Abstract
Anti-inflammatory properties may contribute to the pharmacological effects of angiotensin II receptor blockers (ARBs), a leading therapeutic class in the management of hypertension and related cardiovascular and renal diseases. That possibility, supported by consistent evidence from in-vitro and animal studies showing pro-inflammatory properties of angiotensin II, has been evaluated clinically by measuring the effect of ARBs on C-reactive protein and other circulating indices of inflammation (e-selectin, adhesion molecules, interleukin-6, tissue necrosis factor-alpha, monocyte chemoattractant protein-1) of potential clinical relevance, a body of evidence that this paper aims to review.
Introduction
The renin–angiotensin system (RAS; ) is a multi-step peptidergic system by which circulating angiotensinogen, a liver-derived α-glycoprotein derived from liver and other sources such as the kidney, adipose tissue and the heart,Citation1 is cleaved by renin, the rate limiting step in the biological cascade, to form the decapeptide angiotensin (Ang) I. In turn, AngI is transformed by angiotensin-converting enzyme (ACE), a membrane-bound metalloproteinase expressed in high concentrations on the surface of pulmonary endothelial cells,Citation1 into the octapeptide AngII, the final effector of the RAS. The endocrine RAS, as above summarized, works in concert with local RASs, ie, self-contained, functionally autonomous AngII-generating systems in the heart, the nervous system, reproductive organs, and in interaction with other biological systems, eg, endothelins or nitric oxide.Citation2
Figure 1 The renin–angiotensin system and cascade of bioactive angiotensins.
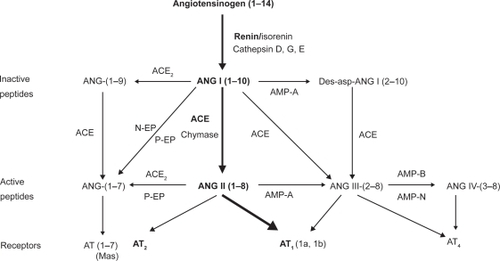
Most of the cardiovascular effects of AngII are mediated by G coupled type 1 receptors (AT1Rs) expressed in the vascular wall and organs such as liver, adrenals, brain, lung, kidney and the heart, that coexist with type 2 receptors mediating vasodilatation, inhibition of cell growth/proliferation and proapoptosis.Citation3 (Pro)renin receptors, which accelerate renin catalytic properties, activate circulating prorenin and stimulate AngII-independent intracellular signaling pathways, have recently been identifiedCitation4 whose more thorough understanding will likely unveil additional pathophysiologic facets of the RAS as a whole ().
Figure 2 Schematic representation of the classical renin–angiotensin system (RAS) and of the emerging concept integrating the (pro)renin receptor and the blocking of the system at different steps by pharmacological compounds.
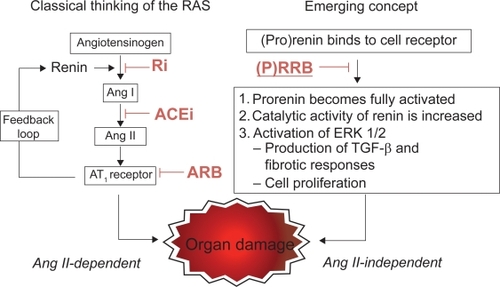
Each step of the biological cascade leading to AngII, the biological effector of the system, can be pharmacologically inhibited by renin inhibitors such as aliskiren, ACE inhibitors (ACEIs) and All AT1R blockers (ARBs) (), these latter triggering a compensatory renin rise due to the disruption of the feedback inhibition of renin production.Citation1–Citation4 The increase in renin activity stimulates the conversion of Ang I and Ang II, which may limit the efficacy of RAS inhibitionCitation3 and the increased renin can also activate the prorenin/renin receptor causing renal and cardiovascular damages independent of Ang IICitation4 (). ARBs constitute a heterogeneous pharmacological class () sharing AT1R antagonismCitation5,Citation6 as a common feature whose clinical profile has been clarified by several published randomized clinical trialsCitation7–Citation29 () in hypertension, cardio-, cerebrovascular disease, diabetes, and others either completedCitation30 or on their way to completionCitation31,Citation32 will further expand our knowledge on this topic.
Table 1 Main pharmacokinetic characteristics of the available ARBs
Table 2 Acronyms of completed and ongoing randomized controlled clinical trials with ARBs
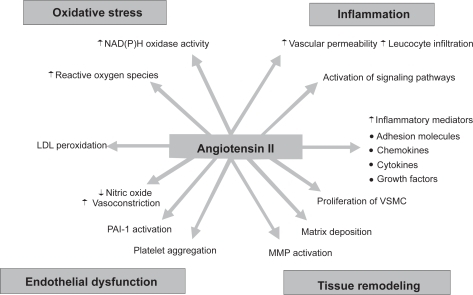
Although primarily ascribable to AT1R antagonism of the vascular, neurohormonal and renal effects of blood-borne and locally produced AngII,Citation1,Citation2 the therapeutic effect of ARBs may be compounded by “pleiotropic” mechanisms related to modulation of the multiform effects of AngII on vascular cells () by which the peptide may accelerate the onset and progression of atherosclerotic vascular disease.Citation33 Growing evidence, in fact, demonstrates the cytokine-like potential of locally-synthesized AngII to act in a paracrine, autocrine, and possibly intracrine manner to promote vascular inflammation, a main component of the atherogenic process (see below). That interesting possibility, generated by a consistent series of in-vitro and animal studies, has stimulated a number of clinical studies focusing on the effect of ARBs on circulating inflammatory indicesCitation34–Citation78 that this paper will discuss.
AngII and vascular inflammation
The classical view of atherosclerosis as a lesion composed by lipid deposits has now been changed to that of a chronic inflammatory disorder triggered and maintained by the production of inflammatory mediators and immune cells involved in the initiation, progression, and rupture of the plaque.Citation79 AngII may promote and amplify that process through the congeries of mechanisms summarized in . In fact, All facilitates adhesion of monocytes and neutrophils to endothelial cells through AT1R-stimulated upregulation of P- and E-selectin expression, thus capturing free-flowing leukocytes from the blood and allowing endothelial rolling. The peptide also stimulates the expression of intercellular (ICAM-1) and vascular (VCAM-1) cellular adhesion molecules by which leukocytes accumulate at the sites of inflammation and infiltrate the endothelial layer by production of chemokines such as monocyte chemoattractant protein-1 (MCP-1) in endothelial and vascular smooth muscle cells, monocytes/macrophages, and cardiac myocytes. AngII also increases the expression of cytokines such as interleukin-6 (IL-6) that activates macrophages and adhesion molecule expression and increases local angiotensinogen generation and thereby local AngII formation in the vascular wall, further amplifying vascular inflammation. AngII, by stimulating platelet binding to endothelial cells and/or leukocytes, contributes to thrombin release, the main effector of platelets, that augments the expression of P-selectin, E-selectin, VCAM-1, and ICAM-1.Citation79,Citation80 It should, however, be clear that the vascular effects of AngII are complex and multiform () and involve several intracellular pathways leading to inflammation and proliferation reviewed in detail elsewhere.Citation33,Citation79
Ang II directly act on NAD(P)H oxidase, an enzyme present in vascular wall cells consisting of membrane and cytoplasmic subunits and a small GTP-binding protein Rac.Citation81 NAD(P)H oxidase generates reactive oxygen species (ROS) that activate nuclear factor kappa B (NFkB), a transcription factor binding specific sequences in the promoter regions of target genes thus inducing transcription of proinflammatory cytokines, chemokines, mediators of inflammation, immune receptors, and adhesion molecules.Citation82 The effect of AngII on NFkB has been documented in endothelial and vascular smooth muscle, glomerular, tubular, and mononuclear cells and its overactivation in tissue of ANGII stimulated animals related to AT1R activation.Citation81 ROS excess also impairs endothelial function by decreasing NO bioavailability by both constitutive (eNOS) and inducible (iNOS) NO synthases, accelerates atherogenesisCitation83 and attenuates BP raise in response to AngII infusion,Citation84,Citation85 a piece of evidence suggestive of a role of inflammatory components in the genesis of essential hypertension.
The effect of ARBs on circulating inflammatory indices
ARBs and C-reactive protein
C-reactive protein (CRP) is a protein synthesized by hepatocytes under the influence of IL-6 within 24–72 hrs after infectious and noninfectious disorders, including myocardial infarction and other acute coronary syndromes. Detection of both CRP mRNA and protein in vascular smooth muscle cells and macrophages within atherosclerotic plaques suggests its de novo synthesis in the vessel wall in which CRP may activate the complement system and/or interact with macrophages and other resident vascular cells.Citation86 Due to its long-term stability during storage, long half-life, lack of diurnal variation as well as lack of age and sex dependence, circulating CRP represents a reliable long-term index of subclinical inflammation provided of predictive power for cardiovascular events in patients with both established coronary artery disease and in primary prevention independent of concomitant factors such as smoking status, diabetes, blood pressure, use of hormone-replacement therapy and low-density lipoprotein (LDL) cholesterol.Citation87
Because of those favorable characteristics for risk stratification, several studies listed in have addressed the effect of ARBs on circulating CRP levels in hypertensive and diabetic patients. The Val-MARC (Valsartan-Managing blood pressure Aggressively and evaluating Reductions in hsCRP) study is probably the more important trial addressing the issue of whether BP reduction per se lowers CRP levels, or whether selective AT1R antagonism through valsartan may have independent effects to reduce CRP levels.Citation69 The study included 1668 patients with stage 2 hypertension randomly allocated to either valsartan alone (160–320 mg/day, n = 836) or valsartan/hydrochlorothiazide (HCTZ, 160–320 mg/12.5 mg/day, n = 832) for a period of six weeks. At the end of treatment, valsartan alone slightly but significantly reduced high sensitivity (hs)CRP levels, an effect maintained over an extended follow-up period albeit with a low level of association with achieved BP. As CRP levels were unchanged in the combined valsartan/HCTZ therapy group, the data were taken as suggestive of a negative interaction of thiazide diuretics with the anti-inflammatory effects of ARBs conclusion. That conclusion contrasts, though, with the results of the VAST (Valsartan/HCTZ versus Amlodipine in STage II hypertensive patients) trial whose primary objective was to determine whether valsartan 160 mg plus HCTZ 25 mg OD would be more effective than monotherapy with amlodipine 10 mg OD.Citation61 Modulation by valsartan of CRP levels was confirmed in other, small-sized studies in patients with hypertension,Citation55 congestive heart failureCitation56 as well as normal subjectsCitation45 although other reports did not confirm those data.Citation58,Citation73,Citation77 For example, Rajagopalan and colleaguesCitation73 found no significant change in hs-CRP in 104 hypertensive patients randomized to 12 weeks valsartan (160 mg daily) as compared with significant reductions in those on combined statin treatment. Galle and colleagues in the VIVALDI trial (inVestigate the efficacy of telmIsartan versus VALsartan in hypertensive type 2 DIabetic patients with overt nephropathy)Citation77 found no influence of valsartan (160 mg) as well as telmisartan (80 mg) on inflammatory parameters in 255 hypertensive patients with diabetic nephropathy and the study was unable to show any effect beyond that due to blood pressure control. Nonsignificant changes in hsCRP were reported with candesartanCitation42,Citation47,Citation60,Citation63 including the CENTRO (CandEsartaN on aTherosclerotic Risk factors) trial, a multicenter, randomized, double blind comparison of candesartan and enalapril, an ACEI, in hypertensive, diabetic patients showing no effect of the ARB (but also enalapril) on hsCRP.Citation60 Similar discrepancies also characterized the effect of telmisartanCitation59,Citation62,Citation65,Citation66,Citation74,Citation78 including the already commented VIVALDI trial.Citation77 Positive results were reported for irbesartan in two studies in coronary heart disease patients,Citation54,Citation57 but their small sample size preludes generalization. Olmesartan was tested in a well designed and carefully conducted prospective, placebo-controlled, double-blind multicenter study by Fliser and colleaguesCitation50 who measured hs-CRP levels and other inflammatory markers in 199 patients with essential hypertension and obesity-related microinflammation. After 12 weeks of therapy, with additional HTCZ if needed, olmesartan decreased hs-CRP (−21.1%; P < 0.02), TNF-α (−13.6%; P < 0.01), IL-6 (−18.0%; P < 0.01) and MCP-1 (−6.5%; P < 0.01). Albeit gathered in a well designed and carefully conducted study, those results need confirmation in additional trials, however. A greater anti-inflammatory effect of olmesartan as compared with telmisartan was recently claimed by Nakayama and colleagues,Citation78 but the conclusion is flawed by the experimental design lacking adequate wash-out prior to randomization. Notably, losartan did not affect CRP in patients with diabetic nephropathy,Citation34 coronary artery disease,Citation38 and hypertension.Citation54 No data are available about the effect of eprosartan.
Table 3 Percent changes in high sensitivity (hs) C-reactive protein (CRP) during ARB treatment
ARBs and circulating adhesion molecules, cytokines, and chemokines
A number of clinical studies have assessed the effect of ARBs on circulating inflammatory markers other than CRP such as E-selectin, a member of the selectin family expressed on the surface of stimulated endothelial cells, and ICAM-1 and VCAM-1, two immunoglobulin-like molecules acting as endothelial ligands to facilitate endothelial adhesion of circulating leukocytes.Citation79 Those biological products circulate in blood as a result of enzymatic cleavage or from shedding of damaged or activated endothelial cells under the influence of proatherogenic stimuli such as hypertension, type 2 diabetes, obesity as well as established peripheral and coronary artery disease.Citation79,Citation88 While the prognostic power of raised s-eSEL is dubious,Citation88 circulating ICAM-1 predicted cardiovascular risk independent of traditional risk factors in the 14,916 healthy men enrolled in the Physicians’ Health Study (PHS),Citation89 as well as in the elderly, apparently healthy subjects of the Atherosclerosis Risk in Communities (ARIC) study.Citation90 On the other hand, VCAM-1 did not predict future cardiovascular risk,Citation90 suggesting important distinctions between the roles of different CAMs in atherogenesis. Evidence has also been gathered in support of the clinical relevance of inflammatory cytokines such as circulating IL-6 and TNF-α, and MCP-1, a chemokine that orchestrates the migration of leukocytes into the intima and within atherosclerotic lesions.Citation79 Increased plasma IL-6 levels were reported early after admission for acute coronary syndromes and associated with a complicated in-hospital course and higher IL-6 levels predicted acute coronary syndromes in apparently healthy men.Citation91 Post-MI elevations of circulating TNF-αCitation92 and MCP-1Citation93 also associated with an increased risk of recurrent coronary events.Citation89
As summarized in , losartan did not affect circulating adhesion molecules in patients with diabetes and/or hypertension and/or coronary artery diseaseCitation34,Citation38,Citation39,Citation53 while a significant decrease was reported only in two, small studies in normal subjects.Citation41,Citation51 The effect of the drug on eSEL, on the other hand, was consistently negative.Citation34,Citation38,Citation39,Citation41,Citation51 The same discrepant behavior was shared by candesartan,Citation35,Citation42,Citation60 the other ARBs frequently used in studies of this kind, while either eprosartanCitation40 or telmisartanCitation65 treatment did not change VCAM-1 levels to a statistically significant extent. No data are available for irbesartan or olmesartan.
Table 4 Percent changes in circulating e-selectin (SEL), intercellular cellular adhesion molecule (ICAM)-1, vascular cellular adhesion molecule (VCAM)-1, interleukin-6 (IL-6), tumor necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1 during ARB treatment
As shown in , similar considerations hold for the effect of ARBs on MCP-1, TNF-α and IL-6.Citation42,Citation47,Citation75
Conclusions
Despite a quite consistent evidence from basic research f ield, the anti-inflammatory effect of ARBs in man, at least to the extent derived from their effect on circulating inflammatory indices, is quite inconsistent, a conclusion that applies even to studies apparently adopting the same drug at similar dosages, comparable patient selection criteria and experimental design. Further limitations derive from the small sample sizes that characterize many of the available studies, heterogeneity of ARBs as a pharmacological class (see ), lack of prospective studies evaluating the relationship between anti-inflammatory effects of ARBs and incident morbid events and the complexity of the effects of AngII on vascular biology (). Additional difficulties derive from the inherent variability of circulating inflammatory indices, a pattern emerging quite clearly from to which genetic factors acting at the individualCitation94 as well as the populationCitation95 level may contribute. Not unlike ARBs, ACEIs showed divergent results,Citation96 sometimes in contrast with the effects of the ARBs. Thus, enalapril but not losartan reduced inflammatory markers in hypertensive and diabetic patients.Citation39 It should also be noted that interference on inflammatory indices is not specific for RAS inhibitors since other classes of cardiovascular drugs such as beta-adrenoceptor blocking drugs,Citation97,Citation98 statinsCitation99 as well as nonpharmacological interventions such as exercise training, weight lossCitation94 and nutritional factorsCitation100 may influence CRP levels. Suggestions have also been raised about a beneficial effect of intensive blood pressure and lipid treatment per se.Citation101 Moreover, the validity of circulating inflammatory markers as a surrogate end-point for an underlying inflammatory process is unclear since the relationship with their activity at the local level is unknown. Importantly, modifications in circulating CRP, even when highly consistent such as in the case of statins,Citation99 have dubious pathophysiological significance since decrements in hsCRP were associated with either no change,Citation102,Citation103 or improved cardiovascular prognosis.Citation104 As a matter of fact, the LDL- and CRP-lowering effect of statinsCitation99,Citation105 are closely intertwined, possibly as an expression of their metabolic effect on the liver. For these reasons, no firm conclusions can be drawn about their effect at this point and further studies are needed.
Disclosure
The authors report no conflicts of interest in this work.
References
- InagamiTA memorial to Robert Tiegerstedt: the centennial of renin discoveryHypertension19983269539579856956
- PaulMPoyan MehrAKreutzRPhysiology of local renin-angiotensin systemsPhysiol Rev200686374780316816138
- de GasparoMCattKJInagamiTWrightJWUngerTInternational union of pharmacology. XXIII. The angiotensin II receptorsPharmacol Rev200052341547210977869
- NguyenGContrepasAThe (pro)renin receptorsJ Mol Med200886664364618322668
- IsrailiZHClinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertensionJ Hum Hypertens200014Suppl 1S73S8610854085
- SongJCWhiteCMPharmacologic, pharmacokinetic, and therapeutic differences among angiotensin II receptor antagonistsPharmacotherapy20002013013910678291
- PittBPoole-WilsonPASegalRfor the Losartan Heart Failure Survival Study ELITE IIEffect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE IILancet200035592151582158710821361
- LewisEJHunsickerLGClarkeWRfor the Collaborative Study GroupRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med20013451285186011565517
- BrennerBMCooperMEde ZeeuwDfor the RENAAL Study InvestigatorsEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med20013451286186911565518
- ParvingHHLehnertHBrochner-MortensenJfor the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study GroupThe effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetesN Engl J Med201;3451287087811565519
- CohnJNTognoniGfor the Valsartan Heart Failure Trial InvestigatorsA randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failureN Engl J Med2001345231667167511759645
- DahlöfBDevereuxRBKjeldsenSEfor the LIFE Study GroupCardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenololLancet20023599311995100311937178
- VibertiGWheeldonNMfor the Microalbuminuria Reduction with Valsartan (MARVAL) Study InvestigatorsMicroalbuminuria reduction with valsartan in patients with type 2 diabetes mellitusCirculation2002106667267812163426
- DicksteinKKjekshusJfor the OPTIMAAL Study GroupEffects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist LosartanLancet2002360933575276012241832
- LithellHHanssonLSkoogIfor the SCOPE Study GroupThe Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trialJ Hypertens200321587588612714861
- SchraderJLüdersSKulschewskiAfor the Acute Candesartan Cilexetil Therapy in Stroke Survivors Study GroupThe ACCESS Study: evaluation of Acute Candesartan Cilexetil Therapy in Stroke SurvivorsStroke20033471699170312817109
- YusufSPfefferMASwedbergKfor the CHARM Investigators and CommitteesEffects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved TrialLancet2003362938677778113678871
- PfefferMAMcMurrayJJVelazquezEJValsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or bothN Engl J Med2003203491893190614610160
- LindholmLHPerssonMAlaupovicPCarlbergBSvenssonASamuelssonOMetabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study)J Hypertens20032181563157412872052
- JuliusSKjeldsenSEWeberMfor the VALUE trial groupOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet200436394262022203115207952
- BarnettAHBainSCBouterPfor the Diabetics Exposed to Telmisartan and Enalapril Study GroupAngiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathyN Engl J Med2004351191952196115516696
- SchraderJLüdersSKulschewskiAfor the MOSES Study GroupMorbidity and Mortality After Stroke, Eprosartan Compared with Nitrendipine for Secondary Prevention: principal results of a prospective randomized controlled study (MOSES)Stroke20053661218122615879332
- MochizukiSDahlöfBShimizuMfor the Jikei Heart Study groupValsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality studyLancet200736995711431143917467513
- ONTARGET InvestigatorsYusufSTeoKKTelmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med2008358151547155918378520
- YusufSDienerHCSaccoRLfor the PRoFESS Study GroupTelmisartan to prevent recurrent stroke and cardiovascular eventsN Engl J Med2008359121225123718753639
- Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) InvestigatorsYusufSTeoKEffects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trialLancet200837296441174118318757085
- SjølieAKKleinRPortaMfor the DIRECT Programme Study GroupEffect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trialLancet200837296451394140218823656
- MassieBMCarsonPEMcMurrayJJfor the I-PRESERVE InvestigatorsIrbesartan in Patients with Heart Failure and Preserved Ejection FractionN Engl J Med2008359232456246719001508
- HallerHVibertiGCMimranAPreventing microalbuminuria in patients with diabetes: rationale and design of the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) studyJ Hypertens200624240340816508590
- DisertoriMLatiniRMaggioniAPfor the GISSI-AF InvestigatorsRationale and design of the GISSI-Atrial Fibrillation Trial: a randomized, prospective, multicentre study on the use of valsartan, an angiotensin II AT1-receptor blocker, in the prevention of atrial fibrillation recurrenceJ Cardiovasc Med (Hagerstown)200671293816645357
- ConnollySYusufSBudajAfor the ACTIVE InvestigatorsRationale and design of ACTIVE: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular eventsAm Heart J200615161187119316781218
- KonstamMAPoole-WilsonPADicksteinKDrexlerHJusticeSJKomajdaMDesign of the heart failure endpoint evaluation of AII-antagonist losartan (HEAAL) study in patients intolerant to ACE-inhibitorEur J Heart Fail200810989990618768350
- HunyadyLCattKJPleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin IIMol Endocrinol200620595397016141358
- AndersenSSchalkwijkCGStehouwerCDParvingHHAngiotensin II blockade is associated with decreased plasma leukocyte adhesion molecule levels in diabetic nephropathyDiabetes Care20002371031103210895868
- TsutamotoTWadaAMaedaKAngiotensin II type 1 receptor antagonist decreases plasma levels of tumor necrosis factor alpha, interleukin-6 and soluble adhesion molecules in patients with chronic heart failureJ Am Coll Cardiol200035371472110716475
- KhanBVNavalkarSKhanQARahmanSTParthasarathySIrbesartan, an angiotensin type 1 receptor inhibitor, regulates the vascular oxidative state in patients with coronary artery diseaseJ Am Coll Cardiol20013861662166711704378
- NavalkarSParthasarathySSantanamNKhanBVIrbesartan, an angiotensin type 1 receptor inhibitor, regulates markers of inflammation in patients with premature atherosclerosisJ Am Coll Cardiol200137244044411216960
- PrasadAKohKKSchenkeWHRole of angiotensin II type 1 receptor in the regulation of cellular adhesion molecules in atherosclerosisAm Heart J2001142224825311479463
- JilmaBLi-Saw-HeeFLWagnerOFBeeversDGLipGYEffects of enalapril and losartan on circulating adhesion molecules and monocyte chemotactic protein-1Clin Sci2002103213113612149103
- RahmanSTLautenWBKhanQANavalkarSParthasarathySKhanBVEffects of eprosartan versus hydrochlorothiazide on markers of vascular oxidation and inflammation and blood pressure (renin-angiotensin system antagonists, oxidation, and inflammation)Am J Cardiol200289668669011897210
- RajagopalanSBrookRMehtaRHEffect of losartan in aging related endothelial impairmentAm J Cardiol200289556256611867042
- WassmannSHilgersSLaufsUBohmMNickenigGAngiotensin II type 1 receptor antagonism improves hypercholesterolemia-associated endothelial dysfunctionArterioscler Thromb Vasc Biol20022271208121212117739
- AgarwalRProinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockadeAm J Physiol (Renal Physiol)20032844F863F86912505865
- BaykalYYilmazMICelikTEffects of antihypertensive agents, alpha receptor blockers, beta blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and calcium channel blockers, on oxidative stressJ Hypertens20032161207121112777959
- DandonaPKumarVAljadaAAngiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-kappa B, in mononuclear cells of normal subjects: evidence of an antiinflammatory actionJ Clin Endocrinol Metab20038894496450112970329
- DohiYOhashiMSugiyamaMTakaseHSatoKUedaRCandesartan reduces oxidative stress and inflammation in patients with essential hypertensionHypertens Res200326969169714620923
- KohKKAhnJYHanSHPleiotropic effects of angiotensin II receptor blocker in hypertensive patientsJ Am Coll Cardiol200342590591012957441
- LautenWBKhanQARajagopalanSUsefulness of quinapril and irbesartan to improve the anti-inflammatory response of atorvastatin and aspirin in patients with coronary heart diseaseAm J Cardiol20039191116111912714159
- TakedaTHoshidaSNishinoMTanouchiJOtsuKHoriMRelationship between effects of statins, aspirin and angiotensin II modulators on high-sensitive C-reactive protein levelsAtherosclerosis2003169115515812860262
- FliserDBuchholzKHallerHfor the EUropean Trial on Olmesartan and Pravastatin in Inflammation and Atherosclerosis (EUTOPIA) InvestigatorsAntiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammationCirculation200411091103110715313950
- GraningerMReiterRDruckerCMinarEJilmaBAngiotensin receptor blockade decreases markers of vascular inflammationJ Cardiovasc Pharmacol200444333533915475831
- KohKKQuonMJHanSHAdditive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patientsCirculation2004110243687369215569835
- SardoMACastaldoMCinquegraniMEffects of AT1 receptor antagonist losartan on sICAM-1 and TNF-alpha levels in uncomplicated hypertensive patientsAngiology200455219520315026875
- SchiefferBBunteCWitteJComparative effects of AT1-antagonism and angiotensin converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery diseaseJ Am Coll Cardiol200444236236815261932
- YasunariKMaedaKWatanabeTComparative effects of valsartan versus amlodipine on left ventricular mass and reactive oxygen species formation by monocytes in hypertensive patients with left ventricular hypertrophyJ Am Coll Cardiol200443112116212315172423
- AnandISLatiniRFloreaVGfor the Val-HeFT InvestigatorsC-reactive protein in heart failure prognostic value and the effect of valsartanCirculation2005112101428143416129801
- BiasucciLMLombardiMPiroMDi GiannuarioGLiuzzoGCreaFIrbesartan significantly reduces C reactive protein concentrations after 1 month of treatment in unstable anginaHeart200591567067115831660
- ManabeSOkuraTWatanabeSFukuokaTHigakiJEffects of angiotensin II receptor blockade with valsartan on pro-inflammatory cytokines in patients with essential hypertensionJ Cardiovasc Pharmacol200546673573916306795
- MiuraYYamamotoNTsunekawaSReplacement of valsartan and candesartan by telmisartan in hypertensive patients with type 2 diabetes: metabolic and antiatherogenic consequencesDiabetes Care200528375775815735228
- RoseiEARizzoniDMuiesanMLfor the CENTRO (CandEsartaN on aTherosclerotic Risk factors) study investigatorsEffects of candesartan cilexetil and enalapril on inflammatory markers of atherosclerosis in hypertensive patients with non-insulin-dependent diabetes mellitusJ Hypertens200523243544415662233
- RuilopeLMMalaccoEKhderYKandraABonnerGHeintzDEfficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: the VAST studyClin Ther200527557858715978306
- KoulourisSSymeonidesPTriantafyllouKComparison of the effects of ramipril versus telmisartan in reducing serum levels of high-sensitivity C-reactive protein and oxidized low-density lipoprotein cholesterol in patients with type 2 diabetes mellitusAm J Cardiol200595111386138815904653
- SchramMTvan IttersumFJSpoelstra-de ManAAggressive antihypertensive therapy based on hydrochlorothiazide, candesartan or lisinopril as initial choice in hypertensive type II diabetic individuals: effects on albumin excretion, endothelial function and inflammation in a double-blind, randomized clinical trialJ Hum Hypertens200519642943715647778
- DerosaGCiceroAFD’AngeloATelmisartan and irbesartan therapy in type 2 diabetic patients treated with rosiglitazone: effects on insulin-resistance, leptin and tumor necrosis factor-alphaHypertens Res2006291184985617345784
- LinkALenzMLegnerDBöhmMNickenigGTelmisartan inhibits beta2-integrin MAC-1 expression in human T-lymphocytesJ Hypertens20062491891189816915040
- NagelJMTietzABGokeBParhoferKGThe effect of telmisartan on glucose and lipid metabolism in nondiabetic, insulin-resistant subjectsMetabolism20065591149115416919531
- NomuraSShouzuAOmotoSNishikawaMFukuharaSIwasakaTEffect of valsartan on monocyte/endothelial cell activation markers and adiponectin in hypertensive patients with type 2 diabetes mellitusThromb Res2006117438539215896827
- OgawaSMoriTNakoKKatoTTakeuchiKItoSAngiotensin II type 1 receptor blockers reduce urinary oxidative stress markers in hypertensive diabetic nephropathyHypertension200647469970516505207
- RidkerPMDanielsonERifaiNfor the Val-MARC InvestigatorsValsartan, blood pressure reduction, and C-reactive protein: primary report of the Val-MARC trialHypertension2006481737916714425
- HanSHKohKKQuonMJLeeYShinEKThe effects of simvastatin, losartan, and combined therapy on soluble CD40 ligand in hypercholesterolemic, hypertensive patientsAtherosclerosis2007190120521116500662
- KohKKQuonMJLeeYAdditive beneficial cardiovascular and metabolic effects of combination therapy with ramipril and candesartan in hypertensive patientsEur Heart J200728121440144717483542
- LiuLZhaoSPZhouHNLiQZLiJXEffect of fluvastatin and valsartan, alone and in combination, on postprandial vascular inflammation and fibrinolytic activity in patients with essential hypertensionJ Cardiovasc Pharmacol2007501505517666915
- RajagopalanSZannadFRadauceanuAEffects of valsartan alone versus valsartan/simvastatin combination on ambulatory blood pressure, C-reactive protein, lipoproteins, and monocyte chemoattractant protein-1 in patients with hyperlipidemia and hypertensionAm J Cardiol2007100222222617631074
- YanoYHoshideSIshikawaJThe differential effects of angiotensin II type 1 receptor blockers on microalbuminuria in relation to low-grade inflammation in metabolic hypertensive patientsAm J Hypertens200720556557217485023
- WhiteMLepageSLavoieJEffects of combined candesartan and ACE inhibitors on BNP, markers of inflammation and oxidative stress, and glucose regulation in patients with symptomatic heart failureJ Card Fail2007132869417395047
- WillemsenJMWesterinkJWDallinga-ThieGMAngiotensin II type 1 receptor blockade improves hyperglycemia-induced endothelial dysfunction and reduces proinflammatory cytokine release from leukocytesJ Cardiovasc Pharmacol200749161217261957
- GalleJSchwedhelmEPinnettiSfor the VIVALDI investigatorsAntiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt nephropathyNephrol Dial Transplant200823103174318318450829
- NakayamaSWatadaHMitaTComparison of effects of olmesartan and telmisartan on blood pressure and metabolic parameters in Japanese early-stage type-2 diabetics with hypertensionHypertens Res200831171318360012
- PackardRRLibbyPInflammation in atherosclerosis: from vascular biology to biomarker discovery and risk predictionClin Chem2008541243818160725
- MarchesiCParadisPSchiffrinELRole of the renin-angiotensin system in vascular inflammationTrends Pharmacol Sci200829736737418579222
- GriendlingKKUshio-FukaiMReactive oxygen species as mediators of angiotensin II signalingRegul Pept20009113212710967197
- BarnesPJKarinMNuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseasesN Engl J Med199733615106610719091804
- FörstermannUOxidative stress in vascular disease: causes, defense mechanisms and potential therapiesNat Clin Pract Cardiovasc Med20085633834918461048
- OrtizMCManriquezMCRomeroJCJuncosLAAntioxidants block angiotensin II-induced increases in blood pressure and endothelinHypertension2001(3 pt2); 3865565911566950
- GuzikTJHochNEBrownKARole of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunctionJ Exp Med2007204102449246017875676
- BlackSKushnerISamolsDC-reactive proteinJ Biol Chem200427947484874849015337754
- VermaSSzmitkoPERidkerPMC-reactive protein comes of ageNat Clin Pract Cardiovasc Med200521293616265340
- RoldánVMarínFLipGYBlannADSoluble E-selectin in cardiovascular disease and its risk factors. A review of the literatureThromb Haemost20039061007102014652631
- RidkerPMHennekensCHRoitman-JohnsonBStampferMJAllenJPlasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy menLancet1998351909688929439492
- HwangSJBallantyneCMSharrettARCirculating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) studyCirculation19979612421942259416885
- RidkerPMRifaiNStampferMJHennekensCHPlasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy menCirculation2000101151767177210769275
- RidkerPMRifaiNPfefferMSacksFLepageSBraunwaldEElevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarctionCirculation2000101182149215310801754
- de LemosJAMorrowDABlazingMASerial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: results from the A to Z trialJ Am Coll Cardiol200750222117212418036447
- ObisesanTOLeeuwenburghCPhillipsTC-reactive protein genotypes affect baseline, but not exercise training-induced changes, in C-reactive protein levelsArterioscler Thromb Vasc Biol200424101874187915271790
- FoxERBenjaminEJSarpongDFEpidemiology, heritability, and genetic linkage of C-reactive protein in African Americans (from the Jackson Heart Study)Am J Cardiol2008102783584118805107
- PrasadKC-reactive protein (CRP)-lowering agentsCardiovasc Drug Rev2006241335016939632
- NagatomoYYoshikawaTKohnoTEffects of beta-blocker therapy on high sensitivity C-reactive protein, oxidative stress, and cardiac function in patients with congestive heart failureJ Card Fail200713536537117602983
- JenkinsNPKeevilBGHutchinsonIVBrooksNHBeta-blockers are associated with lower C-reactive protein concentrations in patients with coronary artery diseaseAm J Med2002112426927411893365
- GenserBGrammerTBStojakovicTSiekmeierRMaerzWEffect of HMG CoA reductase inhibitors on low-density lipoprotein cholesterol and C-reactive protein: systematic review and meta-analysisInt J Clin Pharmacol Ther2008461049751018826864
- GiuseppeRDi CastelnuovoACentrittoFRegular consumption of dark chocolate is associated with low serum concentrations of C-reactive protein in a healthy italian populationJ Nutr2008138101939194518806104
- FelmedenDCSpencerCGChungNARelation of thrombogenesis in systemic hypertension to angiogenesis and endothelial damage/dysfunction (a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial [ASCOT])Am J Cardiol200392440040512914869
- GISSI-HF investigatorsTavazziLMaggioniAPEffect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trialLancet200837296451231123918757089
- KasteleinJJAkdimFStroesESfor the ENHANCE InvestigatorsSimvastatin with or without ezetimibe in familial hypercholesterolemiaN Engl J Med2008358141431144318376000
- RidkerPMFonsecaFAGenestJfor the JUPITER Trial Study GroupBaseline characteristics of participants in the JUPITER trial, a randomized placebo-controlled primary prevention trial of statin therapy among individuals with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive proteinAm J Cardiol2007100111659166418036365
- KinlaySLow-density lipoprotein-dependent and -independent effects of cholesterol-lowering therapies on C-reactive protein: a meta-analysisJ Am Coll Cardiol200749202003200917512355