Abstract
Pulmonary hypertension (PH) is found in a vast array of diseases, with a minority representing pulmonary arterial hypertension (PAH). Idiopathic PAH or PAH in association with other disorders has been associated with poor survival, poor exercise tolerance, progressive symptoms of dyspnea, and decreased quality of life. Left untreated, patients with PAH typically have a progressive decline in function with high morbidity ultimately leading to death. Advances in medical therapy for PAH over the past decade have made significant inroads into improved function, quality of life, and even survival in this patient population. Three classes of pulmonary artery-specific vasodilators are currently available in the United States. They include prostanoids, endothelin receptor antagonists, and phosphodiesterase type 5 (PDE5) inhibitors. In May 2009, the FDA approved tadalafil, the first once-daily PDE5 inhibitor for PAH. This review will outline the currently available data on tadalafil and its effects in patients with PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a rare disorder with reports estimating its prevalence at between 15 and 52 cases per one million patients.Citation1,Citation2 Pulmonary hypertension (PH) has been defined differently based on the modality used to measure pulmonary pressures and hemodynamics. Generally considered the gold standard for the assessment of pulmonary hypertension, direct pulmonary artery catheterization with pressure measurements is preferred to less invasive techniques to confirm the suspected diagnosis. PAH is defined by a mean pulmonary artery pressure (mPAP) of more than 25 mmHg at rest or of more than 30 mmHg with exercise. In PAH, pulmonary capillary wedge pressure or left ventricular end diastolic pressure (in the absence of a mitral valve stenosis) is usually less than 15 mmHg.Citation3,Citation4
Traditional classification of pulmonary hypertension largely differentiated patients with idiopathic or primary cases from patients with PH from secondary causes. This classification scheme had many limitations as it did not adequately describe underlying mechanisms of the disease and their implications for potential treatment options, based on the pathophysiology of the underlying conditions. In 1998, the World Health Organization (WHO) created a classification scheme, often referred to as the “Evian Classification,” which described a variety of disorders causing pulmonary hypertension based on different mechanisms.Citation5 This classification scheme was further refined in 2003 in Venice and most recently in 2008 at the 4th World Symposium on Pulmonary Hypertension in Dana Point, California ().Citation6
Table 1 Dana Point classification of pulmonary hypertension (PAH), 2008
The current WHO classification scheme divides PH into five groups: Group 1 represents disorders resulting in PH such as idiopathic PH, familial forms of PH, drug/toxin-induced PH, PH associated with connective tissue diseases, HIV infection, portal hypertension, congenital heart disease, schistosomiasis, and chronic hemolytic anemia, as well as persistent pulmonary hypertension of the newborn. Group 2 includes causes of PH as a result of left sided heart disease. Group 3 includes patients with chronic hypoxemia and/or lung disease. Chronic thromboembolic disease is categorized in group 4 and group 5 is reserved for those diseases which have multifactorial mechanisms leading to PH.
Treatments for PAH
Most recent pharmacologic treatments of PH have focused on group 1 or PAH. In fact, of the current Food and Drug Administration (FDA)-approved agents for the treatment of PH, all are approved for PAH (group 1) only. Current pharmacologic treatment for PAH include calcium channel blockers, prostanoids, endothelin receptor antagonists (ERA), and phosphodiesterase type 5 (PDE5) inhibitors (). The use of calcium channel blockers in PAH has generally been restricted due to limited benefits, significant side effects, and relatively narrow indications.Citation3 Calcium channel blockers appear to benefit mainly patients with PAH with a positive vasodilator challenge, however, even in those patients, the long-term response to calcium channel blocker treatment is variable.Citation7
Table 2 FDA-approved medications for pulmonary arterial hypertension
ERAs currently approved for use in the US include bosentan and ambrisentan. Endothelin-1 is a potent vasoconstrictor and mitogen likely causing vascular remodeling in susceptible individuals. Both bosentan and ambrisentan have been shown to significantly improve exercise capacity and hemodynamics in PAH.Citation8–Citation12 Bosentan is a nonselective endothelin receptor blocker, whereas ambrisentan blocks only endothelin-A receptors. Liver function abnormalities have been noted with ERAs, in particular with bosentan, thus monthly liver function testing is required.Citation3
Both parenteral and inhaled forms of prostacyclins are currently available for the treatment of PAH. Patients with PAH under produce prostacyclin, which functions to vasodilate, inhibit vascular proliferation, and inhibit platelet aggregation. Epoprostenol, a synthetic prostacyclin, was the first FDA-approved medication for the treatment of PAH. It remains the treatment of choice for advanced disease. Epoprostenol has a very short half-life and must be given continuously with an intravenous pump, typically with a starting dose of 2 ng/kg/min intravenously and titrated upwards to desired clinical effect. Ambulatory pumps have made it possible to treat patients with severe PAH as outpatients. Citation13 Epoprostenol has been shown to improve quality of life, exercise capacity, hemodynamics and even survival in PAH.Citation14–Citation17 Side effects include flushing, body aches, hypotension, and jaw pain. Treprostinil, available in intravenous, subcutaneous, and inhaled formulations, has a longer half-life than epoprostenol, thus allowing a wide variety of routes of delivery and may aid in improving compliance.Citation18–Citation20
PDE5 inhibitors have recently become an attractive first-line choice in the treatment of milder forms of PAH. Sildenafil was first FDA approved in the US for the treatment of erectile dysfunction (ED) in 1998. After its release for the use in ED, sildenafil was found to be an effective pulmonary artery vasodilator.Citation21–Citation23 In 2005, sildenafil was approved for use in PAH at a dose of 20 mg 3 times daily, based in large part to the SUPER trial.Citation24 There has been debate about the appropriate dosing of sildenafil for PAH as many studies have used dosing several-fold higher than the current FDA approved dosing.Citation25–Citation31 While a clear dose-response effect of escalating dosing of sildenafil has not been shown in terms of improvements in exercise capacity, quality of life, or hemodynamics, long-term data in patients receiving 80 mg 3 times daily for 1 year have shown sustained benefit with minimal adverse effects.Citation24 Similar data for the 20 mg dose do not exist. Additionally, when used in combination with ERAs, it has been shown that there are decreased plasma levels of sildenafil, providing more rationale for higher dosages of sildenafil.Citation32 Sildenafil has been shown to improve symptoms of PAH, improve exercise tolerance, improve hemodynamics, and to improve quality of life.Citation24,Citation28,Citation29 Sildenafil inhibits the breakdown of cyclic guanosine monophosphate (cGMP) thus increasing the effects of nitric oxide (NO) resulting in pulmonary vasodilation and inhibition of smooth muscle growth. The mechanism of action of PDE5 inhibitors will be discussed in more detail later. Tadalafil, another PDE5 inhibitor approved for PAH earlier this year, is a once daily medication used at a dose of 40 mg daily. Tadalafil, like sildenafil and vardenafil, was originally developed and marketed as a pharmacologic treatment for erectile dysfunction. Vardenafil appears to be less efficacious than either sildenafil or tadalafil for the treatment of PAH.Citation33 Tadalafil’s longer half-life of 17.5 hours compared with that of sildenafil at 4 to 5 hours allows for a once-daily dosing.
PDE-5 inhibitors for the treatment of erectile dysfunction
Sildenafil was FDA approved in 1998 as the first PDE5 inhibitor for the treatment of ED. Shortly after this, two other PDE5 inhibitors, tadalaf il and vardenaf il, were approved for the treatment of ED. While very few comparative data exist for the various PDE5 inhibitors, there is a large body of double-blind placebo-controlled trials for the various agents.Citation34 A recent meta-analysis showed consistently beneficial short-term efficacy of PDE5 inhibitors in the treatment of ED with good safety profiles.Citation34 Efficacy of treatment for ED is typically based on several validated measures of male sexual performance.Citation34 The International Index of Erectile Function Erectile Functional Domain (IIEF EF) is a scoring system numbered 1–30 with higher numbers correlating with improved sexual function.Citation35 Another assessment tool is the use of questions 2 and 3 of the Sexual Encounter Profile (SEP), which ask first whether or not an adequate erection is obtained to initiate sexual intercourse and second whether or not intercourse is able to be completed.Citation36 The Global Assessment Question (GAQ) asks, “Has the treatment you have been taking during this study improved your erection?”Citation36
Approximately 28 randomized controlled trials (RCTs) observing the efficacy of tadalafil in ED have been recently identified.Citation34 Two recent multi-centered placebo-controlled studies showed marked improvements in sexual performance with the addition of tadalafil given at a daily dose of 20 mg.Citation37,Citation38 Skoumal et al randomized 443 men with ED to receive either tadalafil or placebo.Citation37 Patients in the tadalafil group had significant improvements in sexual function as measured by IIEF EF, SEP, and GAQ. In fact, 64% of patients receiving tadalafil had normal IIEF EF domain scores as opposed to only 16% for those receiving placebo. Porst et al studied 348 patients randomized to tadalafil or placebo for two 4-week treatment intervals.Citation38 Patients were asked to attempt sexual intercourse 24 and 36 hours after dosing with a primary endpoint being the ability to complete sexual intercourse to completion. At 36 hours, 59.2% of patients receiving tadalafil were able to complete intercourse compared with only 28.3% in the placebo arm, P < 0.001. Similar results were observed at 24 hours with successful intercourse reported as 52.9% and 29.1%, respectively (P < 0.001). Another study conducted at 15 US centers randomized 287 patients to receive once-daily tadalafil at 5 mg, 2.5 mg, or placebo for 24 weeks.Citation39 Primary endpoint was a change in IIEF EF Domain score and response to SEP question 2 and 3. Significant improvements in ED were reported in all 3 endpoints with low incidence of adverse events.
The longer half-life of tadalafil (17.5 hours) when compared with sildenafil (4 hours) may represent an attractive option to some patients as it may allow for less frequent dosing and a more sustained benefit. An unbiased, rigorously designed head to head trial among currently approved PDE5 inhibitors for the treatment of ED does not currently exist. Four head to head trials of varying quality are currently published, of which three were funded by Eli Lilly.Citation40–Citation43 Eardley and colleagues conducted a 12-week, open-label, cross-over study comparing tadalafil (10 or 20 mg) to sildenafil (25 mg, 50 mg, or 100 mg).Citation40 No differences were found in IIEF EF domain or SEP 2; however there was a small but statistically significant difference in SEP 3 responses favoring tadalafil (72% vs. 77%, P = 0.003). Additionally, at the conclusion of the study, 71% of patients preferred tadalafil to sildenafil. Two other double-blinded cross-over studies comparing sildenafil 50 mg and tadalafil 20 mg showed similar patient preference for tadalafil at the conclusion of the study; however, no benefit in efficacy was reportedCitation41,Citation42 In a single non-industry sponsored comparison trial of sildenafil (100 mg), vardenafil (20 mg), and tadalafil (20 mg), 132 patients were prospectively enrolled in an open-label, cross over trial over 45 to 60 days with patient drug preference the primary outcome.Citation43 At the end of the trial, 52% preferred tadalafil, 28% chose sildenafil, and 20% chose tardenafil. In terms of efficacy, tadalafil was statistically better than vardenafil measured both by IIEF and the Erectile Dysfunction Inventory for Treatment Satisfaction (EDITS) Questionnaire. A statistically significant difference in favor of tadalafil was also observed when compared to sildenafil as measured by the EDITS questionnaire. While there appears to be a trend towards the superiority of tadalafil over other PDE5 inhibitors for the treatment of ED, these results must be interpreted with caution. Half of these studies were open label and therefore subject to potential patient and investigator bias.Citation40,Citation43 Additionally, two studies used a maximum dose of sildenafil of 50 mg, which may represent a lower effective dose when compared directly with 20 mg of tadalafil.Citation41,Citation42
Pharmacology of tadalafil
Many potential targets for therapeutic intervention in PAH have been identified.Citation44 NO formation in pulmonary artery endothelium is needed to promote pulmonary artery vasodilation and inhibition of smooth muscle cell proliferation. Citation45 The production of cyclic guanosine monophosphate (cGMP) by activation of guanylate cyclase by NO in turn activates protein kinase G (PKG) that decreases pulmonary artery smooth muscle cell calcium and potassium levels leading to pulmonary artery vasodilation, decreased smooth muscle cell proliferation, and increased apoptosis of pulmonary artery smooth muscle cells.Citation31 Patients with PAH have been shown to have both decreased nitric oxide production from the pulmonary artery endothelium and to have increased PDE5 expression in the pulmonary artery smooth muscle cells.Citation46–Citation48 The combined effects of decreased nitric oxide production and increased PDE5 expression ultimately promote pulmonary artery vasoconstriction and increased pulmonary vascular resistance. As PDE5 leads to degradation of cGMP, a selective PDE5 inhibitor would have numerous downstream benefits including pulmonary artery vasodilation, decreased pulmonary vascular resistance and ultimately increased cardiac output. Additionally, PDE5 inhibitors may augment right ventricular function though their inhibition of phosphodiesterase 3.Citation49
Phosphodiesterase type 5 is located primarily in pulmonary artery smooth muscle cells and in the penile circulation. Its main role is to degrade cGMP located in these tissues. The relative paucity of PDE5 in the systemic vasculature makes this an attractive therapeutic target as one would expect minimal systemic vasodilation as opposed to nonselective vasodilators such as calcium channel blockers which cause prohibitive hypotension in most patients with PAH.Citation3
Serum concentrations of tadalafil reach a maximum 2 to 8 hours after ingestion of 40 mg with a mean terminal half-life of 35 hours in patients with PAH. Tadalafil is predominantly metabolized by the liver by CYP3A and eliminated primarily in the feces and urine.Citation50,Citation51 Unlike sildenafil, it is recommended that patients with mild-to-moderate renal or hepatic dysfunction undergo a dose adjustment to 20 mg daily.Citation31 As tadalafil is metabolized by CYP3A, inhibitors of the enzyme such as clarithromycin may lead to elevated serum concentrations.Citation50 Combined usage of bosentan and tadalafil has been shown to lead to a 41.5% decrease in the serum levels of tadalafil with no effect on bosentan.Citation52 Similar effects of combined use of tadalafil and ambrisentan have not been observed.Citation53
PDE-5 inhibition for the treatment of PAH
Numerous studies have shown that sildenafil is efficacious in improving hemodynamics, improving exercise tolerance, and improving quality of life.Citation21–Citation24,Citation28,Citation29 Limited by study size and design, sildenafil, has not been shown to alter the natural history of PAH or to improve survival. Sildenafil’s short half-life necessitates multiple daily dosing schedules and the currently approved dose of 20 mg 3 times daily may be inadequate for some patients.
Tadalafil has a longer half-life than sildenafil, thus allowing once-daily dosing. Starting in 2004, scattered case reports and small case series detailed the successful use of tadalafil for patients with PAH. An initial report in 2004 by Palmieri et al detailed the use of 20 mg tadalafil given every other day in a 72-year-old female patient with PAH who failed epoprostenol infusion.Citation54 The patient ultimately showed improvements in hemodynamics (pulmonary artery systolic pressure by echocardiogram went from 105 mmHg at baseline to 65 mmHg six months later), functional class (New York Heart Association class IV to II over 6 months), and improved gas exchange (partial pressure of oxygen to fraction of inhaled oxygen ratio went from 150 to 300 mmHg). Several other case reports using similar doses had similar results: In 2008, Tay and colleagues reported a case series of 12 patients with PAH who were voluntarily changed from sildenafil at a dose of 100 to 150 mg/day to 10 to 20 mg/day of tadalafil.Citation55 Two small series describing the use of combination therapy with tadalafil have been published. The first published in 2008 by Bendayan et al details the combined use of prostacyclins and tadalafil. The addition of tadalafil to prostacyclins improved the 6-minute walk distance by nearly 60 m and improved functional status in 3 of 4 patients.Citation56 Faruqi and colleagues reported the use of combined tadalafil and sitaxentan (a selective ERA approved in Europe and Australia) in 3 patients with PAH.Citation57 Modest benefits in mean pulmonary artery pressure and 6-minute walk distance were noted after a minimum of 6 months, and, importantly, no serious adverse effects were reported. Given the clinical success of sildenafil in the treatment of PAH and the observed effects of small case series utilizing tadalafil, larger, prospective trials involving tadalafil were inevitable.
Comparisons between PDE-5 inhibitors
In general, head to head comparisons of various PDE5 inhibitors and their effect on the pulmonary circulation are lacking. Ghofrani et al compared the acute effects on hemodynamics and gas exchange of sildenafil (50 mg), vardenafil (10 mg and 20 mg), and tadalafil (20 mg, 40 mg, and 60 mg) on patients with PAH.Citation33 60 patients with New York Heart Association (NYHA) class II to IV symptoms were randomized to receive sildenafil, vardenafil, or tadalafil acutely after receiving short-term inhaled NO. After 120 minutes, all three PDE5 inhibitors showed significant reductions in mean pulmonary artery pressure with a range of −10.0 mmHg to −18.3 mmHg. Only sildenafil and tadalafil were shown to be selective pulmonary vasodilators rather than systemic vasodilators (change in PVR/SVR ratio of −9.3 to −16.0), and only sildenafil was shown to improve oxygenation with an increase in the partial pressure of arterial oxygen of 8.9 mmHg. The authors postulated that the varying effects on hemodynamics and oxygenation were a result of different affinities of the drugs for other PDE subgroups other than 5, which may affect the pulmonary and systemic vasculature differently.
Clinical trials of tadalafil in PAH
In the only randomized, placebo-controlled study examining the use of tadalafil in PAH, Galiè and colleagues reported the effects of tadalafil alone or in combination with bosentan in the treatment of PAH (PHIRST trial).Citation58 Four hundred and six patients were randomized to placebo, 2.5, 10, 20, or 40 mg of tadalafil daily and followed for 16 weeks. Fifty-three percent of enrolled patients were taking bosentan at the time of enrollment. The primary endpoint was the change from baseline in the 6-minute walk test (6MWT) (). Only patients randomized to 40 mg of tadalafil achieved the predetermined level of statistical significance (P < 0.01) with an improvement in 6MWT of 33 m (95% CI, 15–50 m). Patients not taking bosentan improved by 44 m (95% CI, 20–69 m), and those taking bosentan improved by 23 m (95% CI −2 to 48 m). A review of secondary endpoints indicated improvements in time to clinical worsening, incidence of clinical worsening and health related quality of life. The authors suggested a possible drug–drug interaction with bosentan and tadalafil resulting in lower plasma levels of tadalafil, thus explaining the blunted response of tadalafil when used in combination with bosentan.Citation52,Citation58
Figure 1 Change in 6-minute walk distance over 16 weeks, tadalafil versus placebo: results of a 16-week randomized placebo controlled trial of tadalafil for the treatment of pulmonary artery hypertension. Significant improvements in 6-minute walk distance were observed in patients receiving 10, 20 and 40 mg daily of tadalafil compared with placebo.
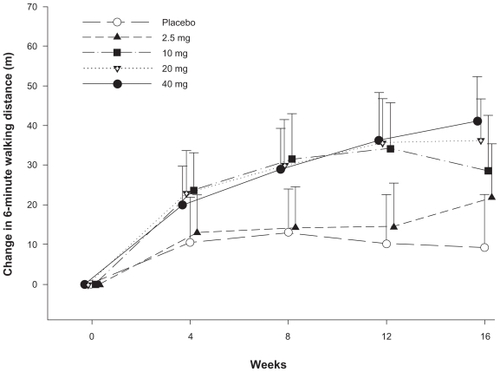
Data from a long-term extension study of the PHIRST trial has yet to be published.Citation58 Tadalafil was generally well tolerated with the most common side effects being headache, myalgias, and flushing.Citation58 A second study published using the initial PHIRST data showed that patients receiving 40 mg daily of tadalafil for PAH had significant improvements in quality of life compared with placebo.Citation59 Self-reported health status and patient reported health-related quality of life using the Short Form 36 (SF-36) and EuroQOL (EQ-5D) were measured at baseline, week 8 and week 16. Interestingly, improvements observed in 6MWT did not correlate with improvements in quality of life measures.
There remain several unanswered questions regarding the use tadalafil in PAH. Mortality benefit for PAH which has only been shown only with the use of epoprostenol, is not known for tadalafil or other PDE5 inhibitors.Citation14,Citation15 Small sample size, short trial duration, and the relative rarity of PAH has made studying survival challenging. A recent meta-analysis examining the impact of treatment on PAH utilizing prostanoids, ERAs, and PDE5 inhibitors was recently published.Citation60 Twenty-one randomized controlled trials were included in the analysis which showed an overall relative risk of mortality of 0.57 (0.35, 0.93) when receiving PAH specific therapy compared with placebo. Subset analysis revealed 5 RCTs which utilized PDE5 inhibitors (all using sildenafil – PHIRST data not included). There was a relative risk of death of 0.39 (0.14, 1.05) compared with placebo when patients were given PDE5 inhibitors, which nearly met statistical significance. This meta-analysis, while provocative, is limited by the relatively short duration of included studies and caution must be made in attributing a potential survival benefit to the use of tadalafil for PAH as the PHIRST data were not included.
Conclusion
The availability of several classes of PAH specific medications has revolutionized the treatment of PAH over the past two decades. Improvements in symptoms, exercise tolerance, quality of life, and survival have transformed the management this patient population from that of hopelessness to one of great promise. Tadalafil, the first once-daily PDE5 inhibitor FDA approved for the treatment of PAH, has been shown to improve exercise tolerance, pulmonary hemodynamics, and quality of life. The drug is well tolerated and carries with it a favorable side effect profile. Preliminary data on its use in combination therapy have been promising. Longer-term studies examining the durability of its benefits are ongoing. Lastly, the role of tadalafil in patients with pulmonary hypertension in groups II–V has not been clearly defined, with more studies needed to establish a role, if any, in these patient populations.
Disclosures
The authors declare no conflicts of interest.
References
- HumbertMSitbonOChaouatAPulmonary arterial hypertension in France: results from a national registryAm J Respir Crit Care Med20061731023103016456139
- PeacockAJMurphyNFMcMurrayJJCaballeroLStewartSAn epidemiological study of pulmonary arterial hypertensionEur Respir J20073010410917360728
- ChinKMRubinLJPulmonary Arterial HypertensionJ Am Coll Cardiol2008511527153818420094
- BarstRJMcGoonMTorbickiADiagnosis and differential assessment of pulmonary arterial hypertensionJ Am Coll Cardiol20044312 Suppl S40S47S15194177
- FishmanAPClinical classification of pulmonary hypertensionClin Chest Med20012238539111590835
- SimonneauGRobbinsIMBeghettiMUpdated clinical classification of pulmonary hypertensionJ Am Coll Cardiol2009541 SupplS43S5419555858
- SitbonOHumbertMJaisXLong-term response to calcium channel blockers in idiopathic pulmonary arterial hypertensionCirculation20051113105311115939821
- RubinLJBadeschDBBarstRJBosentan therapy for pulmonary arterial hypertensionN Engl J Med200234689690311907289
- ChannickRBadeschDBTapsonVFEffects of the dual endothelin receptor antagonist bosentan in patients with pulmonary hypertension: a placebo-controlled studyJ Heart Lung Transplant20012026226311250530
- GalièNOlschewskiHOudizRJAmbrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy Studies (ARIES) GroupAmbrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2Circulation20081173010301918506008
- McLaughlinVVSitbonOBadeschDBSurvival with first-line bosentan in patients with primary pulmonary hypertensionEur Respir J20052524424915684287
- SitbonOMcLaughlinVVBadeschDBSurvival in patients with class III idiopathic pulmonary arterial hypertension treated with first line oral bosentan compared with an historical cohort of patients started on intravenous epoprostenolThorax2005601025103016055621
- RubinLJMendozaJHoodMTreatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trialAnn Intern Med19901124854912107780
- BarstRJRubinLJMcGoonMDCaldwellEJLongWALevyPSSurvival in primary pulmonary hypertension with long-term continuous intravenous prostacyclinAnn Intern Med19941214094158053614
- BarstRJRubinLJLongWAA comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study GroupN Engl J Med19963342963028532025
- ShapiroSMOudizRJCaoTPrimary pulmonary hypertension: improved long-term effects and survival with continuous intravenous epoprostenol infusionJ Am Coll Cardiol1997303433499247503
- HigenbottamTButtAYMcMahonAWesterbeckRSharplesLLong-term intravenous prostaglandin (epoprostenol or iloprost) for treatment of severe pulmonary hypertensionHeart1998801511559813561
- TapsonVFGomberg-MaitlandMMcLaughlinVVSafety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trialChest200612968368816537868
- SimonneauGBarstRJGalieNContinuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trialAm J Respir Crit Care Med200216580080411897647
- BarstRJGalieNNaeijeRLong-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinilEur Respir J2006281195120316899485
- PrasadSWilkinsonJGatzoulisMASildenafil in primary pulmonary hypertensionN Engl J Med2000343134211183578
- MichelakisETymchakWLienDWebsterLHashimotoKArcherSOral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: comparison with inhaled nitric oxideCirculation20021052398240312021227
- SastryBKSNarasimhanCReddyNKRajuBSClinical efficacy of sildenafil in primary pulmonary hypertension, I: a randomized, placebo- controlled, double-blind, crossover studyJ Am Coll Cardiol2004431149115315063421
- GalièNGhofraniHATorbickiASildenafil citrate therapy for pulmonary arterial hypertensionN Engl J Med20053532148215716291984
- HoeperMMMarkevychISpiekerkoetterEWelteTNiedermeyerJGoal-oriented treatment and combination therapy for pulmonary arterial hypertensionEur Respir J20052685886316264047
- HoeperMMWelteTSildenafil citrate therapy for pulmonary arterial hypertensionN Engl J Med20063541091109316525151
- SimonneauGRubinLJGalieNfor the PACES Study GroupAddition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trialAnn Intern Med200814952153018936500
- Pepke-ZabaJGilbertCCollingsLBrownMCJSildenafil improves health-related quality of life in patients with pulmonary arterial hypertensionChest200813318318918187744
- SinghTRohitMGroverAMalhotraSVijayvergiyaRA randomized, placebo-controlled, double-blind, crossover study to evaluate the efficacy of oral sildenafil therapy in severe pulmonary artery hypertensionAm Heart J2006151851.e1851e516569546
- Authors/Task Force Members, Galie N, Hoeper MM, Humbert M, et al. ESC Committee for Practice Guidelines (CPG), Vahanian A, Auricchio A, Bax J, et alGuidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT)Eur Heart J2009302493253719713419
- ArcherSLMichelakisEDPhosphodiesterase type 5 inhibitors for pulmonary arterial hypertensionN Engl J Med20093611864187119890129
- PaulGAGibbsJSBoobisARAbbasAWilkinsMRBosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertensionBr J Clin Pharmacol20056010711215963102
- GhofraniHAVoswinckelRReichenbergerFDifferences in hemodynamic and oxygenation responses to three different phosphodiesterase- 5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective studyJ Am Coll Cardiol2004441488149615464333
- TsertsvadzeAFinkHAYazdiFOral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysisAnn Intern Med200915165066119884626
- RosenRCRileyAWagnerGOsterlohIHKirkpatrickJMishraAThe international index of erectile function (IIEF): a multidimensional scale for as- sessment of erectile dysfunctionUrology1997498228309187685
- BrockGBMcMahonCGChenKKEfficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analysesJ Urol20021681332133612352386
- SkoumalRChenJKulaKBrezaJCalomfirescuNBassonBREfficacy and treatment satisfaction with on-demand tadalafil (Cialis) in men with erectile dysfunctionEur Urol20044636236915306109
- PorstHPadma-NathanHGiulianoFAnglinGVaraneseLRosenREfficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trialUrology20036212112512837435
- RajferJAliottaPJSteidleCPFitchWP3rdZhaoYYuATadalafil dosed once a day in men with erectile dysfunction: a randomized, double-blind, placebo-controlled study in the USInt J Impot Res2007199510316871272
- EardleyIMironeVMontorsiFRalphDKellPWarnerMRAn open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naïve to phosphodiesterase 5 inhibitor therapyBJU Int2005961323133216287454
- von KeitzARajferJSegalSMurphyADenneJCostiganTA multicenter, randomized, double-blind, crossover study to evaluate patient preference between tadalafil and sildenafilEur Urol20044549950715041116
- GovierFPotempaAJKaufmanJDenneJKovalenkoPAhujaSA multicenter, randomized, double-blind, crossover study of patient preference for tadalafil 20 mg or sildenafil citrate 50 mg during initiation of treatment for erectile dysfunctionClin Ther20032527092314693299
- TolràJRCampanaJMCiutatLFMirandaEFProspective, randomized, open-label, fixed-dose, crossover study to establish preference of patients with erectile dysfunction after taking the three PDE-5 inhibitorsJ Sex Med2006390190916942534
- HumbertMSitbonOSimonneauGTreatment of pulmonary arterial hypertensionN Engl J Med20043511425143615459304
- BudhirajaRTuderRMHassounPMEndothelial dysfunction in pulmonary hypertensionCirculation200410915916514734504
- GiaidASalehDReduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertensionN Engl J Med19953332142217540722
- MurrayFMacLeanMRPyneNJIncreased expression of the cGMP-inhibited cAMP-specific (PDE3) and cGMP binding cGMP-specific (PDE5) phosphodiesterases in models of pulmonary hypertensionBr J Pharmacol20021371187119412466227
- WhartonJStrangeJWMøllerGMAntiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cellsAm J Respir Crit Care Med200517210511315817798
- NagendranJArcherSLSolimanDPhosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractilityCirculation200711623824817606845
- Tadalafil (Adeirca) for pulmonary arterial hypertensionMed Lett Drugs Ther200951878819890245
- ForgueSTPattersonBEBeddingAWTadalafil pharmacokinetics in healthy subjectsBr J Clin Pharmacol20066128028816487221
- WrishkoREDingemanseJYuADarsteinCPhillipsDLMitchellMIPharmacokinetic interaction between tadalafil and bosentan in healthy male subjectsJ Clin Pharmacol20084861061818305126
- SpenceRMandagereAHarrisonBDuftonCBoinpallyRNo clinically relevant pharmacokinetic and safety interactions of ambrisentan in combination with tadalafil in healthy volunteersJ Pharm Sci2009984962497419455620
- PalmieriEAAffusoFFazioSLemboDTadalafil in primary pulmonary arterial hypertensionAnn Intern Med200414174374415520445
- TayELGeok-MuiMKPoh-HoonMCYipJSustained benefit of tadalafil in patients with pulmonary arterial hypertension with prior response to sildenafil: a case series of 12 patientsInt J Cardiol200812541641717407795
- BendayanDShitritDKramerMRCombination therapy with prostacyclin and tadalafil for severe pulmonary arterial hypertension: a pilot studyRespirology20081391691818811891
- FaruqiSFathiHMoriceAHCombination of sitaxentan and tadalafil for idiopathic pulmonary arterial hypertension following relapse on bosentanInt J Cardiol2009125 [Epub ahead of print]
- GalièNBrundageBHGhofraniHAPulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study GroupTadalafil therapy for pulmonary arterial hypertensionCirculation20091192894290319470885
- Pepke-ZabaJBeardsworthAChanMAngalakuditiMTadalafil therapy and health-related quality of life in pulmonary arterial hypertensionCurr Med Res Opin2009252479248519686085
- GalièNManesANegroLPalazziniMBacchi-ReggianiMLBranziAA meta-analysis of randomized controlled trials in pulmonary arterial hypertensionEur Heart J20093039440319155250