Abstract
The human ear imparts critical form and function and remains one of the most challenging facial features to reconstruct. Over the past century, surgeons have developed numerous techniques and materials for total auricular reconstruction. Refined costal cartilage techniques have remained the gold standard for the past half-century. Recent advancements with novel materials, tissue engineering and 3D printing provide immense potential; however, prohibitive costs and regulatory steps remain as barriers to clinical translation.
The form and function of the human ear impart a critical component to the entirety of a child's face and remain one of the most challenging features to reconstruct due to its complex 3D geometry. Auricular deformities drastically affect the sensory and social development of a child. As the ear canal and middle ear ossicular chain develops in parallel with that of the external ear pinna, aural atresia and a maximal conductive hearing loss often accompanies the external ear malformation [Citation1]. Additionally, the external ear provides functional support for prescription glasses. Patients with microtia often have poor, misaligned support for their glasses, failing to aid in vision [Citation2]. Children with auricular malformations develop self-awareness of their differences at approximately 4 years of age. Failure to restore the size, shape and function of the ear can result in traumatic bullying and teasing by peers, which becomes more psychologically damaging as the child ages [Citation3].
Over the past century, surgeons have devised techniques and materials to progress total auricular reconstruction in cases of congenital malformations and trauma. Although technological advances provide promise for improved patient outcomes, several challenges to widespread implementation remain. In this review, we aim to highlight the contributions that have led to current reconstructive techniques. We then look ahead to where the future of auricular reconstruction lies and challenges preventing near future translation.
Seminal techniques
The earliest techniques for whole ear reconstruction in the 20th century utilized homologous cartilage grafts or molds. Gillies pioneered a series of reconstructions with cartilage grafts from patients’ mothers [Citation4]. Young introduced the use of precast fibrocartilage, where the diced strips of cartilage were encased in an auricle-shaped mold, buried in abdominal subcutaneous tissue for 3 months and transplanted for use in ear reconstruction [Citation5]. Aufricht and Peer further applied this technique [Citation6,Citation7]. Both of these types of implants did not maintain shape under the taught auricular skin grafts and experienced progressive resorption and distortion [Citation8,Citation9].
The refining costal cartilage techniques
Tanzer revolutionized total auricular reconstruction by using carved autologous costal cartilage (A), originally in a six-stage method with subsequent technique modifications paring down to three to four total surgeries [Citation10–13]. His use of a template drawn on x-ray film allowed for mirroring of a child's normally developed ear in the reconstructed ear cartilage scaffold. Furthermore, he defined key anatomic planes and landmarks, which have been central to subsequent surgical advancements.
Current techniques (A) rib cartilage and (B) MedPor, and future techniques (C) porous bioscaffold.
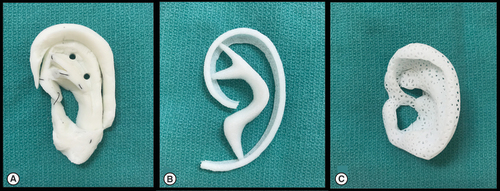
Brent built upon Tanzer's technique by placing the cartilage scaffold under unscarred skin before transposing the lobule [Citation14–18]. His work explored the use of expansile frameworks to maximize skin coverage and suction drainage to reduce dead space and subsequent hematoma and secondary fibrosis formation. Nagata refined this technique to a two-stage reconstruction with the first three steps innovated by Brent consolidated into a singular first stage and the ear elevation completed in the second stage [Citation19]. In a series of landmark papers, he detailed refinements specific for the lobular-, conchal- and small concha-type microtia [Citation20–23]. Firmin compared the techniques developed by Brent and Nagata through 352 patients seen in her surgical practice. She concluded that both surgical techniques result in satisfactory results with Nagata's approach having improved aesthetics in fewer, yet more technically difficult, stages [Citation24]. Firmin added her own suggested improvements with superb outcomes. These intricate techniques require a high degree of expertise with few surgeons achieving acceptable outcomes.
This model of reconstruction possesses several benefits. Most notably, the autologous reconstructed ears maintain their shape, even in cases of severe trauma years to decades after the initial surgery [Citation13,Citation18]. Furthermore, a well-reconstructed ear provides significantly improved psychosocial outcomes in patients [Citation25]. However, this technique is not without challenges. The harvesting of costal cartilage often comes with significant postoperative pain, splinting, potential chest wall deformity and scarring [Citation26,Citation27]. Secondary reconstructions have universally poorer outcomes due to the existing scar tissue and the decreased quantity of suitable tissue for use in the reconstruction [Citation16]. The reliance on the artistry and experience of the surgeon make this reconstruction method less universally accessible and can lead to poor outcomes in an unskilled surgeon's hands. Despite these challenges, the use of costal cartilage remains the gold standard for the majority of reconstructive surgeons [Citation28].
Alloplastic high-density porous polyethylene
Researchers continue to develop alternative materials for total ear reconstruction to avoid the morbidity associated with the costal cartilage method. Most notably, MedPor, an implantable, high-density porous polyethylene, has been widely studied and used for a variety of craniofacial reconstructions, including microtia repair (B) [Citation29,Citation30]. This alloplastic material distinguishes itself from other implants, although competitor products have recently emerged such as SuPor and OmniPor. Multiple animal and human studies have demonstrated its biocompatibility, notably its capacity for rapid tissue ongrowth [Citation31,Citation32]. Furthermore, this material does not resorb or degenerate, which are complications that plagued early alloplastic implants.
Reinisch has pioneered the use of MedPor frameworks in auricular reconstruction, publishing the results from 1178 such cases completed over the past 25 years [Citation33]. His method allows for earlier reconstruction in children as young as 3 years of age since a rib graft is not required. The human ear reaches approximately 90% of its adult size by 8 years old [Citation1]. The future size of the child's ear is predicted and an appropriately sized MedPor framework can be selected to anticipate for this growth. Furthermore, this surgery can be performed in the outpatient setting due to minimal postoperative pain secondary to the lack of rib harvest. Typically, only one stage is necessary since the MedPor implant can be modified to project the auricle, which traditionally requires an additional surgery months later [Citation33].
However, auricular reconstruction with MedPor is not without risks. Patients undergoing repair with this implant experience a reported 4–6.31% complication rate in even the most experienced of hands. These include postoperative infections, framework fracture, compression ischemia and framework exposure [Citation34–36]. These complications often result in multiple subsequent operations and revisions, exposing the patient to additional surgical risks. Furthermore, only a single, modifiable ear construct is available to meet the needs of all pediatric and adult patients requiring reconstruction, and the MedPor implant does not have the capacity for growth or expansion in pediatric patients.
Future directions: tissue engineering & 3D-printed scaffolds
Tissue engineering is a promising alternative to autologous rib grafts or synthetic material constructs through the use of novel, 3D-printed bioscaffolds (C). These solutions have multiple future clinical applications, including whole auricular reconstruction [Citation37–39]. Ideally, materials can be generated based on established biomechanical properties observed in tissues [Citation40]. These bioscaffolds have the potential to restore craniofacial structure, with proposed patient- and tissue-specific methods previously detailed by Zopf et al. that avoid the morbidity of the costal reconstruction method and the limited patient specificity of the MedPor reconstruction method [Citation41].
Many research groups have worked to characterize a suitable scaffold material. Ideally, these materials should be biocompatible while also maintaining a stable auricular shape. Bichara et al. published an extensive review of the previously studied tissue-engineered auricle-shaped constructs [Citation37]. Synthetic and natural porous and hydrogel-based polymers have been developed, including polyglycolic acid, polylactic acid, fibrin gels and fibrous collagens. These scaffolds have been implanted in a variety of nude and immunocompetent animal models with varying levels of success [Citation42–58].
A noted challenge of these materials is the construct contraction. Zhou et al. and Pomerantseva et al. proposed the incorporation of a titanium wire to address the issue of contraction within the bioresorbable, porous and collagen chondrocyte-seeded scaffold [Citation59,Citation60]. However, the propensity of a metal foreign body to extrude through thin, pediatric temporoparietal skin remains a significant concern with this approach, even when encased in the scaffold. Several biomechanical forces are at action. The dehiscence and extrusion of alloplastic materials through the skin are most likely related to the low mechanical stiffness of the skin in relation to the construct. Furthermore, the development of the contracture is related to forces generated by the myocontractile process of scarring.
In order to address these biomechanical concerns, Zopf et al. proposed using a slowly resorbing polycaprolactone (PCL) scaffold with microporous architecture designed to resist contraction in the initial phase of native extracellular matrix deposition [Citation41]. Additionally, it demonstrated adequate shape definition upon implantation in a porcine model. Shieh et al. have previously demonstrated long-term PCL shape retention in nude mice for up to 10 months; however, once implanted in immunocompetent rabbits, they were severely distorted and demonstrated inflammatory cell infiltration histologically [Citation49].
Achieving confluence of cartilage growth with the appropriate cell numbers and the optimal cell type for seeding is yet to be determined. It is estimated that 250 million chondrocytes would be necessary to produce an ear scaffold confluent with cartilage, though further data is necessary to support this conjecture. Our group is investigating co-culturing chondrocytes with pluripotent stem cells that would decrease the number of chondrocytes necessary. Kang et al. suggested a bioprinting solution, interposing scaffold material and chondrocytes [Citation61]. This method demonstrated impressive histologic cartilage growth, yet the gross appearance of the ear framework exhibited limited anatomic detail, a critical outcome, and the in vivo subcutaneous appearance was not published. This approach also depends on the inclusion of cell and growth factor components, thus escalating regulatory requirements and making the approach difficult for immediate clinical translation. The dependence on prohibitively expensive additive manufacturing techniques disallows widespread dissemination, limiting their use to resource-rich academic centers.
Several obstacles to human clinical implementation remain. Current approaches to ear tissue engineering do not allow a feasible pathway for clinical translation and remain cost and resource prohibitive. The efforts to date have not led to clinically practical solutions, and the methods developed have significant regulatory challenges. For example, in the USA, MedPor is considered a class II device; however, once chondrocytes are added to a scaffold, it becomes a class III device and requires clinical trials and significant US FDA investigation. Although these steps are important to ensure the safety and efficacy of these novel bioscaffolds, they may take many years to complete and require high upfront research costs, making the device initially cost prohibitive and unlikely to garner interest and support.
Conclusion
The potential to innovate within the field of auricular reconstruction remains great. Although surgeons have attempted to use a variety of novel materials to produce improved outcomes, the costal cartilage auricular reconstruction method remains the gold standard of currently available options for patient care. Tissue engineering and 3D printing modalities provide immense potential for future innovation and improved more consistent patient outcomes; however, prohibitive cost and clinical translation barriers pose new challenges before these potentially revolutionary options become available to patients.
Future perspective
In the next 5–10 years, we anticipate further innovation in materials and additive manufacturing techniques that will allow for more rapid production of auricular cartilage that will maintain original design characteristics without deformation over time. These goals are achievable through the optimization of tissue engineering designs and processes, either through scaffold-based engineering or bioprinting. We envision the ability to provide a child with a symmetric-appearing ear while avoiding the complications related to current surgical methods. Additionally, we hope to provide surgeons with easy-to-use tools to produce consistently excellent outcomes. Moreover, our group is developing methods that would allow the ability to provide these benchmarks in low-resource settings.
In the setting of increased patient-specific, tissue-engineered devices, regulatory bodies are charged with the challenge of ensuring the safety of a device with slightly varying design features. Recent efforts demonstrate promise and interest in developing a device development pathway that includes early consultation with the FDA through a presubmission process. Overcoming these inherent regulatory challenges will require significant efforts, both by regulatory bodies and developers, to produce innovative and safe devices.
Seminal techniques
Early auricular reconstruction techniques utilized molded diced cartilage, but the results were plagued by resorption and distortion.
Refining costal cartilage techniques
The use of carved costal cartilage for auricular reconstruction was pioneered by Tanzer and progressively improved by Brent, Nagata and Firmin.
The downsides to this method include significant postoperative pain, splinting, potential chest wall deformity and scarring secondary to the rib harvest.
Alloplastic high-density porous polyethylene
MedPor, an implantable, high-density porous polyethylene, has been widely studied and used for a variety of craniofacial reconstructions, including microtia repair.
This material does not resorb or degenerate, which are complications that affected early alloplastic implants.
Complications with this material include postoperative infections, framework fracture, compression ischemia and framework exposure.
Tissue engineering & 3D-printed scaffolds
Tissue engineering is a promising alternative to autologous rib grafts or synthetic material constructs through the use of novel, 3D-printed bioscaffolds.
Despite numerous designs of tissue-engineered auricle-shaped constructs, few maintain a stable auricular shape and achieve confluence of cartilage growth with the appropriate cell numbers, and the optimal cell type for seeding is yet to be determined.
Future perspective
In the next 5–10 years, we anticipate further innovation in materials and additive manufacturing techniques that will allow for more rapid production of auricular cartilage that will maintain original design characteristics without deformation over time.
Overcoming inherent regulatory challenges will require significant efforts, both by regulatory bodies and developers, to produce innovative and safe devices.
Financial & competing interests disclosure
Grant funding provided by NIH T32 DC005356. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Flint PW , HaugheyBH, RobbinsKTet al. Cummings Otolaryngology: Head and Neck Surgery . Elsevier Health Sciences, PA, USA, 2998–3005 (2014).
- Eng H , ChiuRS. Spectacle fitting with ear, nose and face deformities or abnormalities. Clin. Exp. Optom.85(6), 389–391 (2002).
- Johns AL , LucashRE, ImDD, LewinSL. Pre and post-operative psychological functioning in younger and older children with microtia. J. Plast. Reconstru. Aesthet. Surg.68(4), 492–497 (2015).
- Gillies HD . Reconstruction of the external ear with special reference to the use of maternal ear cartilage as the supporting structure. Rev. Chir. Structive.7, 169 (1937).
- Young F . Cast and precast cartilage grafts: their use in the restoration of facial contour. Surgery15, 735 (1944).
- Aufricht G . Total ear reconstruction preliminary report. Plast. Reconstr. Surg.2(4), 297–306 (1947).
- Peer LA . Reconstruction of the auricle with diced cartilage grafts in a vitallium ear mold. Plast. Reconstr. Surg.3(6), 653–666 (1948).
- Steffensen WH . Comments on reconstruction of the external ear. Plast. Reconstr. Surg.16(3), 194–200 (1955).
- Converse JM . The absorption and shrinkage of maternal ear cartilage used as living homografts: follow-up report of 21 of Gillies’ patients. Reconstructive Plastic Surgery (2nd Edition). ConverseJM ( Ed.). Saunders, PA, USA, 308 (1977).
- Tanzer RC . Total reconstruction of the external ear. Plast. Reconstr. Surg.23(1), 1–5 (1959).
- Tanzer RC . An analysis of ear reconstruction. Plast. Reconstr. Surg.31(1), 16–30 (1963).
- Tanzer RC . Total reconstruction of the auricle: the evolution of a plan of treatment. Plast. Reconstr. Surg.47(6), 523–533 (1971).
- Tanzer RC . Microtia – a long-term follow-up of 44 reconstructed auricles. Plast. Reconstr. Surg.61(2), 161–166 (1978).
- Brent B . Ear reconstruction with an expansile framework of autogenous rib cartilage. Plast. Reconstr. Surg.53(6), 619–628 (1974).
- Brent B . The correction of microtia with autogenous cartilage grafts: I. The classic deformity. Plast. Reconstr. Surg.66(1), 1–2 (1980).
- Brent B , ByrdHS. Secondary ear reconstruction with cartilage grafts covered by axial, random, and free flaps of temporoparietal fascia. Plast. Reconstr. Surg.72(2), 141–151 (1983).
- Brent B . Auricular repair with autogenous rib cartilage grafts: two decades of experience with 600 cases. Plast. Reconstr. Surg.90(3), 355–374 (1992).
- Brent B . Technical advances in ear reconstruction with autogenous rib cartilage grafts: personal experience with 1200 cases. Plast. Reconstr. Surg.104(2), 319–334 (1999).
- Nagata S . A new method of total reconstruction of the auricle for microtia. Plast. Reconstr. Surg.92(2), 187–201 (1993).
- Nagata S . Modification of the stages in total reconstruction of the auricle: Part I. Grafting the three-dimensional costal cartilage framework for lobule-type microtia. Plast. Reconstr. Surg.93(2), 221–230 (1994).
- Nagata S . Modification of the stages in total reconstruction of the auricle: Part II. Grafting the three-dimensional costal cartilage framework for concha-type microtia. Plast. Reconstr. Surg.93(2), 231–242 (1994).
- Nagata S . Modification of the stages in total reconstruction of the auricle: Part III. Grafting the three-dimensional costal cartilage framework for small concha-type microtia. Plast. Reconstr. Surg.93(2), 243–253 (1994).
- Nagata S . Modification of the stages in total reconstruction of the auricle: Part IV. Ear elevation for the constructed auricle. Plast. Reconstr. Surg.93(2), 254–266 (1994).
- Firmin F . Ear reconstruction in cases of typical microtia. Personal experience based on 352 microtic ear corrections. Scand. J. Plast. Reconstr. Surg. Hand. Surg.32(1), 35–47 (1998).
- Steffen A , WollenbergB, KönigIR, FrenzelH. A prospective evaluation of psychosocial outcomes following ear reconstruction with rib cartilage in microtia. J. Plast. Reconstr. Aesthet. Surg.63(9), 1466–1473 (2010).
- Thomson HG , KimTY, EinSH. Residual problems in chest donor sites after microtia reconstruction: a long-term study. Plast. Reconstr. Surg.95(6), 961–968 (1995).
- Ohara K , NakamuraK, OhtaE. Chest wall deformities and thoracic scoliosis after costal cartilage graft harvesting. Plast. Reconstr. Surg.99(4), 1030–1036 (1997).
- Breugem CC , StewartKJ, KonM. International trends in the treatment of microtia. J. Craniofac. Surg.22(4), 1367–1369 (2011).
- Shanbhag A , FriedmanHI, AugustineJ, von RecumAF. Evaluation of porous polyethylene for external ear reconstruction. Ann. Plast. Surg.24(1), 32–39 (1990).
- Cenzi R , FarinaA, ZuccarinoL, CarinciF. Clinical outcome of 285 Medpor grafts used for craniofacial reconstruction. J. Craniofac. Surg.16(4), 526–530 (2005).
- Sclafani AP , RomoT3rd, SilverL. Clinical and histologic behavior of exposed porous high-density polyethylene implants. Plast. Reconstr. Surg.99(1), 41–50 (1997).
- Williams JD , RomoT, SclafaniAP, ChoH. Porous high-density polyethylene implants in auricular reconstruction. Arch. Otolaryngol. Head Neck Surg.123(6), 578–583 (1997).
- Reinisch J . Ear reconstruction in young children. Facial Plast. Surg.31(6), 600–603 (2015).
- Wellisz T . Reconstruction of the burned external ear using a Medpor porous polyethylene pivoting helix framework. Plast. Reconstr. Surg.91(5), 811–818 (1993).
- Frodel JL , LeeS. The use of high-density polyethylene implants in facial deformities. Arch. Otolaryngol. Head Neck Surg.124(11), 1219–1223 (1998).
- Romo T , PrestiPM, YalamanchiliHR. Medpor alternative for microtia repair. Facial Plast. Surg. Clin. North Am.14(2), 129–136 (2006).
- Bichara DA , O'SullivanNA, PomerantsevaIet al. The tissue-engineered auricle: past, present, and future. Tissue Eng. Part B Rev.18(1), 51–61 (2011).
- Otto IA , MelchelsFP, ZhaoXet al. Auricular reconstruction using biofabrication-based tissue engineering strategies. Biofabrication7(3), 032001 (2015).
- Zopf DA , HollisterSJ, NelsonME, OhyeRG, GreenGE. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med.368(21), 2043–2045 (2013).
- Zopf DA , FlanaganCL, NasserHBet al. Biomechanical evaluation of human and porcine auricular cartilage. Laryngoscope125(8), E262–E268 (2015).
- Zopf DA , MitsakAG, FlanaganCL, WheelerM, GreenGE, HollisterSJ. Computer-aided designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction. Otolaryngol. Head Neck Surg.152(1), 57–62 (2015).
- Vacanti CA , CimaLG, RatkowskiD, UptonJ, VacantiJP. Tissue engineered growth of new cartilage in the shape of a human ear using synthetic polymers seeded with chondrocytes. MRS Online Proc. Lib. Arch.252, 367–374 (1991).
- Cao Y , VacantiJP, PaigeKT, UptonJ, VacantiCA. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast. Reconstr. Surg.100, 297–302 (1997).
- Ting V , SimsCD, BrechtLEet al. In vitro prefabrication of human cartilage shapes using fibrin glue and human chondrocytes. Ann. Plast. Surg.40, 413–420 (1998).
- Saim AB , CaoY, WengYet al. Engineering autogenous cartilage in the shape of a helix using an injectable hydrogel scaffold. Laryngoscope110, 1694–1697 (2000).
- Haisch A , KlaringS, GrogerA, GebertC, SittingerM. A tissue-engineering model for the manufacture of auricular-shaped cartilage implants. Eur. Arch. Otorhinolaryngol.259, 316–321 (2002).
- Kamil SH , KojimaK, VacantiMP, BonassarLJ, VacantiCA, EaveyRD. In vitro tissue engineering to generate a human-sized auricle and nasal tip. Laryngoscope113, 90–94 (2003).
- Isogai N , AsamuraS, HigashiTet al. Tissue engineering of an auricular cartilage model utilizing cultured chondrocyte–poly(L-lactide-ε-caprolactone) scaffolds. Tissue Eng.10, 673–687 (2004).
- Shieh SJ , TeradaS, VacantiJP. Tissue engineering auricular reconstruction: in vitro and in vivo studies. Biomaterials25(9), 1545–1557 (2004).
- Kamil SH , VacantiMP, AminuddinBS, JacksonMJ, VacantiCA, EaveyRD. Tissue engineering of a human sized and shaped auricle using a mold. Laryngoscope114, 867–870 (2004).
- Xu JW , JohnsonTS, MotarjemPM, PerettiGM, RandolphMA, YaremchukMJ. Tissue-engineered flexible ear-shaped cartilage. Plast. Reconstr. Surg.115, 1633–1641 (2005).
- Isogai N , MorotomiT, HayakawaSet al. Combined chondrocyte–copolymer implantation with slow release of basic fibroblast growth factor for tissue engineering an auricular cartilage construct. J. Biomed. Mater. Res. A74, 408–418 (2005).
- Neumeister MW , WuT, ChambersC. Vascularized tissue-engineered ears. Plast. Reconstr. Surg.117, 116–122 (2006).
- Isogai N , NakagawaY, SuzukiKet al. Cytokine-rich autologous serum system for cartilaginous tissue engineering. Ann. Plast. Surg.60, 703–709 (2008).
- Yanaga H , ImaiK, FujimotoT, YanagaK. Generating ears from cultured autologous auricular chondrocytes by using two-stage implantation in treatment of microtia. Plast. Reconstr. Surg.124, 817–825 (2009).
- Kusuhara H , IsogaiN, EnjoMet al. Tissue engineering a model for the human ear: assessment of size, shape, morphology, and gene expression following seeding of different chondrocytes. Wound Repair Regen.17, 136–146 (2009).
- Liu Y , ZhangL, Zhouet al. In vitro engineering of human ear-shaped cartilage assisted with CAD/CAM technology. Biomaterials31, 2176–2183 (2010).
- Hwang NS , ImSG, WuPBet al. Chondrogenic priming adipose-mesenchymal stem cells for cartilage tissue regeneration. Pharm. Res.28, 1395–1405 (2011).
- Zhou L , PomerantsevaI, BassettEKet al. Engineering ear constructs with a composite scaffold to maintain dimensions. Tissue Eng. Part A17(11–12), 1573–1581 (2011).
- Pomerantseva I , BicharaDA, TsengAet al. Ear-shaped stable auricular cartilage engineered from extensively expanded chondrocytes in an immunocompetent experimental animal model. Tissue Eng.22(3–4), 197–207 (2015).
- Kang HW , LeeSJ, KoIK, KenglaC, YooJJ, AtalaA. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nature Biotechnol.34(3), 312–319 (2016).
