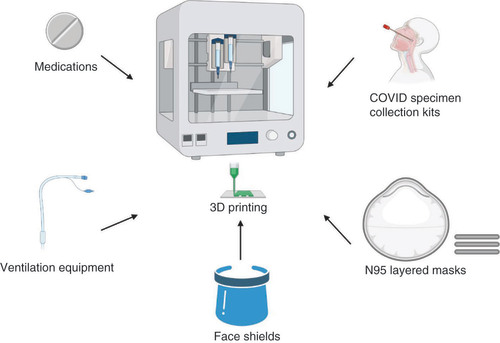
Abstract
The novel coronavirus, COVID-19, created a pandemic with significant mortality and morbidity which poses challenges for patients and healthcare workers. The global spread of COVID-19 has resulted in shortages of personal protective equipment (PPE) leaving frontline health workers unprotected and overwhelming the healthcare system. 3D printing is well suited to address shortages of masks, face shields, testing kits and ventilators. In this article, we review 3D printing and suggest potential applications for creating PPE for healthcare workers treating COVID-19 patients. A comprehensive literature review was conducted using PubMed with keywords “Coronavirus disease 2019”, “COVID-19”, “severe acute respiratory syndrome coronavirus 2”, “SARS-CoV-2”, “supply shortages”, “N95 respirator masks”, “personal protective equipment”, “PPE”, “ventilators”, “three-dimensional model”, “three-dimensional printing” “3D printing” and “ventilator”. A summary of important studies relevant to the development of 3D-printed clinical applications for COVID-19 is presented. 3D technology has great potential to revolutionize healthcare through accessibility, affordably and personalization.
The COVID-19 outbreak was first reported in Wuhan, China in December 2019, resulting in a worldwide public health threat [Citation1]. The race to obtain medical supplies reflects a global panic over a dwindling supply of N95 respirator masks, face shields, ventilators, testing kits and other personal protective equipment (PPE) [Citation1–4]. Adequate production of PPE is essential during the COVID-19 pandemic to protect healthcare workers from viral transmission. 3D printing can be used to create intricate architectures to aid with these shortages. 3D printing is an integrated approach to robotic fabrication, using computer-aided design (CAD) systems to deposit layers of biomaterials (within external anatomy, within internal anatomy and replacement parts for devices) [Citation5–10]. The success of a medical device is not only dependent on the type of biomaterial used for its fabrication but also on the structural integrity and quality (defect free) of the printing parts. Additive manufacturing technologies have opened new opportunities for manufacturing and production paradigms [Citation9–13]. The primary advantage of using additive manufacturing is for on-demand and redistributed manufacturing to circumvent the supply chain disruption. Moreover, additive manufacturing allows for lower energy costs, reduced waste and is affordable. Ideal biomaterials should be readily printable, mechanically stable and biocompatible [Citation5–7]. With ongoing materials research used in 3D technology, there is potential for innovative and cost-effective applications for addressing this current global crisis. Furthermore, the primary advantage of using additive manufacturing is for on-demand and redistributed manufacturing to circumvent the supply chain disruption. This review summarizes the key elements and advantages of 3D technologies that can be used to create 3D-printed tools to protect healthcare workers during the COVID-19 pandemic.
3D-printing techniques
Extrusion-based printing
Extrusion-based printing utilizes print nozzles that extrude material by air pressure or mechanical force, with continuous printing in a layer-by-layer design for controlled and accurate deposition. Synthetic polymers that are commonly used in extrusion printing include, acrylonitrile butadiene styrene (ABS), polyurethane polyvinylpyrrolidone, polyvinyl alcohol and polylactic acid [Citation9,Citation10].
The most common type of extrusion-based printing utilized is fused-deposition modeling (FDM) [Citation6–12]. FDM is fast, effective, and allows for easy integration with different CAD softwares. FDM uses thermoplastic filaments that pass through multiple heated printer nozzles and can therefore print multiple types of materials simultaneously [Citation9,Citation10]. Nylon, ABS, polylactic acid, polyvinyl alcohol, polycarbonate (PC) and polycaprolactone can be printed by FDM [Citation5–10]. Furthermore, FDM can be utilized to build constructs in a timely manner with 3D accuracy and excellent mechanical properties [Citation5–12]. Thus, FDM can be used to create customized patient and physician-specific medical devices, such as masks, face shields, ventilator valves that can be used during the COVID-19 pandemic.
Material sintering
Material-sintering 3D technology is used to fuse powdered biomaterial into solid objects via physical (UV/laser/electron beam) or chemical (binding liquid) sources [Citation14]. Common material sintering processes are called stereolithography (SLA) and selective laser sintering (SLS) [Citation1–11].
SLA is the most often utilized to create prototypes layer by layer using photochemical processes to cross-link polymers, also called photopolymerization [Citation14–16]. SLA is not only rapid and cost effective, but the versatility of 3D printing provides a myriad of clinical applications. Commonly used materials include resins, polyvinyl cinnamate, polyamide (PA), polyisoprene, polyimides and other photopolymers [Citation14–16].
SLS uses a high-power laser beam to fuse the powdered materials in a layer-by-layer pattern to create an object. A high-power beam is controlled by CAD software and guides the printer to trace a cross section of the object onto the powder. The laser heats the powder either to just below its boiling point (sintering) or above its boiling point (melting), which merges the particles in the powder together into a solid form [Citation17,Citation18]. Thereafter, sequential layers of powder are fused together, and the process continues until the complete object has been printed.
Both SLA and SLS have remarkable capability in the creation of customized medications and other tools to address the PPE shortage ().
Masks
N95 respirators masks are >95% efficient at filtering 0.3-μm airborne particles and require a fit test to ensure an adequate face seal [Citation19–30]. The CDC currently recommends N95 masks for healthcare workers caring for COVID-19 patients [Citation29]. 3D facial laser scanning combined with 3D printers can be used to scan exact facial parameters and create customized N95 face seals for improved mask comfort and fit. Important research studies relevant to the development of 3D-printed clinical applications for COVID-19 are shown in . Potential research studies relevant to the development of 3D-printed clinical applications for COVID-19 are shown in .
Table 1. Research studies relevant to the development of 3D-printed clinical applications for COVID-19.
Table 2. Potential research studies relevant to the development of 3D-printed clinical applications for COVID-19.
3D imaging
N95 respirator masks are typically manufactured in small and medium sizes only and may not match perfectly with individual head and facial parameters. An imperfect fit may be uncomfortable for the user, and more concerning is that the mask is ineffective in blocking contaminated air. Therefore, comfort and fit are two important parameters for respirator design and development. Cai et al. created face seal prototypes with acrylonitrile butadiene styrene plastic using a FDM 3D printer. Anthropometric data of the chin arc, jawline, face and nose shape and lengths, and nose protrusion measurements were collected via 3D laser scanning method to create a tailored seal [Citation27]. Three test subjects showed improved contact pressure compared with a 3M© 8210 N95 FFR respirator masks [Citation27]. Moreover, a personalized mask can be designed to account for the presence of facial hair length and density.
displays 3D-imaging technologies and key findings of several studies used to create N95 mask seals [Citation19–28]. Han et al. successfully developed respirator prototypes via digital face modeling and used rapid prototyping to print silicon respirators [Citation21]. Niezgoda et al. used a stereophotogrammetry technique to collect 3D facial geometry of subjects with and without wearing a molded, cup-shaped N95 filtering facepiece respirators [Citation23]. Standard size flat fold and cup-shaped N95 filtering facepiece respirators had significantly different seal pressures (p < 0.01). Thus, imaging technologies provide recommended construction of optimally fitted respirator seals and masks that can be worn by healthcare workers treating COVID-19 patients.
Table 3. Displays the 3D-imaging technology and key findings of several studies used to create N95 mask seals.
3D printing
Standard N95 masks consist of filtration material consisting of electrostatic nonwoven polypropylene (PP) fibers which are semi-rigid, light and fatigue resistant. The semi-crystalline structure may cause significant damage and distortions of the 3D-printed parts upon cooling thereby making 3D printing difficult; however, a combination polymer can help to support the creation of N95 masks. Material extrusion 3D printing was used to design a 3D-printable thermoplastic elastomeric material from a blend of PP and styrene-(ethylene-butylene)-styrene (SEBS) [Citation30]. PP is commonly used for various industrial applications due to its processability, printability, recyclability and durability. SEBS is a thermoplastic with low processing temperature, good elasticity and low distortion during Material extrusion 3D printing [Citation29,Citation54]. Therefore, the PP/SEBS blend provides better printability and flexibility for N95 mask design. Furthermore, the thermoplastic elastomer ratio allows for customizing the durability and elasticity of the biomaterial for better-fitted 3D masks [Citation27–30,Citation54,Citation55].
Setz et al. investigated the morphology of the PP/SEBS blend via scanning electron microscopy and transmission electron microscopy [Citation56,Citation57]. Using scanning electron microscopy, cryofracture surfaces did not dislodge any particles, thereby confirming good compatibility between SEBS and PP. Transmission electron microscopy micrographs demonstrated that SEBS diffuses into PP resulting in biomaterial crosslinking and contributing to increased interfacial strength and elongation.
Haigh et al. demonstrated use of PP microfibers in a 3D melt electrospinning printer. Several sequential fiber layers of material were printed to accurately obtain the 3D form with fiber diameters as small as of 16.4 ± 0.2 μm [Citation42]. Thus, 3D-printing procedures may allow for the creation of biocompatible N95 masks that are comparable to industrial manufacturing brands. displays 3D-printing techniques, materials and applications for N95 3D-printed masks.
McAvoy et al. developed a frame for N95 masks, using biomaterials and 3D-printing technologies [Citation31]. The authors designed a mask frame consisting of two 3D-printed side pieces, malleable wire links that users press against their face, and cut lengths of elastic material that wrap around the head to hold the frame and mask in place. The masks passed qualitative fit testing varying from 48 to 92% (depending on mask model and tester). For individuals for whom a mask passed testing, 75–100% (average: 86%) also passed testing with a frame holding the mask in place [Citation31].
Moore-Imbrie et al. developed a solution for the COVID-19 N95 mask shortage by designing a mask adaptor that maintains the N95 seal standard. Several designs were 3D-printed and optimized based on filter surface area, seal efficacy, and N95 respirator multiplicity; the final design was a 3D-printed soft silicone base and a 3D-printed rigid cartridge to seal one-quarter of a 3M 1860 N95 mask [Citation32]. All participants passed computerized qualitative mask fit testing (6/6). The PortaCount Respirator Fit Tester was used to measure the concentration of microscopic particles outside the mask and leakage inside the mask. The ratio of these two numbers is the fit factor that is used for assessment of standard N95 mask seal. The overall fit factor measured was 148 ± 29, with 100 as the standard pass level for an 1860 N95 mask. In addition, the open-source files are publicly available for other researchers to utilize.
Swennen et al. produced 3D-printed personalized masks by utilizing multiple filtration units [Citation30]. These individualized 3D masks consist of two 3D-printed PA composite components (a face mask and a filter membrane) and disposable components (a head fixation band and a filter membrane). The authors used CAD to measure the face mask based on individual facial scans [Citation30].
Face shields
Face shields are PPE devices used to protect facial areas and associated mucous membranes (eyes, nose, mouth) from sprays and splatter of body fluids. Face shields offer an additional layer of protection from COVID-19 droplets, with sufficient space room to wear a protective N95 mask underneath. Face shields are comprised of three components, a robust headband, a durable plastic shield and an elastic band. PC, polyethylene, polyester, polyvinyl chloride, polyethylene terephthalate, polylactic acid and other synthetic polymers are commonly used to make surgical face shields and can be 3D printed via FDM [Citation58,Citation59]. shows the different biomaterials and 3D technology that can be used to construct a face shield design.
There are several advantages of 3D printing for manufacturing face shields. Due to their simple design, 3D printers can easily provide thousands of face shields per day. Additionally, the biomaterials used to create these shields can be easily sanitized for repeated wear if necessary. Specifically, antimicrobial polymers allow engineers to prototype face shields and other critical medical devices [Citation59].
Ventilation equipment
Globally, one of the biggest challenges amid the COVID-19 crisis is when the number of critical care patients exceeds the available medical infrastructure. Based on data from Wuhan, China, 56% of COVID-19 patients that were admitted to the intensive care unit (ICU) required noninvasive ventilation (NIV) and 76% required further orotracheal intubation and invasive mechanical ventilation [Citation45,Citation46]. Therefore, ventilation devices are in high demand during the COVID-19 pandemic.
Ventilator valves/adaptors
Ventilator valves are attachments used to deliver oxygen at fixed concentrations for patients with acute respiratory distress, including COVID-19 patients. 3D-printing technology can be used via a filament extrusion system or a polymer-laser powder/SLS bed fusion process to print single-use valve sets [Citation46,Citation60]. 3D printers can be used to design the different elements of the valve using biomaterials such as PA, polysulfone, PC, silicone rubber, nylon and PA 12 (PA12) [Citation45,Citation46,Citation59–61]. Furthermore, these disposable valves eliminate time-consuming sterilization.
Mechanical bag valve mask
3D-printed emergency-respiration custom adapters and valves can be used to connect to mechanical bag valve mask (BVM) or artificial manual-breathing unit bags (AMBU) [Citation46,Citation62–64]. This mechanical BVM is meant for short-term emergency ventilation of COVID-19 patients while more critical patients would require long-term (>2 weeks) ventilation with controlled settings based on humidification, oxygen, filtration and pressure adjustments. One major advantage is that 3D-printed respirator adapters and valves are scalable with a production rate between 50 to 100 units per day [Citation62–64]. It is also possible to add layers of automation sensors and customized regulation of air pressure and flow allowing for disease-specific and patient-tailored respiratory support implementations [Citation63]. Moreover, these masks are very well-fitted to the user’s face which is particularly important to prevent emission of aerosols. Dhanani et al. performed in vivo and in vitro testing in pigs using a 3D modular ventilator [Citation41]. The AMBU bag was connected to a wall oxygen source using a flow meter. The authors demonstrated comparable mechanical efficiency of the test ventilator compared with a standard ventilator [Citation41]. The 3D ventilator is low cost and can be rapidly produced, but limitations include lack of data on plateau pressure (alveolar pressure) and positive end-expiratory pressure.
Noninvasive ventilation
NIV therapy or continuous positive airway pressure uses face masks, nasal masks or mouthpieces to provide both oxygenation and ventilation support. NIV has been proposed for treatment of less-severe COVID-19 patients that do not require ventilators given the increasing demand for ICU beds for critically ill patients during this pandemic [Citation41,Citation61–64]. The WHO and the CDC recommend that NIV should be utilized in a negative-pressure isolation room for patients [Citation63].
Makowski et al. developed customized respirators according to the anthropometric dimensions via 3D-scanning and -printing techniques [Citation44]. These measurements were detected using a hand-held 3D scanner and the digital model of the facepiece was matched to the user’s face via CAD software. Thereafter, SLS was used to print tailored facepieces from thermoplastic polyurethane [Citation63]. These respirators were very well-fitted and did not cause any facial imprints or contact dermatitis. The application of 3D facial scanning and printing techniques for designing and fabricating customized facepieces are a viable choice for development of respiratory protective devices, such as an NIV mask for COVID-19 patients. Some advantages of NIV include, less intensive monitoring and more efficient use of scarce medical resources like ICU beds [Citation41,Citation62–64]. displays 3D-printing techniques, materials and applications for ventilation equipment.
COVID-19 specimen collection kit
Nasopharyngeal (NP) swabs are flexible rods with bristled ends that are inserted into the nasal cavity to sample cells and mucus. Oropharyngeal (OP) swabs are used to collect specimens by swabbing the patient’s posterior pharynx and tonsillar area. Creating 3D-printed NP and OP test swabs would help increase COVID-19 testing capacity for patients worldwide. Testing swabs can be made from a flexible polymer, like dacron, nylon flocked, rayon, polyester or surgical guide resin, with customized formulations resulting in a wide range of mechanical, optical and thermal properties [Citation65–67]. Moreover, swab bud lattice fibers can be made in customized fashion using 3D engineering [Citation44,Citation65].
An ideal NP and OP swab should have: efficient capillary hydraulics between the brush strands allowing to maximal absorption, a tip with perpendicular brush-like texture allowing the flocked nylon to efficiently dislodge and collect cells and mucus and a tip with an open lattice structure, allowing rapid automatic elution that releases the sample immediately when immersed in viral transport medium [Citation65]. In addition, different sized (small, medium, large) nasal swabs can be printed for variations in nostril size to minimize patient discomfort. shows the different biomaterials and 3D technology that can be used to construct NP and OP swabs.
Oland et al. performed a clinical validation study on 3D-printed NP swabs for the diagnosis of COVID-19 [Citation38]. Seventy adult patients (37 COVID-19 positive and 33 COVID-19 negative) underwent consecutive diagnostic reverse transcription PCR testing with a flocked swab followed by one or two 3D-printed swabs. The ‘lattice swab’ demonstrated 93.3% sensitivity and 96.8% specificity and the ‘origin KXG’ demonstrated 83.9% sensitivity and 100% specificity. Thus, the authors concluded that 3D-printed NP swab results have high concordance with the control swabs [Citation38].
Arjunan et al. developed 3D-printed auxetic NP swabs with the aim of reducing patient pain and discomfort [Citation39]. These specific swabs can shrink under axial resistance thus allowing the swab to navigate through the nasal cavity with significantly less stress on the surrounding tissues [Citation39].
One of the major advantages of using 3D technology to print NP and OP swabs is a production rate of 2000–3000 a day [Citation66]. Another advantage is that synthetic swabs have a more effective sample release process when placed into a culture medium.
Medications
3D technology such as fused filament, powder extrusion, gel extrusion, SLS and SLA allows for fabrication of printed medications for COVID-19 patients that are in short supply. On 31 March 2020, the US FDA added the antimalarial drugs, hydroxychloroquine and chloroquine, potential treatments for COVID-19, to its shortage list due to increased demand [Citation38,Citation65–67]. In addition, there has been an exponential increase for the antiviral drug, remdesivir, which is also in limited supply [Citation49–52,Citation68,Citation69].
Hsiao et al. reported applications and challenges in applying 3D-printing technology to oral solid dosage forms production [Citation70,Citation71]. They noted that since 2018, other studies have shown multicomponent controlled-release polypills and custom capsule devices for sustained drug release, such as SLS, direct-powder extrusion and electrohydrodynamic printing [Citation47,Citation48,Citation72–74]. They noted that many of repurposing drug candidates for COVID-19 have poor aqueous solubility and that oral administration of these drugs would need specific bioavailability enabling formulations, such as amorphous solid dispersion, for drug efficacy. Since specific formulations are necessary for anti-viral drugs, FDM printing and amorphous solid dispersion using hydrophilic polymers could be suitable [Citation70]. Some limitations in these approaches are the high drug melting point that is needed for printing [Citation70].
Multicompartment and multilayer 3D printing can be used for fixed doses or combination of two or more anti-viral therapeutics for COVID-19. It would be important to embed drugs in 3D-printed dosage forms that would then provide a barrier for physical and chemical degradation. The major challenges in 3D-printed medication are the synergy between drug formation and for selection of 3D-printing technology (i.e., ink-jet powder). Moreover, scalability, expense and time are other challenges.
Fused-filament 3D printing is a versatile delivery system used to fabricate tablets containing drug doses customized to individual patients or specific drug-release profiles. Goyanes et al. created tablets that had excellent mechanical properties and little thermal degradation [Citation53,Citation75–92]. Furthermore, dissolution tests showed that release profiles were dependent on the drug-fill percentages. Therefore, FF 3DP can be an exceptional solution for fabricating personalized-dose medicines or dosages with controlled-release profiles for COVID-19 patients.
SLA has been used to create 3D-printed medicine in several reports [Citation15,Citation16,Citation53,Citation78–90]. In one study, SLA was used to fabricate drug-loaded tablets with modified-release characteristics [Citation78]. The medications were successfully printed, and dissolution simulations of the GI tract showed that the 3D-printed drug release from the tablets was dependent on the drug formation, but independent of dissolution pH. Thus, SLA is a remarkable tool for manufacturing of drug-loaded tablets with distinct release profiles [Citation78].
SLS have remarkable capability in the creation of customized medications and other tools to address the PPE shortage [Citation17,Citation18,Citation81,Citation82]. Specifically, Fina et al. used SLS to successfully produce printed pills that showed no evidence of drug degradation [Citation17]. Although there are no specific antivirals or vaccines for treatment of COVID-19, several well-characterized drugs are being considered as therapies [Citation2,Citation3,Citation83–86]. It is feasible to use 3D medication-printing technology to effectively and quickly print, remdesivir, chloroquine and hydroxychloroquine pills [Citation2,Citation49,Citation50,Citation52]. Thus, 3D medication printing has great potential within the pharmaceutical industry in general, but also in the optimization of supply and distribution chains to aid in the treatment of COVID-19 patients.
Limitations
There are several potential challenges for the development and approval of 3D printing of medical devices during the pandemic. First, medical devices need to be highly regulated for safety and efficacy; in-house expertise is of particular concern. Second, standard safety and quality measures of 3D-printing labs must be optimized. In regards to the pandemic, medical centers that have partnerships between 3D-printing resources and hospitals would need to follow specific safety protocols. This includes sterilization processes using newly-printed medical devices. Third, intellectual property remains a concern and thus regulators and policy makers must establish partnerships. Some other top 3D-printing concerns include part quality (integrity, strength and aesthetics), costs of biomaterials, printers and other equipment and cost of pre- and postprocessing. Scalability is also a challenge because mass production might be limited due to printing times, which can be typically a few hours.
For 3D imaging, there are inherent limitations. Specifically, understanding the 3D-imaging technology for personalized facial scanning for PPE may be challenging however 3D-imaging tutorials may be available for healthcare facilities and there may be options for virtual facial scanning to print N95 masks.
The biggest potential bottleneck for 3D printing is lack of standardization and potential for low-quality products. Printing technologies lack universal standards and thus many manufacturers and scientists may encounter issues with quality, strength and reliability of products. Thus, an industry-wide standardization for 3D printing and manufacturing is necessary. In addition, printing hardware failure and irregular maintenance frequency are a bottlenecks that merit consideration.
Conclusion
Amid the rapidly progressing COVID-19 outbreak, there have been PPE shortages globally. 3D-printing technology is well suited to address COVID-19-related shortages by creating several low-cost medical equipments from cost-effective and readily-available polymers. However, processing time, clinical testing and skills shortage are potential barriers to creating 3D-printed medical equipment. Synthetic polymers needed for 3D-printed PPE are exact or very similar in biomaterial composition to the standard manufacturing grade products (i.e., N95 respirator masks, mask filters, NP and OP swabs, ventilator adaptors). Thus, 3D technology has great potential to revolutionize healthcare through accessibility, affordability and personalization. While optimizing mitigation strategies, 3D-printing technology can be used to yield a variety of tools that front lines healthcare workers can use in the fight against the COVID-19.
Future perspective
Although 3D printing offers significant contributions to the healthcare, there are still unanswered questions on regulations for point-of-care manufacturing. Premarket approval submissions and FDA approvals must meet certain requirements to be fully functional for use. Moreover, these applications may take time to complete testing for approval. Some of the important aspects that need to be considered include, intellectual property for medical parts, a validated manufacturing process that adheres to specifications and regulations and personnel and equipment that are readily available at facilities. As 3D printing is adopted more widely for various applications, regulatory oversight is necessary to ensure safety. During the pandemic, several innovative partnerships with universities rapidly produced medical devices, thus highlighting the possibilities for the future production. Importantly, several organizations have openly sourced their 3D printing, allowing people from all over the world to have access. This open-source model is an attempt for equitable standardization and democratic availability from a software perspective. Over the next 10 years, applications of 3D printing in medicine will continue to grow with the goal of improving patient diagnosis and treatment options, as well as, medical equipment for healthcare systems. This transformative technology has the capability to significantly impact medicine in the coming years.
The global spread of COVID-19 has resulted in shortages of personal protective equipment leaving frontline health workers unprotected and overwhelming the healthcare system.
3D printing allows researchers and engineers to design potential applications for personal protective equipment for healthcare workers treating COVID-19 patients.
There are major developments of 3D-printed clinical applications for COVID-19 that is illustrated in this article.
3D-imaging technology can be used to create N95 mask seals.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Ranney ML , GriffethV, JhaAK. Critical supply shortages – the need for ventilators and personal protective equipment during the COVID-19 pandemic. N. Engl. J. Med.382(18), e41 (2020).
- Marco C , RajnikM, CuomoAet al. Features, evaluation and treatment coronavirus (COVID-19). In: StatPearls [Internet].FL, USA (2020).
- Iacobucci G . Covid-19: doctors still at “considerable risk” from lack of PPE, BMA warns. BMJ368, m1316 (2020).
- Iacobucci G . Covid-19: lack of PPE in care homes is risking spread of virus, leaders warn. BMJ368, m1280 (2020).
- Alomari M , MohamedFH, BasitAWet al. Personalised dosing: printing a dose of one's own medicine. Int. J. Pharm.494, 568–577 (2015).
- Sanderson K . 3D printing: the future of manufacturing medicine. Pharm. J.294(7865), 590–592 (2015).
- Ishack S , LipnerSR. A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends. Dermatol. Surg.46(12), 1500–1505 (2020).
- Goyanes A , FinaF, MartoranaA. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm.527(1-2), 21–30 (2017).
- Azad MA , OlawuniD, KimbellGet al. Polymers for extrusion-based 3D printing of pharmaceuticals: a holistic materials-process perspective. Pharmaceutics12(2), 124 (2020).
- Vaezi M , ZhongG, KalamiHet al. Extrusion-based 3D printing technologies for 3D scaffold engineering. Funct. 3D Tiss. Eng. Scaff.2018, 235–254 (2015).
- He DL , HanFG, WangZet al. A review of 3D printing via fused deposition modeling in pharmaceutics. Acta. Pharm. Sin. B.51(11), 1659–1665 (2016).
- Long J , GholizadehH, LuJet al. Novel biomaterials used in medical 3D printing techniques. Curr. Pharm. Des.9(1), 17 (2018).
- Tappa K , JammalamadakaU. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater.9(1), 17 (2018).
- Mubarak S , DhamodharanD, DivakaranNet al. Enhanced mechanical and thermal properties of stereolithography 3Dprinted structures by the effects of incorporated controllably annealed anatase tio2 nanoparticles. Nanomaterials (Basel)10(1), 79 (2020).
- Healy AV , FuenmayorE, DoranPet al. Additive manufacturing of personalized pharmaceutical dosage forms via stereolithography. Pharmaceutics11(12), E645 (2019).
- Kumar H , KimK. Stereolithography 3D bioprinting. Methods Mol. Biol.2140, 93–108 (2020).
- Fina F , GoyanesA, GaisfordSet al. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm.529(1-2), 285–293 (2017).
- Fina F , MadlaCM, GoyanesAet al. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm.541(1-2), 101–107 (2018).
- Lei Z , YangJ, ZhuangZ. Headform and N95 filtering facepiece respirator interaction: contact pressure simulation and validation. J. Occup. Environ. Hyg.9(1), 46–58 (2012).
- Lei Z1 , YangJ, ZhuangZ. A novel algorithm for determining contact area between a respirator and a headform. J. Occup. Environ. Hyg.11(4), 227–237 (2014).
- Han DH , RhiJ, LeeJ. Development of prototypes of half-mask facepieces for Koreans using the 3D digitizing design method: a pilot study. Ann. Occup. Hyg.48(8), 707–714 (2004).
- Krishnamurthy H , SenD. Deriving statistical fit contours and shape of an aerosol mask from 3D head scans. Int. J. Hum. Fac. Model. Simul.2(4), 293–313 (2011).
- Niezgoda G , KimJ, RobergeR, BensonS. Flat fold and cup-shaped N95 filtering facepiece respirator face seal area and pressure determinations: a stereophotogrammetry study. J. Occup. Environ. Hyg.10(8), 419–424 (2013).
- Zhuang Z , BensonS, ViscusiD. Digital 3-D headforms with facial features representative of the current US workforce. Ergonomics53(5), 661–671 (2010).
- Cai M , ShenH, ZhangLXet al. Study of contact characteristics between a respirator and a headform. J. Occup. Environ. Hyg.13(3), D50–D60 (2016).
- Cai M , ShenS, LiH. The effect of facial expressions on respirators contact pressures. Comput. Method Biomech. Biomed. Engin.20(10), 1122–1131 (2017).
- Cai M , LiH, ShenSet al. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J. Occup. Environ. Hyg.15(3), 226–234 (2018).
- Roberge RJ , NiezgodaG, BensonS. Analysis of forces generated by n95 filtering facepiece respirator tethering devices: a pilot study. J. Occup. Environ. Hyg.9(8), 517–523 (2012).
- Centers for Disease Control and Prevention . NIOSH-approved n95 particulate filtering facepiece respirators. http://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/n95list1.html
- Swennen GRJ , PottelL, HaersPE. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int. J. Oral Maxillofac. Surg.49(5), 673–677 (2020).
- McAvoy M , BuiAN, HansenCet al. 3D Printed frames to enable reuse and improve the fit of N95 and KN95 respirators. medRxivdoi:10.1101/2020.07.20.20151019 (2020) ( Epub ahead of print).
- Imbrie-Moore AM , ParkMH, ZhuY, PaulsenMJ, WangH, WooYJ. Quadrupling the n95 supply during the COVID-19 crisis with an innovative 3D-printed mask adaptor. Healthcare (Basel)8(3), 225 (2020).
- Wesemann C , PieralliS, FretwurstTet al. 3D printed protective equipment during COVID-19 pandemic. Materials (Basel)13(8), 1997 (2020).
- Sapoval M , GaultierAL, DelGiudice Cet al. 3D-printed face protective shield in interventional radiology: evaluation of an immediate solution in the era of COVID-19 pandemic. Diagn. Interv. Imaging101(6), 413–415 (2020).
- Lemarteleur V , FouquetV, LeGoff Set al. 3D-printed protected face shields for health care workers in COVID-19 pandemic. Am. J. Infect. Control11, 389–391 (2020).
- Sananès N , LodiM, KochAet al. 3D-printed simulator for nasopharyngeal swab collection for COVID-19. Eur. Arch. Otorhinolaryngol.278(7), 2649–2651 (2020).
- Ford J , GoldsteinT, TrahanS, NeuwirthA, TatorisK, DeckerS. A 3D-printed nasopharyngeal swab for COVID-19 diagnostic testing. 3D Print. Med.6(1), 21 (2020).
- Oland G , GarnerO, deSt Maurice A. Prospective clinical validation of 3D printed nasopharyngeal swabs for diagnosis of COVID-19. Diagn. Microbiol. Infect. Dis.99(3), 115257 (2021).
- Arjunan A , ZahidS, BaroutajiA, RobinsonJ. 3D printed auxetic nasopharyngeal swabs for COVID-19 sample collection. J. Mech. Behav. Biomed. Mater.114, 104175 (2020).
- Ayyıldız S , MuratA, VedatDet al. 3d-printed splitter for use of a single ventilator on multiple patients during covid-19. 3D Print. Addit. Manuf.7(4), 181–185 (2020).
- Dhanani J , PangG, PincusJet al. Increasing ventilator surge capacity in COVID 19 pandemic: design, manufacture and in vitro-in vivo testing in anaesthetized healthy pigs of a rapid prototyped mechanical ventilator. BMC Res. Notes13(1), 421 (2020).
- Haigh JN , DargavilleTR, DaltonPD. Additive manufacturing with polypropylene microfibers. Mater. Sci. Eng. C. Mater. Biol. Appl.77, 883–887 (2017).
- Cabrini L , LandoniG. A novel non-invasive ventilation mask to prevent and manage respiratory failure during fiberoptic bronchoscopy, gastroscopy and transesophageal echocardiography. Heart Lung Vessel.7(4), 297–303 (2015).
- Makowski K , OkrasaM. Application of 3D scanning and 3D printing for designing and fabricating customized half-mask facepieces: a pilot study. Work63(1), 125–135 (2019).
- Ñamendys-Silva SA . Respiratory support for patients with COVID-19 infection. Lancet Respir. Med.8(4), e18 (2020).
- Nickson C . Bag-valve-mask (BVM) ventilation (2019). https://litfl.com/bag-valve-mask-bvm-ventilation/
- Maroni A , MelocchiA, PariettiFet al. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release268, 10–18 (2017).
- Fina F , MadlaCM, GoyanesAet al. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm.541, 101–107 (2018).
- CBS News . Shortage of possible coronavirus treatment puts others at risk. (2020). http://www.cbsnews.com/news/coronavirus-drugs-remdesivir-hydroxychloroquine-shortage-risk/
- Erman M . States work to limit prescriptions of potential coronavirus drugs. Reuters (2020). http://www.reuters.com/article/us-health-coronavirus-usa-pharmacies/states-work-to-limit-prescriptions-of-potential-coronavirus-drugs-idUSKBN2190XC
- GILEAD Corporation . Emergency access to remdesivir outside of clinical trials. Gilead Sciences (2020). http://www.gilead.com/purpose/advancing-global-health/covid-19/emergency-access-to-remdesivir-outside-of-clinical-trials
- ASHP Foundation . Hydroxychloroquine sulfate tablets. (2020). http://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortage-Detail.aspx?id=646
- Formlabs Inc . The ultimate guide to stereolithography (SLA) 3D printing. Formslab (2020). https://archive-media.formlabs.com/upload/SLA_Guide.pdf
- Banerjee SS , BurbineS, KodihalliNet al. 3D-printable PP/SEBS thermoplastic elastomeric blends: preparation and properties. Polymers (Basel)11(2), 347 (2019).
- Banerjee SS , BhowmickAK. Novel nanostructured polyamide 6/fluoroelastomer thermoplastic elastomeric blends: influence of interaction and morphology on physical properties. Polymer54, 6561–6571 (2013).
- Setz S , StrickerF, DuschekT. Morphology and mechanical properties of blends of isotactic or syndiotactic polypropylene with SEBS block copolymers. J. Appl. Polym. Sci.59, 1117–1128 (1996).
- Setz S , StrickeF, KresslerJ. Morphology and mechanical properties of blends of isotactic or syndiotactic polypropylene with SEBS block copolymers. J. Appl. Polym. Sci.86(2), 359–365 (1996).
- Roberge RJ . Face shields for infection control: a review. J. Occup. Environ. Hyg.13(4), 235–242 (2016).
- Centers for Disease Control and Prevention . Strategies to allocate ventilators from stockpiles to facilities (2019). http://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/ventilators.html
- The Physics arXiv . How to build a mechanical ventilator for a few hundred euros. (2020). http://www.discovermagazine.com/technology/how-to-build-a-mechanical-ventilator-for-a-few-hundred-euros
- 3dadept . COVID-19: spain approves first medical 3D printed ventilator. (2020). https://3dadept.com/covid-19-spain-approves-first-medical-3d-printed-ventilator-next-step-is-to-produce-100-ventilators-per-day/
- Custom 3D printed noninvasive ventilation mask . Pediatric sleep apnea (2020). http://www.clinicalconnection.com/clinical-trials-from-other-databases/full-listing-from-other-databases/418052/44158316/custom-3-d-printed-noninvasive-ventilation-mask
- Bersten AD . Best practices for noninvasive ventilation. CMAJ183(3), 293–294 (2011).
- Hill NS . Where should noninvasive ventilation be delivered?Respir. Care54(1), 62–70 (2009).
- BD Corporation . BD flocked swabs. (2020). http://www.bd.com/en-us/offerings/capabilities/specimen-collection/swab-based-specimen-collection/bd-flocked-swabs
- Dawood FS , JaraJH, EstripeautD. What is the added benefit of oropharyngeal swabs compared to nasal swabs alone for respiratory virus detection in hospitalized children aged <10 years?J. Infect. Dis.212(10), 1600–1603 (2015).
- Northwell Heath Group . Northwell 3D prints nasal swabs critical to COVID-19 testing. (2020). http://www.northwell.edu/news/in-the-news/northwell-3d-prints-nasal-swabs-critical-to-covid-19-testing
- Brittney Manchester . Coronavirus (COVID-19) update: daily roundup March 31, 2020. http://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-march-31-2020
- Herper M . Gilead pauses access to experimental COVID-19 drug due to ‘overwhelming demand’. StatNews (2020). http://www.statnews.com/2020/03/22/gilead-suspends-access-to-experimental-covid-19-drug-remdesivir/
- Hsiao WK , LorberB, PaudelA. Can 3D printing of oral drugs help fight the current COVID-19 pandemic (and similar crisis in the future)?Expert Opin. Drug Deliv.17(7), 899–902 (2020).
- Hsiao W-K , LorberL, ReisamerHet al. 3D printing of oral drugs: a new reality or hype? Expert Opin. Drug Deliv. 15(1), 1–4 (2018).
- Haring AP , TongY, HalperJet al. Programming of multicomponent temporal release profiles in 3D printed polypills via core-shell, multilayer, and gradient concentration profiles. Adv. Healthcare Mater.7, 1800213 (2018).
- Goyanes A , AllahhamN, TrenfieldSJet al. Direct powder extrusion 3D printing: fabrication of drug products using a novel single-step process. Int. J. Pharm.567, 118471 (2019).
- Wu S , AhmadZ, LiJ-Set al. Fabrication of flexible composite drug films via foldable linkages using electrohydrodynamic printing. Mater. Sci. Eng. C.108, 110393 (2020).
- Khaled SA , BurleyJC, AlexandeMR. Desktop 3D printing of controlled release pharmaceutical bilayer tablets Author links open overlay panel. Int. J. Pharm.461(1-2), 105–111 (2014).
- Goyanes A , BuanzAB, BasitAW. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm.476(1-2), 88–92 (2014).
- Goyanes A , BuanzAB, HattonGB. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm.89, 157–162 (2015).
- Wang J , GoyanesA, GaisfordS. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm.503(1-2), 207–212 (2016).
- Martinez PR , GoyanesA, BasitAW, GaisfordS. Influence of geometry on the drug release profiles of stereolithographic (sla) 3D-printed tablets. AAPS Pharm. Sci. Tech.19(8), 3355–3361 (2018).
- Zhang S , LiM, NaijiaH. Stereolithography 3D printing of lignin-reinforced composites with enhanced mechanical properties. ACS Omega4(23), 20197–20204 (2019).
- Schmid M , WegenerK, AmadoA. Materials perspective of polymers for additive manufacturing with selective laser sintering. J. Mater. Res.29, 1824–1832 (2014).
- Yuan RLS , ZhangW, ZhengH. 3D printing of mixed matrix films based on metal-organic frameworks and thermoplastic polyamide 12 by selective laser sintering for water applications. ACS Appl. Mater. Interfaces11(43), 40564–40574 (2019).
- Lin Q , ZhaoS, GaoD. A conceptual model for the coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China with individual reaction and governmental action. Int. J. Infect. Dis.93, 211–216 (2020).
- Information for clinicians on therapeutic options for patients with COVID-19 (2020). http://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html
- Coronavirus (COVID-19) update (2020). http://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundup-april-7-2020
- Liu C , ZhouQ, LiYet al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci.6(3), 315–331 (2020).
- Richardson P , GriffinI, TuckerCet al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet395(10223), e30–e31 (2020).
- Zhang X , ZhuZ, NiZet al. Inexpensive, rapid fabrication of polymer-film microfluidic autoregulatory valve for disposable microfluidics. Biomed. Microdevices19(2), 21 (2017).
- Pavani K , MahabaleswaraH, JosephKet al. Tracheal intubation through laryngeal mask airway Ctrach™ with polyvinyl chloride tube: comparison between two orientations of the tracheal tube. Anaesthesiol. Clin. Pharmacol.33(4), 473–479 (2017).
- Yu I , ChenRK. A feasibility study of an extrusion-based fabrication process for personalized drugs. J. Pers. Med.10(1), 16 (2020).
- Rocha CR , ShemelyaCM, WickerRBet al. Novel ABS-based binary and ternary polymer blends for material extrusion 3D printing. J. Mater. Res.29, 1859–1866 (2014).
- Lamichhane S , ParkJBP, SohnDH. Customized novel design of 3d printed pregabalin tablets for intra-gastric floating and controlled release using fused deposition modeling. Pharmaceutics11(11), 564 (2019).