography (CT) or magnetic resonance imaging (MRI) have been developed. These imaging methods, either without exogenous contrast agents (MRI only) or with exogenous contrast agents (both CT and MRI) have become increasingly popular over the past few years as data have suggested that their accuracy, in some clinical settings, might approach that of the accepted standard diagnostic method, catheter X-ray angiography (XRA) using iodinated contrast agents (CitationRieker et al 1997; CitationGrist 2000; CitationTan et al 2002).
MRI is a safe, non-invasive, and widely available imaging technique that has experienced rapid growth over the past decade. Magnetic resonance angiography (MRA), as a more recent development in MRI, uses tailored acquisition sequences to highlight blood flow and is widely used to assist in the management of patients with vascular diseases, especially in the brain. In many vascular beds like peripheral vessels, however, non-contrast MRA is not used routinely in clinical practice due to shortcomings of unenhanced MRA.
What is gadofosveset?
Gadofosveset is the first intravascular contrast agent approved for use with MRA in the European Union, Switzerland, Turkey, Canada, and Australia. Gadofosveset reversibly binds to human serum albumin, providing significantly higher relaxivity and extended intravascular enhancement compared to existing extracellular magnetic resonance contrast agents ().
Table 1 Relaxivity (r1) in human plasma at 37 °C (mM-1s-1)
MS-325 is the product development code for the drug product containing trisodium-{(2- (R)-(4,4-diphenylcycloh exyl)phosphonooxymethyl)-diethylenetriaminepentaacetato) (aquo) gadolinium(III)} (INN (international non-proprietary name) = gadofosveset trisodium) as the active substance. Gadofosveset is commercially available as Vasovist® (Bayer Schering Pharma AG, Berlin/Germany). Gadofosveset injection is composed of an aqueous solution (244 mg/mL, 0.25 mmol/L) of drug substance, gadofosveset trisodium, and a small amount of ligand excipient, fosveset, to ensure minimal free gadolinium in solution. The drug substance consists of a stable gadolinium diethylenetriaminepentaacetic acid (Gd-DTPA) chelate substituted with a diphenylcyclohexyl-phosphate group. Gadofosveset injection is a clear, colorless to slightly yellow solution in which the pH has been adjusted to 6.5 to 8.0. The density is 1.12 g/mL and the osmolality ranges from 700 to 950 mOsm/kg at 37 °C. The viscosity of MS-325 injection ranges from 2.7 to 3.3 cps at 20 °C. The molecular formula is C33H40GdN3Na3O15P and the molecular weight for the anhydrous form is 975.88. Gadofosveset is administered either by a hand or power injector to deliver a dose of 0.03 mmol/kg (in 7–20 seconds).
Overview of the clinical development program
Prior to approval in the European Union, gadofosveset underwent extensive evaluation of the safety and efficacy of the drug. The clinical development program for efficacy included two phase II studies and four phase III studies. In phase II studies, approximately 300 patients were evaluated to define optimal dose for MRA. The optimal dose for MRA was found to be 0.03 mmol/kg.
The clinical effectiveness of gadofosveset was demonstrated through analysis of efficacy data of 672 patients that were included in four adequate and well-controlled phase III studies. Vascular beds representative of areas of turbulent blood flow (AIOD: aorto-iliac occlusive disease), flow to an organ (renal artery disease), and slow flow (pedal arterial disease) were studied. In all of these studies, the fundamental methodology was the same:
Each study was designed to evaluate and quantify the improvement provided by the administration of gadofosveset compared with the MRA device alone (TOF-MRA) using XRA as the standard of reference (SOR);
All images were acquired using prospectively defined imaging protocols;
The efficacy evaluation of all studies included blinded reading and 3 independent blinded readers for each study were used. These blinded readers had no prior affiliation with the sponsor, and had not participated in other gadofosveset studies;
A total of 32 independent blinded readers (24 radiologists, 8 vascular surgeons) were used in the four studies. Vascular surgeons were asked to evaluate the images for management decisions;
Images were randomized and no other clinical information was provided to the blinded readers;
Three different blinded readers evaluated XRA images to develop the SOR (2 readers plus 1 adjudicator). No consensus reading was performed in determining the SOR.
Summary of efficacy results of four phase III studies
Gadofosveset reduced the rate of uninterpretability significantly and improved the diagnostic confidence (CitationGoyen et al 2005; CitationRapp et al 2005). Fewer than 2.3% were uninterpretable for gadofosveset-enhanced MRA, versus approximately 16% for 2D-TOF. For comparison, 2.8% of the vessels were deemed uninterpretable on XRA. For all readers in all studies, the vascular surgeon readers agreed with XRA more often when using gadofosveset-enhanced MRA than when using pre-contrast MRA (range of improvements: 1%–36%). For six of the eight readers, the improvement was substantial (>15%) and statistically significant (p < 0.001).
Safety data for gadofosveset
Safety data in 767 patients (505 males and 262 females) receiving 0.03 mmol/kg bw dose have been reported. There were no clinically significant trends found in adverse events, laboratory assays, vital signs, ECGs, or oxygen saturation. Gadofosveset has a good safety profile and can be safely administered as an intravenous bolus injection. The overall rate and experience of adverse events was comparable to placebo and were similar to that reported in clinical trials for other Gd chelates.
Clinical applications of gadofosveset-enhanced MRA
Considering the enormous success of extracellular gadolinium-based contrast agents for contrast-enhanced MRA, what is likely to be the role for an intravascular contrast agent such as gadofosveset? The answer lies in the key question: can gadofosveset be used in first-pass (arterial) imaging with equal effect? As the answer appears to be yes, this suggests that gadofosveset can be used instead of the current extracellular contrast agents for first-pass arterial imaging (CitationKlessen et al 2007) (𠈓). The crucial advantage of gadofosveset, ie, the presence of persistent high intravascular enhancement significantly greater than with extracellular agents, can be exploited to acquire additional high-resolution images in the steady-state which lead to a better delineation of vessel pathology. Steady-state imaging offers the possibility of depicting the entire vascular system without relevant extravasation of the contrast medium from the intravascular space. The extended diagnostic window of gadofosveset makes the examination more convenient because it is less dependent on the bolus dynamics.
Figure 1 Gadofosveset-enhanced MRA data of a 30-year old healthy proband: after contrast agent injection and by using new multi-channel MR scanners, a MRA in dynamic or first-pass phase with purely arterial contrast can be realized for both the carotid arteries and the lower thigh arteries (A, spatial resolution: 1.0 mm3 voxel size, subtracted maximum-intensity projections (MIP), acquisition time: 29 seconds). In the following acquisition performed during the equilibrium phase, very high spatial resolutions of below 0.1 mm3 voxel size can be achieved, which, at a satisfactory signal-to-noise ratio, permit sufficient differentiation between arterial and venous vascular structures despite the small vascular diameter and the close proximity of the structures (B, C (enlarged view), 0.074 mm3 voxel size, acquisition time: 6:15 minutes, arrows: veins; arrow heads: arteries). Image: courtesy Konstantin CitationNikolaou, reproduced from Mathias Goyen (ed.). 2006. Vasovist – the first intravascular contrast agent for MR angiography. ABW-Wissenschaftsverlag Berlin.
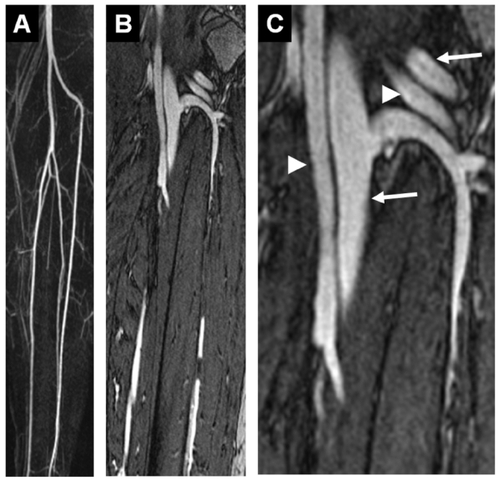
Figure 2 Example of a healthy proband whole-body MRA: compilation of a gadofosveset-enhanced whole-body MRA with acquisition of the carotid arteries (A) and the lower thigh arteries (B) in the dynamic and first-pass phase (for acquisition parameter refer to ), as well as acquisition of the thoracic area (C, voxel size 1.0 mm3, acquisition time: 37 seconds), abdomen (D, voxel size 1.0 mm3, acquisition time: 35 seconds). The signal-to-noise ratio that can be achieved in the equilibrium phase is even sufficient for satisfactory imaging of the coronary arteries (E, voxel size: 0.7 mm3, navigator technology, acquisition time: 3:30 min). Ao: aorta; arrow: right coronary artery. Image courtesy Konstantin CitationNikolaou, reproduced from Mathias Goyen (ed.). 2006. Vasovist – the first intravascular contrast agent for MR angiography, ABW-Wissenschaftsverlag Berlin.
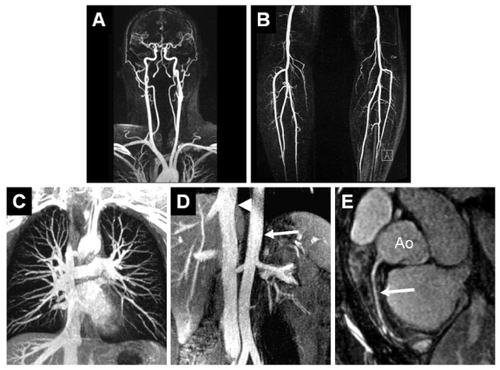
Figure 3 Sixty-four-year-old male presented with known peripheral vascular disease. The MRA suggests an occlusion of the fibular artery at the level of the distal calf on the first-pass image: the vessel cannot be identified even on the curved MPR (A) due to venous overlay (A). However, curved MPR of the higher spatial resolution steady-state images (B allows for clear identification of the distal fibular artery next to the fibular vein and allows for depiction of the distal collateral filling of the posterior tibial artery. Image courtesy Winfried Willinek, Department of Radiology, University of Bonn/Germany).
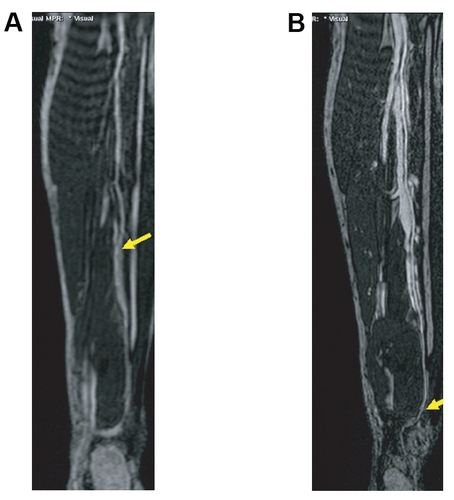
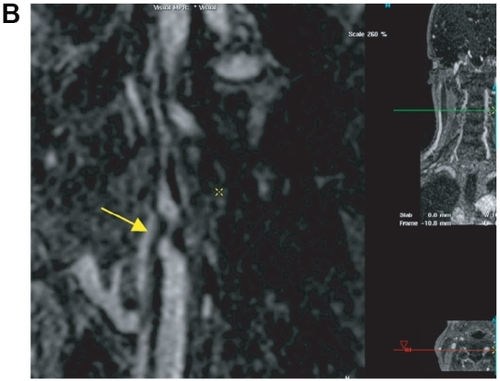
Figure 4 Patient with symptomatic abdominal aortic aneurysm referred for peripheral MRA. The high grade stenosis of the left internal carotid artery was confirmed by 64 multi-slice CTA and the patient was discharged after successful thrombendarterectomy and aneurysm repair. After first-pass MRA of the abdomen and lower extremities (A), an ultra-sonographically suspected stenosis of the left internal carotid artery is confirmed by the 0.66 mm isotropic resolution steady-state gadofosveset-enhanced MRA (B, arrow). This approach facilitates the pre-operative work-up of patients with systemic vascular disease without the need of a second contrast injection or a separate MR-examination. Image courtesy Winfried Willinek, Department of Radiology, University of Bonn/Germany.

General considerations
Gadofosveset can be used in exactly the same way as extracellular agents with regard to first-pass imaging. The advantage of using gadofosveset for first-pass imaging lies in its much higher relaxivity (CitationRohrer et al 2005). This means that a higher signal-to-noise ratio can be obtained when parameters are kept identical or, conversely, that spatial resolution can be increased while maintaining the same signal-to-noise ratio. The truly interesting property of gadofosveset, however, is its much longer intravascular residence time. Equilibrium imaging is possible because, despite the fact that dilution of the injected contrast medium after first arterial passage leads to a T1 increase of the blood pool compared with the first pass, the value is still much lower than that of fat. CitationHartmann et al (2006) estimated that T1 of blood in the equilibrium phase, 3–5 minutes after injection of 0.03 mmol/kg gadofosveset, is about 130 ms, increasing to about 150 ms after 10–15 minutes. This prolonged T1 reduction offers the opportunity to obtain images of the vascular tree up to about 45–60 minutes after injection. The extended imaging window can be used to acquire images with much higher spatial resolution without a significant loss of vessel-to-background contrast (). In clinical practice this means that scan duration is no longer determined by the transient T1 shortening, but by the capacity of the patient to sustain a breath-hold or to remain motionless. A possible drawback of using gadofosveset is the simultaneous enhancement of venous structures close to arteries. This phenomenon is a well-known problem at first-pass imaging, often resulting in images that cannot be used for clinical decision-making. However, because equilibrium phase images can be acquired at much higher spatial resolution – often with a 5–15-fold decrease in voxel size compared with first-pass protocols – arteries can be readily separated from accompanying veins.
Practical aspects
The use of gadofosveset has reduced the deleterious consequences of missing the bolus in the first pass. If, for whatever reason, acquisition in the first pass fails, images can always be obtained in the equilibrium phase because of the prolonged intravascular retention. Although prolonged intravascular retention is highly advantageous, it is not recommended to perform a test bolus when using gadofosveset because of this property. If possible, it is better to acquire a dynamic series of acquisitions using a time-resolved MRA technique and to evaluate the data set with the best selective arterial opacification. The most commonly used format to display 3-D MRA data is the maximum intensity projection (MIP). Although MIP is an elegant way to collapse a 3-D volumetric data set into a 2-D projection, review of cross-sectional images remains an integral part of the evaluation, especially for data acquired in the equilibrium phase. The MIP algorithm works best when using thin-slab or curved subvolume selections. In whole-volume MIPs, contrast-enhancing organs or other vascular structures may superimpose over smaller arteries when they have higher signal intensities along a particular viewing path. When working with equilibrium-phase images, the use of thin-slab sub-volume MIPs can be particularly useful. Another helpful technique for the precise evaluation of vessel morphology, especially when evaluating equilibrium phase data, is curved multiplanar reformation (cMPR) along the axis of the arterial segment of interest. Most post-processing workstations offer the ability to interactively generate a cMPR while scrolling through source images. This technique is particularly useful to obtain views of eccentric stenoses, and as a basis to generate views perpendicular to the central axis of the vessel to measure cross-sectional area reduction in stenoses.
Gadofosveset-enhanced MRA of the abdomen
For the diagnostic assessment of the abdominal vasculature, contrast-enhanced (CE)-MRA – together with computed tomography angiography (CTA) – has become the clinically accepted standard of reference and has replaced conventional digital subtraction angiography. In abdominal aortic aneurysm or dissection, CE-MRA allows a simultaneous assessment of the aneurysmal extent and involvement of the renal, visceral or iliac arteries. With gadofosveset, the first pass of the compound can be used for time-resolved MRA, allowing a dynamic assessment of the perfusion of the visceral organs and visualization of blood-flow differences in the true and false lumen of aortic dissection (CitationSchoenberg et al 1999). In patients with endovascular repair of abdominal aortic aneurysm, blood-pool agent-enhanced MRA might be more accurate for the detection of endoleakage than contrast-enhanced CT (CitationErsoy et al 2004). In patients with atherosclerotic disease, the potential to increase the spatial resolution during the steady state promises a higher accuracy for the detection of vascular stenoses than conventional MRA with extracellular contrast agents. In patients with aortoiliac disease, CitationVogt et al (2007) reported a higher agreement regarding stenosis location and degree of stenosis of gadofosveset-enhanced MRA and DSA compared with a non-contrast, time-of-flight MRA.
In a study by CitationNikolaou et al (2006), gadofosveset-enhanced MRA showed a sensitivity of 97%–100% and specificity of 96%–100% for the assessment of high-grade stenosis in different vascular territories (eg, carotid or renal arteries) compared with the clinical standard of reference. In this study the intermodality agreement between the gadofosveset-enhanced imaging and reference CE-standard imaging data was 90%–93%.
CE-MRA is the clinical gold standard for the detection of renal artery stenosis (RAS). Due to the limited breath-hold capacity of the patients, imaging has to take place during suspended breath-hold, limiting acquisition time and thereby reducing the spatial resolution. While proximal RAS can still be safely detected, fibromuscular dysplasia (FMD) may potentially evade detection. Gadofosveset as a blood pool contrast agent may overcome this limitation of MRA by facilitating longer respiratory-gated MRA acquisitions. Due to the widespread use of 3-D post-processing tools, the venous signal does not interfere with diagnostic image reading. In addition, the first pass of the contrast agent can be used to measure renal perfusion (CitationMichaely et al 2006). Due to the high relaxivity of gadofosveset, only a fraction of the amount of gadolinium that would be required for extracellular contrast agents is needed. This is of particular interest in view of the recently described disease ‘nephrogenic systemic fibrosis’, which is more likely to occur when higher doses of gadolinium are administered (CitationLeiner et al 2007). Gadofosveset may also be valuable in patients who are being evaluated as potential renal donors, as the entire abdomino-pelvic arterial and venous system can be examined after a single injection of contrast agent. This eases workflow and should eventually be more cost-efficient.
Gadofosveset-enhanced MRA of the thorax
The assessment of the pulmonary arteries for pulmonary embolism is an interesting application for gadofosveset-enhanced MRA. Although CT is nowadays the first-line imaging tool for the assessment of patients with suspected pulmonary embolism, contrast-enhanced MRI is very appealing as it allows for a comprehensive radiation-free assessment of pulmonary perfusion and direct visualization of embolic material in the pulmonary arteries using a single contrast agent injection. Moreover, MR venography of the deep venous system can be added for the assessment of underlying deep venous thrombosis without an additional contrast agent (CitationFink et al 2004). Recently, an animal study indicated that the higher relaxivity of a blood pool contrast agent together with the increased signal-to-noise ratio of 3 T effectively supports highly accelerated, parallel acquisition, time-resolved pulmonary MRA (CitationNael et al 2007).
For cardiac imaging, the direct visualization of coronary arteries is a major challenge for MRI. Although multislice-CT coronary angiography is now the preferred non-invasive imaging technique for the assessment of coronary disease, blood-pool contrast agents may change the role of CE-MRA. Animal and volunteer studies of coronary MRA using blood-pool agents have been reported by different authors using different blood-pool contrast agents. Though heterogeneous in their set-up, all studies reported a significant advantage in coronary imaging compared with extracellular contrast agents (CitationShoenberg et al 1999; CitationLi et al 2001; CitationHuber et al 2003; CitationSakuma et al 2005; CitationZheng et al 2005). A final assessment of the clinical value of this method is still pending.
Gadofosveset-enhanced MRA of the peripheral vasculature/whole-body MRA
CE-MRA of the peripheral vasculature has evolved over the past few years from an experimental imaging modality to a technique that is now widely applied in clinical practice. The recent introduction of gadofosveset expands the diagnostic armamentarium of the radiologist by opening up new opportunities in the field of peripheral MRA. The higher relaxivity and prolonged intravascular residence time of gadofosveset yield better first-pass image quality, as well as the possibility of obtaining additional steady-state MRA data. The latter property will lead to a fundamental paradigm shift in MR imaging of the vasculature, enabling the migration to equilibrium-phase ultra-high spatial resolution imaging sequences. Initially, there were concerns in regard to the presence of venous overlay on the steady-state 3D-CE-MRA data sets, particularly for arteries with small vessel calibre and close-by coursing veins such as in the distal calves. However, with today’s optimized surface coils and the use of PAT, isotropic voxel sizes of less than 100 μm3 can now be acquired in reasonable scan time of approximately 5 min (CitationNikolaou et al 2006) (, ). Due to the absence of motion artefacts in the peripheral arteries, exquisite image quality can be achieved allowing already for visual artery-vein separation despite the close proximity of theses vessels. Artery-vein separation can be further enhanced by the use of semi-automated software, which is currently under preparation by different vendors. Gadofosveset-enhanced MR imaging of the venous system is ideally suited to the detection of venous thromboembolic disease. Deep venous thrombi, even below the knee, are readily detected (CitationLi et al 2007) Furthermore, in patients with massive thromboembolic occlusion of central veins, the collateral pathways can reliably be visualized in a fashion that is superior to conventional X-ray-based venography.
With the introduction of whole-body MRI scanners with multiple independent rf-channels and inbuilt coil systems for large anatomic coverage this approach becomes attractive for assessment of the entire vasculature by a combination of multi-station first-pass 3D-CE-MRA and steady-state 3D-CE-MRA. This shifts the focus of MRA away from solely displaying vascular anatomy to a more disease specific imaging approach. One example includes the systemic assessment of atherosclerotic disease, which has been shown as an arising application for screening of cardiovascular risk factors in preliminary studies (CitationKramer et al 2005) (-). This systemic angiographic assessment is also of high interest for patients with vasculitis such as Takayasu arteriitis that manifests in multiple different locations. Here, the combination of first-pass 3D-CE-MRA of the carotid and renal arteries in combination with high-resolution assessment of the inflammatory stenoses could improve the overall diagnostic work-up of the patients and replace a more time-intensive, multi-step, multi-modality approach.
Conclusion
Gadofosveset-enhanced MRA is safe and well tolerated in patients with vascular disease, effective for the detection of vascular stenosis and aneurysms, significantly more accurate (both more sensitive and specific) than non-contrast MRA for the diagnosis of vascular stenoses, and similar to conventional angiography for the overall characterization of vascular disease, without the need for catheterization. Gadofosveset has the potential to open new horizons in diagnostic MRA by increasing the spatial resolution and the robustness of MRA examinations and facilitating the examination of multiple vascular beds.
References
- CriquiMHFronekABarrett-ConnorEThe prevalence of peripheral arterial disease in a defined populationCirculation198571510153156006
- ErsoyHJacobsPKentCKBlood pool MR angiography of aortic stent-graft endoleakAm J Roentgenol20041821181615100115
- FinkCLeySPuderbachM3D pulmonary perfusion MRI and MR angiography of pulmonary embolism in pigs after a single injection of a blood pool MR contrast agentEur Radiol2004141291614997336
- GoyenMEdelmanMPerreaultPMR angiography of aortoiliac occlusive disease: a phase III study of the safety and effectiveness of the blood-pool contrast agent MS-325Radiology20052368253316020554
- GristTMRA of the abdominal aorta and lower extremitiesJ Magn Reson Imaging200011324310676618
- HartmannMWiethoffAJHentrichHRInitial imaging recommendations for Vasovist angiographyEur Radiol200616Suppl 2B152316802439
- HuberMEPaetschISchnackenburgBPerformance of a new gadolinium-based intravascular contrast agent in free-breathing inversion-recovery 3D coronary MRAMagn Reson Med2003491152112509826
- KlessenCHeinPAHuppertzAFirst-pass whole-body magnetic resonance angiography (MRA) using the blood-pool contrast medium gadofosveset trisodium: comparison to gadopentetate dimeglumineInvest Radiol2007426596417700282
- KramerHSchoenbergSONikolaouKCardiovascular screening with parallel imaging techniques and a whole-body MR imagerRadiology20052363001015987982
- LeinerTHerbornCUGoyenMNephrogenic systemic fibrosis is not exclusively associated with gadodiamideEur Radiol2007171921317458550
- LiDZhengJWeinmannHJContrast-enhanced MR imaging of coronary arteries: comparison of intra- and extravascular contrast agents in swineRadiology2001218670811230638
- LiWSalanitriJTuttonSLower extremity deep venous thrombosis: evaluation with ferumoxytol-enhanced MR imaging and dual-contrast mechanism – preliminary experienceRadiology20072428738117325072
- MichaelyHJSchoenbergSOOesingmannNRenal artery stenosis: functional assessment with dynamic MR perfusion measurements – feasibility studyRadiology20062385869616436819
- NaelKSalehRNyborgGKPulmonary MR perfusion at 3.0 Tesla using a blood pool contrast agent: Initial results in a swine modelJ Magn Reson Imaging200725667217154181
- NikolaouKKramerHGrosseCHigh-spatial-resolution multistation MR angiography with parallel imaging and blood pool contrast agent: initial experienceRadiology20062418617217032914
- RappJHWolffSDQuinnSFAortoiliac occlusive disease in patients with known or suspected peripheral vascular disease: safety and efficacy of Gadofosveset-enhanced MR angiography – multicenter comparative phase III studyRadiology200523671815987963
- RiekerODuberCNeufangACT angiography versus intraarterial digital subtraction angiography for assessment of aortoiliac occlusive diseaseAm J Roentgenol1997169113389308477
- RohrerMBauerHMintorovitchJComparison of magnetic properties of MRI contrast media solutions at different magnetic field strengthsInvest Radiol2005407152416230904
- SakumaHIchikawaYSuzawaNAssessment of coronary arteries with total study time of less than 30 minutes by using wholeheart coronary MR angiographyRadiology20052373162116126921
- SakumaHIchikawaYChinoSDetection of coronary artery stenosis with whole-heart coronary magnetic resonance angiographyJ Am Coll Cardiol200648194695017112982
- SchoenbergSOWunschCKnoppMVAbdominal aortic aneurysm. Detection of multilevel vascular pathology by time-resolved multiphase 3D gadolinium MR angiography: initial reportInvest Radiol199934648910509243
- TanKTvan BeekEJBrownPWMagnetic resonance angiography for the diagnosis of renal artery stenosis: a meta-analysisClin Radiol2002576172412096862
- VogtFMHerbornCUParsonsECDiagnostic performance of contrast-enhanced MR angiography of the aortoiliac arteries with the blood pool agent Vasovist: initial results in comparison to intra-arterial DSARofo20071794122017385136
- VogtMTWolfsonSKKullerLHLower extremity arterial disease and the aging process: a reviewJ Clin Epidemiol199245529421588358
- ZhengJLiDMaggioniFSingle-session magnetic resonance coronary angiography and myocardial perfusion imaging using the new blood pool compound B-22956 (gadocoletic acid): initial experience in a porcine model of coronary artery diseaseInvest Radiol2005406041316118554