Abstract
TOGETHER investigated whether targeting multiple cardiovascular (CV) risk factors using single-pill amlodipine/atorvastatin (AML/ATO) and therapeutic lifestyle changes (TLC) results in greater blood pressure (BP)/lipid control and additional reduction in estimated cardiovascular disease (CVD) risk compared with blood pressure intervention only using amlodipine (AML) + TLC. TOGETHER was a 6-week, randomized, double-blind, double-dummy trial using hypertensive participants with additional CV risk factors without CVD/diabetes. Participants were randomized to either AML/ATO (5 to 10/20 mg) + TLC or AML (5 to 10 mg) + TLC. The primary end point was the difference in proportion of participants attaining both BP (<140/90 mm Hg) and low-density lipoprotein cholesterol (LDL-C) (<100 mg/dL) goals at week 6. At week 6, 67.8% of participants receiving AML/ATO + TLC attained the combined BP/LDL-C goal versus 9.6% with AML + TLC (RD [A–B]: 58.2; 95% CI [48.1 to 68.4] P < 0.001; OR: 19.0; 95% CI 9.1 to 39.6; P < 0.001). Significant reductions from baseline in LDL-C, total cholesterol and triglycerides and estimated 10-year Framingham risk were also observed. Treatment with AML/ATO was well tolerated. In conclusion, a multifactorial CV management approach is more effective in achieving combined BP/LDL-C targets as well as CV risk reduction compared with BP intervention only in this patient population.
Introduction
Approximately one in three Americans are affected by cardiovascular disease (CVD).Citation1 Hypertension is an important modifiable cardiovascular (CV) risk factor that commonly clusters with other CV risk factors, such as dyslipidemia, smoking, and diabetes. These risk factors independently add to the overall risk of CV events.Citation2 Although patients with hypertension and additional CV risk factors could potentially benefit significantly from the treatment of their absolute CV risk,Citation3 the dominant paradigm in routine clinical practice is often focusing on each risk factor in isolation rather than simultaneously. There are many reasons for this isolated approach, both historical and practical, eg, current CV risk factor guidelines which traditionally have been focused on individual risk factors rather than taking an integrated approach addressing a patient’s absolute CV risk; and concern about pill burden by physicians and patients.Citation4,Citation5
Concern about an increasing pill burden may also negatively affect adherence to co-administered antihypertensive and lipid-lowering medication;Citation6 in turn, improved adherence to co-administered antihypertensive and lipid-lowering therapy at levels seen in clinical trials may result in incremental cost savings compared with ‘real-world’ adherence levels.Citation7 By reducing pill burden, combination pills containing both an antihypertensive and a lipid-lowering agent may therefore improve the management of CV risk and improve the cost-effectiveness of the dual therapy.
Therapeutic lifestyle changes (TLC) are an integral part of current hypertension guidelines.Citation4 For patients at low to moderate CV risk, they recommend the use of pharmacotherapy if patients do not attain their blood pressure (BP) therapeutic goals through TLC alone.Citation4
The aim of this randomized, double-blind, double-dummy, controlled trial was to investigate whether a multiple risk factor management strategy using AML/ATO single-pill therapy + TLC results in greater BP/lipid control than the traditional approach of BP intervention only using amlodipine + TLC, in patients at low-to-moderate CV risk who would not be identified as candidates for statin therapy by current guidelines.
Methods
Study participants
Participants who were enrolled in the trial were ≥21 years of age, had hypertension, but no history of CVD or diabetes, and had ≥2 of the following CV risk factors: age ≥45 years if male; ≥55 years if female; current smoker; a family history of premature coronary heart disease (CHD) in a first-degree relative; high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL (<1.0 mmol/L); or a waist circumference of 102 cm (≥40 inches) if male or 88 cm (≥35 inches) if female. All participants were previously treated with AML 5 mg, with BP either controlled or stage 1 hypertension (systolic BP [SBP] ≤ 159 mm Hg and diastolic BP [DBP] ≤ 99 mm Hg) or controlled BP at 10 mg of amlodipine, in addition to a fasting low-density lipoprotein cholesterol (LDL-C) level at screening of ≥100 mg/dL and ≤170 mg/dL. Participants were excluded if they had a history of CHD, stroke, pulmonary vascular disease, or any of the following medications <14 days prior to screening: lipid-lowering drugs; calcium channel blockers other than AML; or >3 antihypertensive agents (including AML).
Trial design
TOGETHER was a 6-week, prospective, randomized, double-blind, double-dummy clinical trial conducted between January 15, 2007 and April 18, 2008 within 38 US clinical sites. Following screening, eligible participants were randomized to either AML (5 to 10 mg) + TLC or AML/ATO single-pill therapy (5 to 10 mg/20 mg) + TLC (). For operational reasons, participants in both treatment arms received a placebo: an amlodipine placebo in the amlodipine/atorvastatin single-pill therapy arm, and an amlodipine/atorvastatin single-pill placebo in the amlodipine arm.
Figure 1 Trial design and flow of participants through the trial.
Abbreviations: AML, amlodipine; ATO, atorvastatin; TLC, therapeutic lifestyle changes.
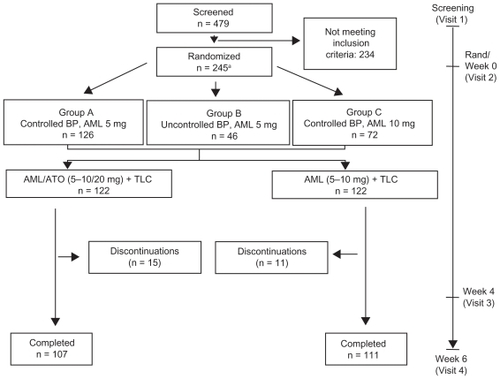
Double-blinding occurred using a central web/telephone computer-based telerandomization system which dispensed two double-blind labeled bottles to participants at each dispensing visit (week 0 and week 4). Participants were instructed to take one tablet from each bottle per day at the same time daily. Randomized participants were stratified based on prior amlodipine dose and BP control, and those who were uncontrolled at 5 mg were uptitrated to 10 mg after randomization.
Blood pressure was measured using a digital blood pressure monitor at each visit. Following a seated resting period (10 to 30 min), three seated BP measurements were taken and the mean of these measurements was calculated.
Serum lipid profiles (LDL-C, total cholesterol, triglycerides, and HDL-C) were determined at each visit. Participants were required to fast for 10 hours prior to the blood test, and were asked not to perform any vigorous exercise the day before the test.
Participants in each treatment arm were counseled on implementing TLC as outlined by the American Heart Association’s Diet, Exercise and Smoking Cessation guidelinesCitation8 at the start of the study for use throughout the entire duration of the trial.
Efficacy measures
The primary end point in this study was the proportion of participants attaining a combined BP goal of <140/90 mm Hg as defined by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7)Citation4 and an optimal LDL-C level (<100 mg/dL) as defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III),Citation9 at week 6. The optimal LDL-C threshold of <100 mg/dL is a considerably more aggressive goal than the level recommended by NCEP ATP III for most patients in this study population.
Secondary end points in this study included the proportion of participants attaining a combined BP goal of <140/90 mm Hg and an LDL-C goal of <100 mg/dL at week 4; the proportion of participants attaining the LDL-C goal of <100 mg/dL at weeks 4 and 6; the proportion of participants attaining the BP goal of <140/90 mm Hg at weeks 4 and 6; change from baseline in SBP, DBP, LDL-C, total cholesterol (TC), triglycerides, and HDL-C at weeks 4 and 6; the predicted 10-year Framingham risk of CHD outcomesCitation10 at weeks 4 and 6; and the safety profile of the AML/ATO single-pill regimen + TLC compared with the AML + TLC regimen. The efficacy population was defined as all randomized participants who took at least one dose of study drug and for whom at least one post-baseline efficacy measurement was available. The safety population was defined as all randomized participants who took at least one dose of study drug.
Adverse events
Adverse events (AEs) were monitored and recorded by the investigator from the time participants took at least one dose of study medication until the end of the treatment period. AEs were summarized by treatment group and severity, and encoded to a system organ class according to the Medical Dictionary for Regulatory Affairs (MedDRA), version 11.0. The investigator also assessed the causal relationship of the AE to the study medication. For any AE considered related to the study medication, follow-up by the investigator was required until the AE ceased or stabilized to levels deemed acceptable by the investigator.
Statistical analysis
Originally, 260 participants were estimated to be required for randomization, assuming a response rate of 35% for the primary efficacy measure in the AML/ATO + TLC arm, 15% in the AML + TLC arm, and a discontinuation rate of no more than 5%, at a significance level of 5% to provide 90% power. The number of randomized participants (n = 245) was considered sufficient, based on the actual treatment effect from a trial with a similar population and design.Citation11
An intent-to-treat (ITT) analysis was conducted including participants who discontinued from the trial during the treatment period. Last observations from the non-completers were carried forward for final analysis. One participant randomized to AML + TLC did not take any study drug and no data were available for this individual beyond the baseline visit. This patient was excluded from any analyses.
For the primary end point analysis, odds ratios (OR), 95% confidence intervals (CI), and the corresponding P-values were calculated based on a Cochran-Mantel-Haenszel (CMH) test stratified by baseline amlodipine dose and BP control. For the mean change from baseline data, difference in least-square (LS) means, its 95% CI, and the corresponding P-values were based on an analysis of covariance (ANCOVA) model with terms for treatment group, randomization stratum, and baseline values as explanatory variables in the model.
Results
Baseline demographics
In total, 245 participants were randomized to the two treatment arms (). However, one randomized participant in the AML + TLC arm did not take any study medication and was excluded from all analyses. Of the 244 participants who took study medication, n = 111 and n = 107 completed the trial in the AML + TLC and AML/ATO + TLC arms, respectively (). The mean age of participants was 56 years (ranging from 24 to 84 years), with more than half of them being white (). There were no significant differences in BP, lipid, and 10-year Framingham CHD risk at baseline between participants in the two treatment arms ().
Table 1 Baseline demographic characteristicsTable Footnotea
BP and LDL-C goal attainment
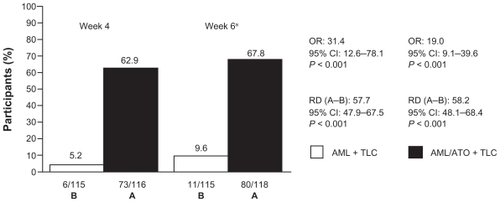
The proportion of participants attaining combined SBP/LDL-C goal at week 6 (ie, the primary end point) was 9.6% in the AML + TLC arm, and 67.8% in the AML/ATO + TLC arm (RD [A–B]: 58.2; 95% CI [48.1 to 68.4]; P < 0.001; OR, 19.0; 95% CI [9.1 to 39.6]; P < 0.001; ). This difference was already apparent at week 4, when 5.2% participants on the AML + TLC-based regimen attained the combined goal compared with 62.9% participants receiving AML/ATO single-pill therapy, along with TLC (RD [A–B]: 57.7; 95% CI [47.9 to 67.5]; P < 0.001; OR, 31.4; 95% CI [12.6 to 78.1]; P < 0.001; ).
Figure 2 Attainment of combined BP/LDL-C goal.
Abbreviations: AML, amlodipine; ATO, atorvastatin; OR, odds ratio; RD, risk difference; TLC, therapeutic lifestyle changes.
LDL-C goal was achieved by 7.0% in the AML + TLC arm at week 4 compared with 82.8% participants in the AML/ATO + TLC arm (RD [A–B]: 75.8; 95% CI [67.4 to 84.2]; P < 0.001; OR 65.5; 95% CI [27.1 to 158.3]; P < 0.001; ); at week 6, 11.3% in the AML + TLC arm were at LDL-C goal versus 83.9% participants on the single-pill combination along with TLC (RD [A–B]: 72.6; 95% CI [63.7 to 81.5]; P < 0.001; OR, 42.0; 95% CI [19.4 to 91.0]; P < 0.001; ). The difference in combined SBP/DBP goal attainment between the two treatment arms was not significant (); however, a 3.3 mm Hg difference in LS means in SBP at week 6 between the two groups was observed ().
Figure 3 Attainment of LDL-C goal.
Abbreviations: AML, amlodipine; ATO, atorvastatin; OR, odds ratio; RD, risk difference; TLC, therapeutic lifestyle changes.
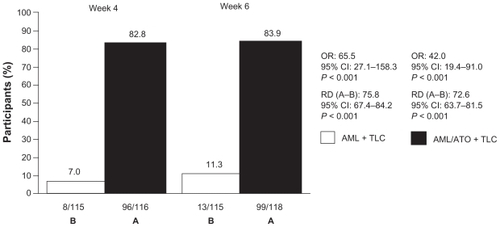
Figure 4 Attainment of SBP/DBP goal.
Abbreviations: AML, amlodipine; ATO, atorvastatin; OR, odds ratio; RD, risk difference; TLC, therapeutic lifestyle changes; SBP, systolic blood pressure; DBP, diastolic blood pressure.
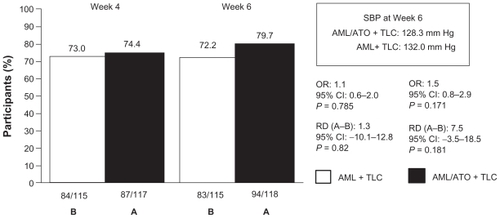
Table 2 Mean (95% CI) changes from baseline in SBP/DBP, various lipid parameters and 10-year Framingham risk for CHD outcomes
Mean changes from baseline in lipids, SBP/DBP, and 10-year Framingham risk
There were significant mean percentage reductions from baseline in LDL-C, TC, and triglycerides in the AML/ATO + TLC arm compared with the AML + TLC arm at weeks 4 and 6 (). There was no difference in DBP between the two treatment groups and no difference in SBP at week 4. However, at week 6 the difference in LS means in SBP was 3.3 mm Hg lower in the AML/ATO arm compared with the AML arm (P = 0.02; ).
In the AML/ATO + TLC arm, the 10-year Framingham risk for CHD at baseline was 8.2% and was reduced to 5.5% and 5.4% at week 4 and week 6, respectively, compared with the AML + TLC arm in which the 10-year Framingham risk was 8.1% at baseline and remained unchanged at week 6. At week 4, the percentage relative reduction from baseline in 10-year Framingham risk for CHD in participants on AML/ATO + TLC was 39.6%, compared with a percentage relative increase of 0.6% in participants on AML + TLC. At week 6, the corresponding percentage relative reduction from baseline was 42.0% in the AML/ATO + TLC arm compared with a percentage relative increase of 4.5% in the AML + TLC arm ().
Safety and tolerability
Of the 244 participants who were included in the safety analysis, 4 discontinued from the trial due to an adverse event or laboratory abnormality, all of which in the AML/ATO + TLC arm (). Of these, 3 participants had AEs which were considered related to study medication: one discontinued due to mild abdominal pain, one due to severe tinnitus, and a third due to moderate abdominal pain and joint sprain.
Table 3 Treatment-emergent all-causality adverse events by system organ class
There were no deaths or serious AEs reported during the conduct of the trial. Overall, treatment-related AEs occurred in 18 (14.8%) and 11 (9.0%) of participants in the AML + TLC and AML/ATO + TLC treatment arms, respectively. The majority of events in both arms were mild. Changes in liver function test and creatine phosphokinase were mild to moderate and similar in incidence and severity with previous reports and known profile of the study medication.
Discussion
In clinical practice, patients with hypertension and additional CV risk factors are usually treated in isolation, ie, by initially focusing on treating their BP, and treating another CV risk factor only after the patient’s BP has been controlled, thereby leaving the patient exposed to considerable residual CV risk in the interim. These results show that simultaneous treatment of BP and lipids using AML/ATO single-pill therapy, along with TLC, provides significant and rapid benefits for helping patients attain their combined BP and lipid goals, with a greater reduction in absolute 10-year Framingham risk for CHD outcomes, compared with an approach based on current guideline recommendationsCitation4 using AML + TLC only.
As expected, significant improvements were observed in attaining LDL-C goals (<100 mg/dL). In fact, in this population LDL-C, not BP, was the driving factor behind the significant benefits that were observed for combined BP/LDL-C goal attainment, presumably as participants were already receiving amlodipine prior to the trial, and consequently any additional improvements in BP goal attainment by the single-pill combination were minor, since BP treatment was equally optimized between the two arms (data not shown). The LDL-C threshold of <100 mg/dL is a considerably more aggressive goal than the one recommended by NCEP ATP III for most patients in this population. The decision to implement the LDL-C goal of <100 mg/dL for every patient in this population was made for operational reasons, as it is not possible to preserve the blind with individually determined therapeutic goals.
The effect of the atorvastatin component of the single-pill combination on LDL-C is also most likely the driving force behind the 2.8% percentage point drop in LS means in the 10-year Framingham risk for CHD events within 6 weeks seen in this trial. There was no percentage reduction in LS means in the 10-year Framingham risk in the AML + TLC arm, presumably because patients had been previously treated with amlodipine, as stated above. This demonstrates that a simultaneous treatment approach using single-pill AML/ATO reduces CV risk burden in this population compared with BP intervention only.
Although the difference in combined SBP/DBP goal attainment between the two treatment arms was not statistically significant in this study, a 4 mm Hg difference in SBP at week 6 was nevertheless observed, despite equal mean doses of amlodipine at week 6 in both treatment arms (data not shown). This may be due to a modest BP-lowering effect of the atorvastatin component of the single-pill combination which has previously been reported for statins.Citation12,Citation13
The participants in the TOGETHER trial consisted of previously treated hypertensive individuals with additional CV risk factors but conventionally normal lipid levels (fasting LDL-C: ≤170 mg/dL [4.4 mmol/L]), in which a statin (in the form of the atorvastatin component in the single-pill AML/ATO study medication) in addition to AML + TLC resulted in improved combined BP/LDL-C goals compared with individuals on AML + TLC alone. Cholesterol lowering in addition to an antihypertensive regimen has been shown to translate into reduced CHD outcomes in a hypertensive population with normal lipid levels (nonfasting TC ≤ 251.4 mg/dL [≤6.5 mmol/L]) in the lipid-lowering arm of the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA),Citation14 indicating that a simultaneous multiple risk factor management strategy results in improved CHD outcomes.
The efficacy and safety of AML/ATO single-pill therapy has been demonstrated in another randomized trial, the Caduet in Untreated Subjects Population (CUSP) trial.Citation11 The CUSP results are highly consistent with our results. In CUSP, similar to TOGETHER, a significant difference in combined BP (<140/90 mm Hg)/LDL-C (<100 mg/dL) goal attainment was reported between a treatment arm receiving AML/ATO + TLC versus a control arm (AML/ATO + TLC, 47.6% versus placebo + TLC, 1.7%; P < 0.001) within a short period of time (4 weeks in CUSP versus 6 weeks in TOGETHER).Citation11 This suggests that AML/ATO single-pill therapy, in addition to TLC, provides CV benefits in both treated and untreated hypertensive individuals within a short period of time.
The results from the TOGETHER trial show that the initiation of a multiple risk factor management strategy using AML/ATO single-pill therapy + TLC leads to improvements in CV risk burden compared with an approach using an AML + TLC-based regimen alone based on current guideline recommendations, in patients previously treated with amlodipine.
Conclusions
AML/ATO single-pill therapy + TLC is more effective in achieving combined BP/LDL-C therapeutic targets than an AML + TLC-based regimen based on current guidelines in treated hypertensive patients with additional CV risk factors, highlighting the utility of a simultaneous treatment approach for the rapid reduction of CV risk burden. The single-pill combination was well tolerated in these patients, in line with previous reports.
Acknowledgments
This study was sponsored by Pfizer Inc. Editorial support was provided by Fiona Nitsche, PhD, of UBC, a medical writer funded by Pfizer Inc.
Disclosures
Dr Grimm receives grants/research support from Pfizer and Novartis; acts as a consultant for Merck, Pfizer, and Novartis; and is on the Speakers Bureau for Pfizer, Merck-Schering Plough, and Novartis.
Dr Malik has no real or perceived conflicts of interest pertaining to this study.
Dr Yunis and Dr Sutradhar are employees of Pfizer Inc.
Dr Kursun was an employee of Pfizer Inc at the time the study was conducted.
References
- Lloyd-JonesDAdamsRCarnethonMHeart Disease and Stroke Statistics 2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics SubcommitteeCirculation2009119e1e16119124668
- KannelWBFifty years of Framingham Study contributions to understanding hypertensionJ Hum Hypertens200014839010723112
- AnsellBJEvidence for a combined approach to the management of hypertension and dyslipidemiaAm J Hypertens2005181249125716245413
- ChobanianAVBakrisGLBlackHRSeventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood PressureHypertension2003421206125214656957
- NCEPATPIIIExecutive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III)JAMA20012852486249711368702
- ChapmanRHBennerJSPetrillaAAPredictors of adherence with antihypertensive and lipid-lowering therapyArch Intern Med20051651147115215911728
- CherrySBBennerJSHusseinMAThe clinical and economic burden of nonadherence with antihypertensive and lipid-lowering therapy in hypertensive patientsValue Health20091248949718783393
- American Heart AssociationDietary approaches to prevent and treat hypertension: a scientific statement by the American Heart AssociationHypertension20064729630816434724
- GrundySMICJMerzCNImplications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III GuidelinesJ Am Coll Cardiol20044472073215358046
- NCEP. National Cholesterol Education Program of the National Institutes of Health National Heart, Lung, and Blood InstituteSpreadsheet-based 10-year risk assessment tool2008
- NeutelJMBestermannWHDyessEMThe use of a single-pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized, placebo-controlled, multi-center studyJ Clin Hypertens2009112230
- GolombBADimsdaleJEWhiteHLReduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trialArch Intern Med200816872172718413554
- FeldsteinCAStatins as antihypertensivesRecent Patents Cardiovasc Drug Discov200839297
- SeverPSDahlöfBPoulterNRPrevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trialLancet20033611149115812686036
Appendix
List of participating investigators
Jon Shapiro (Philadelphia Health Associates); Mandeep Singh Oberoi (Central Jersey Medical Research Center); Ginger Sandler Kubala (Medical Research Unlimited, Inc); Waymon Drummond (Renaissance Clinical Research and Hypertension); Dinh Van Dinh (Harmony Clinical, Inc); Mark Edward Shirley (Omaha Clinical Research, PC); Piotr Z Imiolek (Clifton-Wallington Medical Group); Andrew Brockmyre (Holston Medical Group); Eli M. Roth (Sterling Research Group, Limited); Kris Manlove-Simmons (Clinical Research Solutions, Inc); Michael Friend Wilson (Kaleida Health Millard Fillmore Hospital); Peter A Lodewick (Searle Harris Clinic); Matthew Jay Budoff (Los Angeles Biomedical Research Institute at Harbor); Shelton P Hager (Holston Medical Group); Fatima C Phillips (Accelovance); Z David Skloven (Cardiovascular Associates of Mesa); John V Bernard (Private Practice); Julie A Mullen (Sterling Research Group); Albert Ayerst Carr (Southern Clinical Research and Management, Inc); Michael R Seidner (Green and Seidner Family Practice); Cynthia Becher Strout (Coastal Caroline Research Center); Mel Emiel Lucas (Prime Care Research Associates); Christopher M Chappel (FPA Clinical Research); Scott William Yates (North Texas Medical Research); Robert Jay Weiss (Main Research Associates); Richard Anthony Cottiero (Hypertension and Nephrology, Inc); Susan Levit (SLQC D&T Center); John P Kripsak (Bridgewater Medical Group); Prasad Gupta (Hopewell Valley Medical Group, PA); Stevan A Smallow (Pennsylvannia Research Institute); Michael Schoenwalder (Mercy Medical Group – Woodlake Research); Vibhuti Narain Singh (Suncoast Cardiovascular Research, Inc); Clinton Nicolas Corder (COR Clinical Research, LLC); Barry S Meyer (Ryan Medical Associates); Robert Grady Ashley (Florida Research Network, LLC); Patrick Henry Peters (Texas Medical Research Associates), Hector G Ramirez (Clinical Interventions Research Institute); Philip Alan Levin (Model Clinical Research); Richard Neil Marple (Castlerock Clinical Research Consultants); Dennis Stone Riff (Advanced Clinical Research Institute); Van Q Nguyen (The Heart and Vascular Institute of Florida); Richard Allen Craven (Professional Place Medical Group, LLC); Louis Marino (Buckingham Family Medicine); Joseph R Weinstein (Your Doctors Care); Max Helman (Accelovance); Frederick Tannenggee (Lovelace Scientific Resources, Inc); Ronald A Tachibana (Midtown Primary Care); Alan S Hoffman (Saint Lukes Health System); Wentworth Grantley Jarrett (Medical Research Centers of South Florida, Inc).