Abstract
The chemokine and adhesion molecule fractalkine and its receptor CX3CR1 have emerged as interesting regulators in inflammation and related atherosclerosis. The pro-inflammatory status may be counteracted by appropriate treatment, such as in rehabilitation. We compared serum fractalkine concentrations of 46 patients with coronary heart disease (CHD) and 47 insulin-dependent diabetic patients (IDDM) following rehabilitation with those of 50 control subjects. Following rehabilitation serum fractalkine levels (477 ± 225 pg/mL) in CHD patients were similar to those in control subjects (572 ± 205 pg/mL; P = 0.303), whereas fractalkine levels were lower in IDDM patients (430 ± 256 pg/mL; P = 0.042). No significant difference between CHD and IDDM patients was present (P = 0.319). Postprandial hyperlipemia may influence inflammation; thus, we investigated fractalkine levels four and eight hours after inducing postprandial hyperlipemia. However, we did not find any significant alterations in CHD and diabetic patients, whereas the fractalkine levels in controls were reduced. In vitro, lipofundin used as a hyperlipemic stimulus was added to vessel wall cells and reduced fractalkine levels. Low fractalkine levels in patients attending rehabilitation indicate a beneficial effect of the rehabilitation procedure on innate inflammatory pathways, such as the chemokine and adhesion molecule fractalkine.
Introduction
Atherosclerosis is a multifactorial disease of the blood vessel wall that is strongly influenced by inflammatory pathways,Citation1,Citation2 such as adhesion of leukocytes to the endothelium, and other inflammatory or innate mechanisms.Citation3,Citation4 During atherogenesis, monocytes accumulate in the neointima and subsequently transform into tissue macrophages/foam cells. In addition, other leukocytes, such as mast cellsCitation5 or T-cells,Citation6 are also found in atherosclerotic lesions. The invasion of the vessel wall starts when blood cells adhere to the endothelium. However, the mechanisms underlying the initial activation have still not been completely defined. The leukocyte-endothelial interaction involves rolling, firm adhesion, and migration into the neointima, which is mediated by selectins and other adhesion moleculesCitation7 as well as by chemokines.Citation8 Chemokines attract and direct cells along a chemotactic gradient. Furthermore, they may activate other atherosclerosis-relevant functions of vessel wall cells or leukocytes, for example, by activating adhesion molecules on the endothelium.Citation8,Citation9
The recently discovered chemokine fractalkineCitation10 and its receptor CX3CR1 have emerged as interesting intermediaries in atherosclerosis. Fractalkine is a multidomain protein expressed on the surface of a variety of cells, including activated endothelial and smooth muscle cells.Citation11–Citation15 Fractalkine exhibits features which have not been described for other members of the chemokine family, including its exceptionally large size of 95 kDa and the presence of a transmembrane anchor. Furthermore, a soluble form can be released from its membrane-associated form by extracellular cleavage.Citation16 CX3CR1 is a seven-transmembrane-spanning fractalkine receptor, mediating its signal by G-proteins, as most cytokines do. This receptor mediates the firm adhesion to fractalkine even in the absence of G-protein activationCitation17 and thus plays a role as an adhesion molecule in addition to functioning as a chemokine. In this way, membrane-bound fractalkine contributes to the adhesion of CX3CR1-expressing leukocytes. On the other hand, soluble fractalkine, after enzymatic cleavage and release, has potent chemotactic activity for monocytes and lymphocytes but, like CC chemokines, not for granulocytes.Citation18
Cytokines, including chemokines, and other innate compounds are strongly involved in the inflammatory pathways contributing to atherosclerosis.Citation4,Citation7,Citation8 Thus, evidence has been provided that fractalkine plays a role in various cardiovascular diseases. For example, increased fractalkine levels have been reported in patients after cardiac allograft rejection. In a murine model, allograft survival was prolonged from 7 ± 1 days to 49 ± 30 days (P ≤ 0.0008) by administering fractalkine antibodies.Citation19 These studies identified a critical role for fractalkine in the pathogenesis of acute rejection and suggest that fractalkine inhibition might be a potential therapeutic target. Fractalkine levels have only been rarely determined in patients; however, it has been shown that Alzheimer patients express enhanced fractalkine levels, correlating with the Mini-Mental State Examination (MMSE) score.Citation20 Another study also reported that fractalkine levels are enhanced in Wegener’s granulomatosis.Citation21 In patients with coronary artery disease fractalkine plasma levels were enhanced and then reduced after statin therapy.Citation22 When CX3CR1 genotypes were analyzed in patients with acute coronary syndromes and healthy controls, CX3CR1-I249 heterozygosity was associated with a markedly reduced risk of acute coronary events, independent of established coronary risk factors such as smoking or diabetes.Citation23,Citation24
Both the risk and the rate of development of atherosclerosis are increased in diabetic patients, but the underlying mechanisms are still not completely understood. Epidemiologic and pathophysiologic evidence suggests that lipoproteins, platelets, or soluble clotting factors represent contributing factors. Furthermore, both balance of the prostaglandin-metabolites prostacycline and thromboxane and blood pressure regulation are disturbed. On the other hand, the metabolism and proliferation of arterial smooth muscle cells are modified.Citation25 Many of these alterations accompany hyperinsulinemia and may account for the recent evidence that hyperinsulinemia is a risk factor for atherosclerosis. Cytokines and chemokines may contribute to regulate atherosclerosis-related pathways in diabetes or its associated diseases.Citation26,Citation27 However, little is known about the expression of fractalkine and its receptor in diabetes, although the mRNA of fractalkine and its receptor were upregulated in diabetic rats in the early stages of diabetic nephropathy.Citation28
To the best of our knowledge, fractalkine levels have not yet been analyzed in patients with insulin-dependent diabetes following rehabilitation. An optimized medical treatment consisting of medication such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARBs), and statins plays an important role during rehabilitation. Some of the aforementioned drugs may also exert anti-inflammatory properties, which could contribute to their beneficial effects. Indeed, fractalkine levels were reduced in patients with coronary artery disease after a six-week statin treatment.Citation22 Thus, rehabilitation may normalize levels of fractalkine.
In diabetic patients postprandial hyperlipemia may represent a potential risk factor for atherosclerosis.Citation29 Postprandial hyperlipemia can induce an inflammatory state by activating granulocytes,Citation30 leukocytes,Citation31 or endothelial cells.Citation32 Triglyceride-rich lipids (TRL) may even penetrate the arterial wall and activate vascular smooth muscle cell genes differently, depending on the composition (ie, butter, vegetable oil/fish oil, or olive oil) of the lipid.Citation33 Furthermore, chylomicron remnants containing TRLs have been shown to potently activate smooth muscle cell MCP-1 production.Citation34 Postprandial hyperlipemia may promote inflammation in patients and oppose therapy. However, it is not clear whether therapy during rehabilitation may prevent postprandial hyperlipemia from inducing inflammation.
Thus, in the present study we investigated the expression of serum fractalkine in patients with coronary heart disease (CHD) and patients with insulin-dependent diabetes following optimized medical treatment after a period of rehabilitation. Subsequently, we analyzed the effect of postprandial hyperlipemia. We found that fractalkine levels in CHD and diabetic patients after rehabilitation were comparable to those in control subjects and that the postprandial fractalkine levels were not significantly increased. However, postprandial fractalkine levels in control subjects were reduced, a finding paralleled by in vitro cell culture data. These data indicate that the anti-inflammatory effects of the rehabilitation medication played an important role in the study patients.
Material and methods
Study population
We determined the serum concentrations of fractalkine in 46 nondiabetic patients with angiographically proven CHD, 47 insulin-dependent diabetic patients (IDDM), and 50 healthy controls. The patients were recruited in the “Curschmann Klinik für Rehabilitation” (Timmendorfer Strand/Germany). The control subjects were volunteers (mostly working at the Curschmann Clinic) with no prove of CHD or diabetes mellitus. The study was approved by the ethics committee of the University of Lübeck, Schleswig Holstein, Germany. All patients gave written informed consent.
Blood sampling and clinical laboratory parameters
Blood samples were taken from all subjects under standardized conditions after an overnight period of fasting (time-zz [compared below]). The next blood samples were taken four and eight hours later. All blood samples were immediately centrifuged at 4,000 rpm for 10 minutes, supernatants were extracted and divided into aliquots. All aliquots were stored at −80 °C until analysis. The fractalkine levels in the samples were measured by using a sandwich ELISA (enzyme-linked immunosorbent assay; DuoSet, R&D Systems, Wiesbaden, Germany) according to the manufacturer’s recommendations. Besides fractalkine, atherosclerosis-relevant serum markers of inflammation (C-reactive protein [CRP]) and lipid metabolism (low-density lipoprotein cholesterol [LDL-C]; high-density lipoprotein cholesterol [HDL-C]; total cholesterol [TC]; and triglycerides [TG]) were measured. The lipid markers were routinely determined by standard clinical laboratory analysis.
Induction of postprandial hyperlipemia
Postprandial hyperlipemia was induced by eating a high calorie fatty meal. Briefly, the meal contained a total of 1,265 kcal/m2 body surface area. It was composed of 105 g fat (consisting of approximately 52 g saturated fat, 53 g unsaturated fat, and 300 mg cholesterol), 48 g carbohydrates, and 32 g protein. Probands were instructed to eat the meal within 15 minutes and not to eat any other food until the subsequent blood samples were taken. Before the meal (time-point 0) the diabetic patients received a dose of insulin subcutaneously, which was individually adapted to the meal described above. Glucose levels were measured every two hours until the end of the study period in order to prevent severe hypo- or hyperglycemia. All glucose levels were in an acceptable range; thus, no glucose or further insulin administration was required until the end of the eight-hour study period.
Isolation and culture of human vascular vessel wall cells
Human vascular smooth muscle cells were isolated from unused portions of saphenous veins obtained following bypass surgery, as described previously.Citation35,Citation36 Use of the specimens that would otherwise have been discarded was approved by the local ethics committee. Dulbecco’s modified Eagle’s medium (DMEM; 1 g/L D-glucose) containing 10% fetal calf serum (FCS), 1% antibiotics, and 1% l-glutamine (DMEM medium) was used for cell culture. Upon confluency the cells were subcultured after treatment with trypsin/EDTA (0.05% 0.02%, 10 minutes, 37 °C, Biochrom, Berlin, Germany). We used smooth muscle cells in passages 4 to 7. Stimulation experiments were performed in 24-well culture plates as specified in . Lipofundin LCT-10 solution was obtained from Braun (Melsungen, Germany).
Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS Inc, Chicago, IL, USA). Continuous variables were compared by ANOVA. Chi-squared test was used for categorical variables. Correlation analysis was conducted according to the Pearson method.
Results
Baseline characteristics
The demographic data for the study populations are presented in . Compared with CHD patients and control subjects, the IDDM patients were older. The BMI was not significantly different, and the distribution of sexes was similar among the three groups. More smokers were found among the healthy control subjects than in the two patient groups. On the other hand, more subjects with hypertension or a family history of CHD were present in the patient groups. As anticipated, medical treatment with statins, β-blockers, ACE inhibitors, ARBs, and diuretics was more prevalent in the patient groups. Except for diuretics, medication was similar in the tested patient groups. Healthy controls did not suffer from any obvious atherosclerotic, metabolic, or inflammatory disease.
Table 1 Characteristics of the investigated study populationsTable Footnotea
The fractalkine levels in diabetic patients are lower than in healthy control subjects
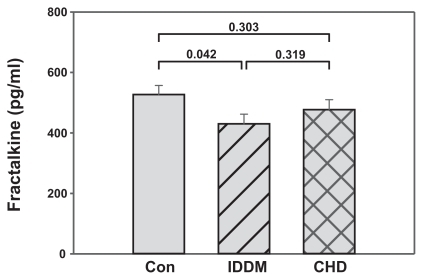
Fractalkine levels have not been measured yet in diabetic patients following rehabilitation. Thus, we analyzed the expression of fractalkine in the IDDM and CHD patients who received optimal medical treatment and underwent rehabilitation for four weeks. The fractalkine levels of the patients were compared to those of the control subjects. A comparison of the fractalkine levels in CHD and IDDM patients after rehabilitation and healthy controls (time-point 0) is shown in . At this time-point fractalkine levels were slightly but not statistically significantly lower in CHD patients (477 ± 225 pg/ml) than in control subjects (527 ± 205 pg/ml; P = 0.303). In contrast, they were lower in IDDM patients than in the controls (430 ± 256 pg/ml; P = 0.042), whereas they were not significantly different from fractalkine levels in CHD patients (IDDM vs CHD; P = 0.319). Thus, fractalkine levels in both patient groups were below or equal to control levels.
Figure 1 Fraktalkine levels in patients following rehabilitaiton are not higher than fractalkine levels in healthy control subjects. Blood samples of diabetic patients (IDDM; n = 47) and coronary heart disease patients (CHD; n = 46) following rehabilitation and blood samples of apparently healthy volunteers (Con; n = 50) were measured by fractalkine ELISA. Mean and standard error of the mean are presented. Significances are given above the columns.
Postprandial hyperlipemia does not reduce fractalkine levels in IDDM and CHD patients
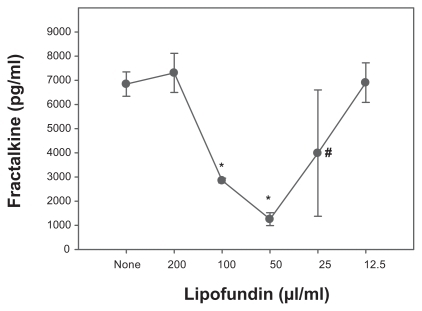
Hyperlipemia can activate inflammatory pathways. However, it was unclear whether postprandial hyperlipemia affects inflammatory parameters in rehabilitation patients as compared to control subjects. Thus, after the first blood sample was taken (time-point 0), a defined postprandial hyperlipemia was induced in the three investigated groups. Four and eight hours after the first blood sampling and eating the defined meal the next samples were taken (time-point 4; time-point 8). At time-point 4 the fractalkine levels were higher in diabetic patients (548 ± 300 pg/mL) than in CHD patients (437 ± 228 pg/mL; P = 0.033); however, they were not different from the control levels (474 ± 203 pg/mL; ). At time-point 8 the fractalkine levels were 432 ± 212 pg/mL in the CHD group, 454 ± 262 pg/mL in the IDDM group, and 393 ± 166 pg/ml in the control group. These values were not significantly different from each other. Comparing each group at the different time-points by ANOVA revealed that only the differences in the values for healthy control subjects were statistically significant (P = 0.004). Post hoc analysis showed that the fractalkine levels at time-point 8 were different from those at time-point 4 (P = 0.046) and time-point 0 (P = 0.001), whereas at time-points 0 and 4 they were not significantly different. In contrast to control subjects, ANOVA analysis of IDDM and CHD patients did not reveal significant differences between the three time-points (P = 0.080 and P = 0.574, for diabetes and CHD, respectively). Post hoc analysis showed the same result at the 0.01 level. However, the IDDM time-points 0 and 4 were different at the 0.05 level (P = 0.033). Taken together, the data indicate that the fractalkine levels were only reduced in control subjects, whereas in CHD the levels remained low and in IDDM patients the levels fluctuated, although the differences were not significant according to ANOVA. In order to investigate the influence of hyperlipemia in vitro we incubated vascular smooth muscle cells, which represent a potential source of fractalkine in atherogenesis, with several lipofundin concentrations. We observed a dose-dependent reduction in fractalkine production ().
Figure 2 Induction of postprandial hyperlipemia reduces fractalkine levels in healthy control subjects, but not in patients following rehabilitation. Blood samples of IDDM and CHD patients following rehabilitation and blood samples of healthy subjects (Con) were collected at time-point 0. Immediately thereafter a postprandial hyperlipemia was induced by a defined meal of 1,285 kcal/m2 body suface (105 g fat + 300 mg cholesterol + 48 g carbohydrates + 32 g protein. At two time-points later (four and eight hours, respectively) blood samples were taken and measured by fractalkine ELISA. Data are presented as a box blot (SigmaPlot). The whiskers and box lines indicate the 10th, 25th, median, 75th, and 90th percentiles. The broken line indicates the mean. Extra symbols indicate outliers. Analysis by one-way ANOVA showed a significant difference between the fractalkine levels at the time-points of the control subjects (P = 0.004), whereas levels for the diabetic (P = 0.080) and the CAD (P = 0.574) patients were not significantly different at the given time-points. Post-hoc analysis at the 0.01 level showed the same result; however, diabetic patients at the time-point 0 and 4 were different at the 0.05 level (P = 0.033).
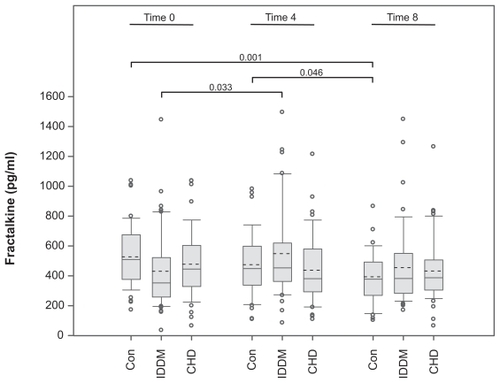
Figure 3 Reduction in fractalkine levels in lipofundin-treated cultures of vessel wall cells. Human vascular smooth muscle cells (20,000 SMC/cm2) were cultured in 24-well plates. After 24 hours the medium was changed and fractalkine production induced by costimulation with interferon-γ and tumor necrosis factor-α (200 U/ml, 20 ng/ml, respectively). Parallel cultures were incubated in the presence of various concentrations of lipofundin. After 24 hours, the supernatants were harvested and measured by fractalkine ELISA. Two experiments provided similar results.
The fractalkine levels in the tested subjects do not correlate with CRP and lipids
The analysis of inflammatory and lipid markers at time-point 0 is presented in . The CRP levels were higher in the diabetic patients (10 ± 16 mg/L) than in CHD patients (6 ± 7 mg/L; P = 0.025) and controls (2 ± 3 mg/L; P ≤ 0.001). All lipid parameters were highest in the healthy control subjects. The differences between controls and IDDM patients were less pronounced than the differences between controls and CHD patients. These data indicate that the lipid-lowering aspect of the IDDM and CHD medication worked properly. The correlation analysis revealed that there was no significant correlation between fractalkine serum levels, CRP, the lipid markers, age, or body mass index (BMI). BMI did not correlate with cholesterol, triglycerides, or LDL either. On the other hand, and as expected, BMI inversely correlated with HDL-C (−0.264; P ≤ 0.001); the high CRP levels inversely correlated with high HDL levels (−0.242; P ≤ 0.001); and cholesterol levels correlated well with triglycerides (0.463; P < 0.001) and LDL-C (0.847; P ≤ 0.001).
Table 2 Classical markers of inflammation and lipid metabolismTable Footnotea
Fractalkine in coronary artery bypass graft surgery and multivessel disease
Among the CHD patients the subgroup of patients who had currently received coronary artery bypass graft (CABG) surgery were analyzed because these patients express the highest pro-inflammatory state prior to rehabilitation. However, in the 14 patients of the CHD group who had received a CABG within two weeks before attending the “Curschmann Klinik für Rehabilitation” (533 ± 288 pg/mL), the fractalkine serum levels were not significantly different from those in the CHD patients not receiving a bypass (459 ± 203 pg/mL; P = 0.349). In addition, a CHD subgroup of patients suffering from multivessel disease with either two (496 ± 237 pg/mL) or three (429 ± 215 pg/mL) stenosed coronary arteries did not express significantly increased fractalkine levels after rehabilitation, as compared to patients with only one (507 ± 231 pg/mL) occluded coronary artery (P = 0.575).
Discussion
Innate inflammatory pathways are important in atherogenesis. Chemokines and adhesion molecules contribute to this process. Disease-associated, enhanced levels of adhesion molecules, cytokines, or chemokines may be reduced after optimal medical treatment during rehabilitation. Thus, we compared the serum levels of fractalkine in patients with CHD or IDDM following rehabilitation with those in healthy control subjects. Furthermore, we investigated the relation between fractalkine and classical risk factors for atherosclerosis in patients with CHD and IDDM in a state of postprandial hyperlipemia. The data show that the fractalkine levels of the patients after rehabilitation were at the control level or lower. Postprandial hyperlipemia reduced the fractalkine levels in control subjects and in vessel wall cells in vitro, whereas postprandial hyperlipemia did not result in significantly different fractalkine levels in diabetic and CHF patients according to ANOVA. These data indicate that the fractalkine levels were similar to control values and that acute hyperlipemia did not alter fractalkine levels in patients, whereas it did so in controls.
Previous studies reported that soluble fractalkine was increased in CHD patients as compared to controls, and that membrane-bound fractalkine was increased in human carotid arteries and in animal models.Citation37,Citation38 In another study serum fractalkine levels were determined:Citation22 These data showed that patients with unstable angina (1,199 ± 112 pg/mL) had enhanced fractalkine levels as compared with control levels (423 ± 25 pg/mL) and that six-month atorvastatin therapy, but not simvastatin therapy, greatly reduced the fractalkine levels in the investigated patients (587 ± 89 pg/mL). These results are in line with the data shown in the present manuscript, which are in the range of those for the controls or statin-treated patients. Fractalkine has been demonstrated to be involved in diabetic nephropathy in murine models. Upregulated fractalkine and CX3CR1 levels were present in the early stage of diabetic kidney disease in rats.Citation28 The aforementioned data suggest that fractalkine and CX3CR1 have an important role in the progression of diabetic nephropathy, functioning as an arrest chemokine in monocyte/macrophage adhesion before migration into the kidney. Blockade of the renin–angiotensin–aldosterone system and treatment with statins currently represent the only clinical strategies for treating the inflammatory process in diabetic nephropathy. Newer strategies include chemokine receptor antagonists and even immunosuppressive therapy, but these are still in the experimental stage. In contrast to the CHD patients, no data exist about the fractalkine levels in untreated diabetic patients. We are currently addressing this question. However, the cited data indicate that fractalkine levels are enhanced in CHD patients. The low fractalkine levels in the same patient group in the present study suggest an analogous reducing effect caused by 1) the rehabilitation process, and/or 2) alleviating the acute status of the disease.
Serum fractalkine levels in CHD and IDDM patients following rehabilitation did not differ from those in healthy control subjects. This may partly be explained by the optimal medical and lifestyle treatment that patients benefit from during rehabilitation. Thus, blood glucose, heart rate, and blood pressure are continuously monitored and promptly corrected. Furthermore, patients profit from physical activity and controlled food intake, ie, lifestyle improvement.Citation39,Citation40
On the other hand, patients with CHD and IDDM in rehabilitation are no longer in an acute phase of the disease. The baseline characteristics of our study population indicate that medical treatment in the two patient groups was more prevalent than in control subjects. The majority (96%) of the CHD and IDDM patients were on statin therapy compared to only 4% in controls. In addition to their lipid-lowering activity, statins may also have anti-inflammatory effects. During the constrictive remodeling status, statins have the ability to stabilize the atherosclerotic plaque.Citation41 In addition, their anti-inflammatory effect was measured by their ability to lower CRP levels.Citation42,Citation43 The lipid and CRP data in the latter publications are in line with those for the control population presented here. The lipid data in the present study patients also indicate that the treatment had a similarly beneficial effect. However, the CRP levels did not reach the control values, which might be explained by the type and dose of statins used in the current study. This may have influenced the expression of the inflammatory parameters.Citation22,Citation41 Other therapeutic agents in atherosclerotic diseases include ACE inhibitors and ARBs. Both agents can reduce the expression of angiotensin I and II receptors on endothelial cells and monocytes. Thereby, they may reduce various inflammatory processes.Citation42,Citation43 ACE inhibitors and ARB therapy was used in 72% of the CHD and IDDM patients, compared to only 10% among the healthy controls. We hypothesize that the low fractalkine levels in the two patient groups may be caused by the combined ACE inhibitor, ARB, and statin treatment.
An approximate measure of treatment efficacy is shown by the differing responses to postprandial hyperlipemia found in the present experiments. Postprandial hyperlipemia significantly reduced fractalkine levels in the controls, whereas it had no effect in the patients. We assume that this lack of response was caused by the anti-inflammatory capacity of the drugs being used. On the other hand, the lower (systemic) fractalkine levels may reflect reduced shedding, which in turn may indicate enhanced vascular inflammation. However, this suggestion remains somewhat speculative since no corresponding data have been published. However, we found that lipid treatment (lipofundin) reduced fractalkine release in vascular smooth muscle cells in a dose-dependent fashion in in vitro experiments. The reduction in the fractalkine levels in the controls also indicates that the selected measurement time-points were adequate. Other reports used similar time-points for postprandial measurements.Citation30,Citation32 On the other hand, we found that cytokines could already be detected in bypass patients after three hours.Citation44 The enhanced fractalkine level at the four-hour time-point may reflect activation of the inflammatory genes by insulin.Citation45,Citation46 However, insulin is also known to have anti-inflammatory effects.
Similarly to other studies we were not able to show any significant correlation between fractalkine levels and other classical risk factors for atherosclerosis, such as lipid metabolism (HDL-C, LDL-C, TC, and TG), age, BMI, or diabetes.Citation22,Citation23 In our study, fractalkine serum levels were not significantly elevated in patients who received CABG surgery within two weeks before attending rehabilitation. This indicates that the acute inflammatory phase in these patients has already subsided. Furthermore, fractalkine levels in patients with two or three occluded coronary arteries were not higher than those in patients with only one stenosed coronary artery. These data suggest that the differences in the inflammatory situations between controls and CHD patients,Citation22 or as suspected here, between controls and diabetic patients are leveled by rehabilitation. Serum fractalkine levels are increased in patients with CHD during the acute inflammatory state of the disease, such as acute myocardial infarction or acute heart failure.Citation22,Citation47 After the acute phase of a cardiovascular inflammatory disease optimized medical treatment, such as that given during rehabilitation, may decrease the inflammatory status, as shown by the similar serum fractalkine levels in patients and healthy controls in this study.
Conclusion
Our results demonstrate the relevance of optimized medical treatment, such as that given during rehabilitation, for patients with CHD and IDDM. The present data show that in CHD and diabetic patients, in whom an activated inflammatory state is expected, inflammation as determined by serum levels of fractalkine was at the control level. Even in an elevated inflammatory state caused by postprandial hyperlipemia, no significant increase in fractalkine serum levels was found in the two patient groups compared to control subjects. The results may be explained by the quiescent disease state, optimized anti-inflammatory treatment with statins, ACE inhibitors and ARBs, and the controlled environment of the CHD and IDDM rehabilitation patients.
Disclosures
The authors report no conflicts of interest in this work. The technical assistance of Ms Claudia Pilowski and Ms Susanne Koch is gratefully acknowledged.
References
- RossRThe pathogenesis of atherosclerosis – an updateN Engl J Med19863144885003511384
- LibbyPInflammation in atherosclerosisNature200242086887412490960
- TedguiAMallatZCytokines in atherosclerosis: pathogenic and regulatory pathwaysPhysiol Rev20068651558116601268
- LoppnowHWerdanKBuerkeMVascular cells contribute to atherosclerosis by cytokine- and innate-immunity-related inflammatory mechanismsInnate Immun200814638718713724
- SunJSukhovaGKWoltersPJMast cells promote atherosclerosis by releasing proinflammatory cytokinesNat Med20071371972417546038
- HanssonGKHolmJJonassonLDetection of activated T lymphocytes in the human atherosclerotic plaqueAm J Pathol19891351691752505620
- BlankenbergSBarbauxSTiretLAdhesion molecules and atherosclerosisAtherosclerosis200317019120314612198
- LusterADChemokines-chemotactic cytokines that mediate inflammationN Engl J Med19983384364459459648
- GersztenREMachFSautyARosenzweigALusterADChemokines, leukocytes, and atherosclerosisJ Lab Clin Med2000136879210945236
- BazanJFBaconKBHardimanGFractalkine: a new class of membrane-bound chemokine with CX3C motifNature199738564448985246
- ImaiTHieshimaKHaskellCIdentification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesionCell1997915215309390561
- HarrisonJKJiangYWeesEAInflammatory agents regulate in vivo expression of fractalkine in endothelial cells of the rat heartJ Leukoc Biol19996693794410614775
- ChapmanGAMooresKEGohilJThe role of fractalkine in the recruitment of monocytes to the endotheliumEur J Pharmacol200039218919510762673
- LudwigABerkhoutTMooresKGrootPChapmanGFractalkine is expressed by smooth muscle cells in response to IFN-gamma and TNF-alpha and is modulated by metalloproteinase activityJ Immunol200216860461211777952
- WongBWWongDMcManusBMCharacterization of fractalkine (CX3CL1) and CX3CR1 in human coronary arteries with native atherosclerosis, diabetes mellitus, and transplant vascular diseaseCardiovasc Pathol20021133233812459434
- HundhausenCMisztelaDBerkhoutTAThe disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesionBlood20031021186119512714508
- HaskellCAClearyMDCharoIFMolecular uncoupling of fractalkine-mediated cell adhesion and signal transduction. Rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activationJ Biol Chem1999274100531005810187784
- MichelsenKSDohertyTMShahPKArditiMTLR signaling: An emerging bridge from innate immunity to atherogenesisJ Immunol20041735901590715528321
- RobinsonLANatarajCThomasDWA role for fractalkine and its receptor (CX3CR1) in cardiac allograft rejectionJ Immunol20001656067607211086038
- KimTSLimHKLeeJYChanges in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and Alzheimer’s diseaseNeurosci Lett200843619620018378084
- BjerkeliVDamasJKFevangBHolterJCAukrustPFrolandSSIncreased expression of fractalkine (CX3CL1) and its receptor, CX3CR1, in Wegener’s granulomatosis--possible role in vascular inflammationRheumatology2007461422142717616549
- DamåsJKBoullierAWaehreTExpression of fractalkine (CX3CL1) and its receptor, CX3CR1, is elevated in coronary artery disease and is reduced during statin therapyArterioscler Thromb Vasc Biol2005252567257216224053
- McDermottDHHalcoxJPSchenkeWHAssociation between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosisCirc Res20018940140711532900
- MoattiDFaureSFumeronFPolymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery diseaseBlood2001971925192811264153
- SteinerGDiabetes and atherosclerosis: an overviewDiabetes198130Suppl 2177028539
- BasuADevarajSJialalIDietary factors that promote or retard inflammationArterioscler Thromb Vasc Biol200626995100116484595
- GravesDTLiuROatesTWDiabetes-enhanced inflammation and apoptosis: impact on periodontal pathosisPeriodontol 200020074512813717850453
- RusterCWolfGThe role of chemokines and chemokine receptors in diabetic nephropathyFront Biosci20081394495517981602
- AlipourAvan OostromAJIzraeljanALeukocyte activation by triglyceride-rich lipoproteinsArterioscler Thromb Vasc Biol20082879279718218988
- WantenGvan Emst-De VriesSNaberTWillemsPNutritional lipid emulsions modulate cellular signaling and activation of human neutrophilsJ Lipid Res20014242843611254755
- van OostromAJRabelinkTJVerseydenCActivation of leukocytes by postprandial lipemia in healthy volunteersAtherosclerosis200417717518215488881
- AlipourAElteJWvan ZaanenHCRietveldAPCabezasMCPostprandial inflammation and endothelial dysfunctionBiochem Soc Trans20073546646917511629
- NorataGDGrigoreLRaselliSPost-prandial endothelial dysfunction in hypertriglyceridemic subjects: molecular mechanisms and gene expression studiesAtherosclerosis200719332132717055512
- BermúdezBLópezSPachecoYMInfluence of postprandial triglyceride-rich lipoproteins on lipid-mediated gene expression in smooth muscle cells of the human coronary arteryCardiovasc Res20087929430318359786
- LoppnowHLibbyPProliferating or interleukin-1-activated human vascular smooth muscle cells secrete copious interleukin-6J Clin Invest1990857317382312724
- ChenLFristerAWangSInteraction of vascular smooth muscle cells and monocytes by soluble factors synergistically enhances interleukin-6 and MCP-1 productionAm J Physiol Heart Circ Physiol2009296987996
- BursillCAChannonKMGreavesDRThe role of chemokines in atherosclerosis: recent evidence from experimental models and population geneticsCurr Opin Lipidol20041514514915017357
- GhilardiGBiondiMLTurriOGuagnelliniEScorzaRInternal carotid artery occlusive disease and polymorphisms of fractalkine receptor CX3CR1: a genetic risk factorStroke2004351276127915118174
- FranklinBATrivaxJEVanheckeTENew insights in preventive cardiology and cardiac rehabilitationCurr Opin Cardiol20082347748618670260
- AlipourAElteJWvan ZaanenHCRietveldAPCastro CabezasMNovel aspects of postprandial lipemia in relation to atherosclerosisAtheroscler Suppl20089394418595782
- NissenSETuzcuEMSchoenhagenPStatin therapy, LDL cholesterol, C-reactive protein, and coronary artery diseaseN Engl J Med2005352293815635110
- PrasadKC-reactive protein-(CRP)-lowering agentsCardiovasc Drug Rev200624335016939632
- ApostolakisSKrambovitisEVlataZKochiadakisGEBaritakiSSpandidosDACX3CR1 receptor is up-regulated in monocytes of coronary artery diseased patients: impact of pre-inflammatory stimuli and renin-angiotensin system modulatorsThromb Res200712138739517521710
- AlbertMADanielsonERifaiNRidkerPMEffect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort studyJAMA2001286647011434828
- WuHPChenCHHsiehHCLiuYCEffects of insulin and glucose on cytokine production from peripheral blood mononuclear cellsChang Gung Med J20083125325918782947
- OkazakiMIwasakiYJingHInsulin enhancement of cytokine-induced coagulation/inflammation-related gene transcription in hepatocytesEndocr J20085596797518614853
- HusbergCNygårdSFinsenAVCytokine expression profiling of the myocardium reveals a role for CX3CL1 (fractalkine) in heart failureJ Mol Cell Cardiol20084526126918585734