SUMMARY
Aims:
In this paper, we examine the long-term consequences of a breast cancer diagnosis on hypothalamic–pituitary–adrenal axis functioning.
Methods:
This study provides a comprehensive analysis of the diurnal and reactive cortisol profiles of breast cancer survivors considered ‘disease-free’ compared with women with no history of breast cancer.
Results:
The results indicate similar diurnal patterns in both groups; however, significant differences in stress reactivity were noted, with breast cancer survivors displaying a relatively flat profile following the acute stress induction. Subjective levels of psychological stress were similar in both groups, suggesting incongruence between perceived stress and the physiological stress response of breast cancer survivors.
Conclusion:
The patterns suggest a progression towards more typical cortisol reactivity with longer time since diagnosis and may reflect some recovery of hypothalamic–pituitary–adrenal axis functioning as time passes.
• Diurnal and reactive cortisol profiles studied in long-term breast cancer survivors.
• No differences in psychological stress experienced between breast cancer survivors and women without a history of cancer.
• ·Normal diurnal cortisol patterns in breast cancer survivors but significant differences in acute stress reactivity (flat profile) relative to control participants.
• ·Patterns indicate a progression towards more typical cortisol reactivity patterns with passage of time.
In recent decades, advances in the fields of screening and treatment have led to the decline in the mortality rate of breast cancer patients. While the increase in survivor rates is encouraging, these results emphasize even more on the importance of investigating the psychological and physical wellbeing of breast cancer survivors later on in their lives. The experience of cancer that encompasses a tumor or tumor cells, difficult treatments, as well as high levels of psychological stress weigh heavily on the biological systems of the body and can lead to their dysregulation [Citation1]. One of the systems that has been shown to be particularly altered by the experience of cancer is the hypothalamic–pituitary–adrenal (HPA) axis [Citation2–5]. However, an important question is whether the HPA axis can recover and go back to its precancer functioning once treatment has terminated and no signs or symptoms of cancer are visible. This is important to address since a compromised HPA axis had been shown to lead to alteration in cardiovascular and immune systems [Citation6]. Furthermore, dysregulation of the HPA axis has been associated with mortality and disease severity in metastatic breast cancer patients [Citation7] and lung cancer patients [Citation8]. In this paper, we examine the long-term consequences of a breast cancer diagnosis on HPA axis functioning.
The function of the HPA axis is to maintain homeostasis and prepare organisms to deal with environmental challenges [Citation9]. It has a baseline circadian activity as well as mechanisms designed to respond to an acute stressful situation [Citation10]. Via release of cortisol and a cascade of other participants, the HPA axis acts permissively in energy metabolism, stress responsiveness and information processing [Citation9]. The activity of the HPA axis can be assessed in a variety of ways, most commonly by measuring cortisol output. In healthy individuals, cortisol diurnal secretion follows a typical and predictable slope that peaks 30–45 min after waking and diminishes throughout the day [Citation11]. However, following an acute stressor, cortisol concentration begins to mount and peaks 10–30 min after stressor cessation and recovery to baseline level is observed 1–2 h afterwards [Citation12,Citation13].
• Circadian patterns of cortisol in breast cancer survivors
Investigation of the circadian rhythm of cancer survivors has received growing consideration over recent decades following its relationship with cancer prognosis and severity [Citation7]. Studies measuring the diurnal rhythms of cortisol in metastatic breast cancer patients have reported an atypical presentation of the cortisol circadian slopes with erratic peaks and troughs as well as flattened profiles [Citation2,Citation5,Citation7,Citation14]. Moreover, dysregulation of the circadian rhythm was associated with disease severity [Citation5,Citation14] and high levels of anxiety [Citation15]. However, no significant alterations were found between the circadian rhythms of recently diagnosed breast cancer survivors [Citation16] and early-stage breast cancer survivors [Citation17].
Diurnal rhythms were also investigated in breast cancer survivors who had completed all treatments with the exception of hormonal therapy [Citation18,Citation19]. In the Porter et al. study [Citation18], breast cancer participants were 3–5 years post diagnosis and most were diagnosed with a cancer stage of 1 or 2. Two sets of saliva samples were collected – a baseline sample 1 month before a routine mammogram (e.g., baseline) as well as the day before, the day of a mammogram and the day after the test. Results indicated that at baseline, breast cancer survivors presented with higher levels of diurnal cortisol than that of women in the control group. However, these levels were lower than the values obtained around the mammogram period. These results suggest that alteration of the diurnal rhythmicity may be present in breast cancer survivors that are considered disease free. Furthermore, these results are consistent with the hypocortisolism hypothesis that suggests lower levels of cortisol in the presence of an acute stressor. Bower et al. [Citation19] investigated the role of fatigue in the cortisol diurnal rhythms of breast cancer survivors considered cancer free. Their research participants were 1–5 years post-diagnosis. The results indicated that breast cancer survivors with persistent fatigue had flatter diurnal cortisol rhythms compared with their counterparts without fatigue.
• Cortisol reactivity
Cortisol reactivity following an acute stressor was assessed in breast cancer survivors via the use of laboratory protocols design to induce a moderate stress response, such as the TSST [Citation3,Citation20] and a protocol in which the research participant was asked to prepare a story about a threatening situation (e.g., accused of stealing in a shop) [Citation4]. The results were mixed. In Spiegel et al.’s study, metastatic breast cancer survivors were found to have a blunted response to acute stress compared with women without a history of cancer [Citation3], whereas in van der Pompe et al.’s study, the opposite was found. Finally, in Bower et al.’s study [Citation20], the results indicated that the fatigued breast cancer survivors had a significantly blunted stress response compared with that of the nonfatigued group. One of the variables that may account for some of the discrepancy seen in these results relates to how participants perceived the stressor in these studies. Given the high levels of stress experienced by breast cancer survivors, their appraisal of the level of stress may be different than that of women without a history of cancer [Citation4].
Overall, these results suggest that metastatic breast cancer survivors have a more altered pattern of cortisol secretion than that observed in newly diagnosed or early-stage breast cancer survivors. However, some alteration of the levels of the diurnal and cortisol reactivity has also been reported in breast cancer survivors who were considered cancer-free.
Other factors that may have an impact on cortisol secretion patterns include time since diagnosis, stage of disease and cancer-related fatigue. While these studies have evaluated breast cancer survivors with less than 5 years since initial diagnosis, no study has yet investigated the cortisol secretion pattern of long-term breast cancer-free survivors (more than 5 years since initial diagnosis). Furthermore, no study has combined the assessment of diurnal cortisol and cortisol reactivity in a laboratory setting in disease-free breast cancer survivors and women without a history of cancer.
Using a cross-sectional design, the aim of the present study was to provide a comprehensive analysis of the diurnal cortisol secretion profiles and their patterns following an acute stressor in breast cancer survivors with no evidence of disease otherwise and to compare these to women with no history of breast cancer. Factors associated with breast cancer were also assessed in order to evaluate their relationship with cortisol patterns; these included time since diagnosis, cancer stage, fatigue, sleep quality and subjective levels of psychological stress. The unique contribution of this study is the inclusion of perceived stress and both diurnal and reactive patterns of cortisol using a standardized laboratory protocol in the same individuals.
Methods
• Participants
Breast cancer survivors and women without a history of breast cancer were recruited for this study. To be included in the breast cancer survivor group, women needed to satisfy the four following inclusion criteria: diagnosis of breast cancer, completion of all local and/or systemic adjuvant therapy at least 6 months earlier, with the exception of hormonal treatment, considered cancer free and fluent in English. Exclusion criteria were the following: no previous history of other cancers, with the exception of noninvasive skin cancer and cervical cancer and no other major disabling medical or psychiatric conditions that could interfere with quality of life. Inclusion criteria for the control group were the following: women aged between 29 and 80 years old, and fluent in English. Exclusion criteria for the control group were the following: no previous history of cancers, with the exception of non-invasive skin cancer and cervical cancer and no other major disabling medical or psychiatric condition that could interfere with quality of life.
Instruments
• Trier Social Stress Test
The Trier Social Stress Test (TSST) is a widely used tool designed to induce moderate stress in a laboratory setting [Citation13]. It is a task that combines high levels of social-evaluative threat and uncontrollability [Citation21]. The TSST consists of a mock interview in which the research participant is asked to deliver a free-speech as well as to perform an arithmetic task in front of a panel of judges that provides no feed-back [Citation13]. The TSST has been found to be very effective in inducing a cortisol response, with roughly a two- to threefold rise in salivary cortisol levels in a majority of tested participants [Citation22].
Measures of stress
• Salivary cortisol
Saliva samples were assayed in duplicate for cortisol using commercially available highly-sensitive enzyme linked immunosorbent assay kits and the protocol designed by Salimetrics, State College, PA, USA.
• Visual Analog Scale
The Visual Analog Scale (VAS) is a 100-mm bipolar line that measures a characteristic across a continuum. Participants mark a spot on the line that indicates their subjective feeling [Citation23]. In the present study, subjective appraisal of stress was measured with the statement “I feel stressed.” The scale was anchored from 0 = ‘not at all’ to 100 = ‘very much.’ Scores were determined by measuring the distance from the left end to the appraisal mark.
• Bidimensional Fatigue Scale
The Bidimensional Fatigue Scale (BFS) is a questionnaire developed by Chalder et al. [Citation24]. It has 11 items with seven items assessing physical fatigue and four items assessing mental fatigue. Each item is answered on a 4-point scale and the total scores can range between 0 and 33 with higher scores indicating greater fatigue. The BFS also has a cut-off score of 11 meaning that scores of 11 and greater indicate significant fatigue [Citation25].
• Sleep quality
The participants were asked to report the number of hours they slept the night before day 1, the night before day 2 and the night before their laboratory visit. The average number of hours of sleep was calculated for each participant and was used as an indicator of their sleep quality.
• Socio-demographic questionnaire
The socio-demographic questionnaire entailed questions regarding general history, health history and habits known to influence cortisol concentrations. In addition, the breast cancer survivors were also questioned regarding their breast cancer history.
Procedure
This study comprised two parts. For the first part, each participant was asked to provide five saliva samples at home at specific times (waking, 30 min after waking, noon, 16h00 and 21h00) on each of two consecutive days. The purpose was to assess the cortisol diurnal pattern. For the second part, each participant was asked to participate in a laboratory protocol (the TSST) designed to elicit a moderate stress response as indexed by a change in baseline cortisol levels.
Participants were recruited via multiple sources to target both breast cancer survivors and women with no history of cancer in a similar age range. Ads were placed on radio and in newspaper and cancer-related magazines. Those who had indicated an interest in the study were contacted via phone. During the first telephone conversation, a summary of the study was provided and eligibility of potential participants was verified. Eligible participants were scheduled at their convenience for two laboratory visits at the University of Ottawa (Ontario, Canada). The first visit served to obtain informed consent and to provide specific instructions on how and when to collect at-home saliva samples. Participants were provided with a container of labeled salivettes to use at each time point. To collect the samples, participants were asked to rinse their mouths with water and to wait at least 10 min before collection to avoid sample dilution. They were instructed to place the swab directly under their tongues and to wait for 3 min, after which the swab was returned to the salivette and stored in the refrigerator. Participants were asked to avoid alcohol for 24 h before sample collection, to refrain from eating a major meal within 60 min of sample collection, to avoid dairy products for 20 min before sample collection and to avoid foods with high sugar or acidity (citrus), or caffeine content, immediately before sample collection. They were also asked to avoid exercise/working out/training 1 h before sample collection and to avoid brushing their teeth immediately before sample collection (guidelines based on Salimetrics protocol [Citation26]). Participants were provided with a booklet in which they were asked to indicate the time at which they collected the sample as well as any associated mistakes. At the end of the first visit, the second laboratory visit was scheduled, roughly within 7 days after completion of the in-home saliva collection.
• Second visit: laboratory stress protocol
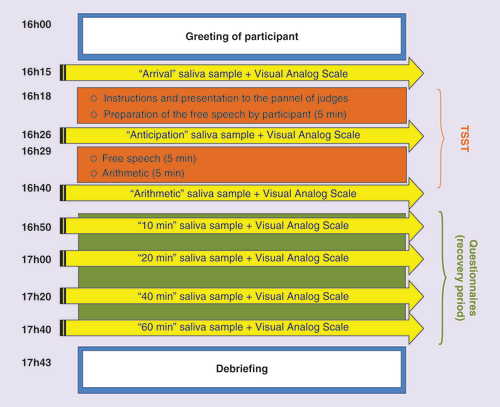
presents an overview of the second laboratory visit. The visit was scheduled at the end of the day, beginning between 15h00 and 17h00 and lasted approximately 2 h; this period was chosen to coincide with the time at which cortisol concentrations are at their lowest values and the function relating time to cortisol concentration is relatively flat. On the day of the laboratory visit, the participant was escorted to the test room and invited to share the saliva collection experience and report any problems. The participant was then asked to drink a glass of water and relax for 10 min. The consumption of water was added to the protocol to increase the odds of having sufficient saliva for sampling during the TSST. The participant was then asked to provide a saliva sample (sample labeled ‘arrival’) and to rate her subjective stress level on the VAS. Once completed, the participant was led to a second test room in order to introduce the mock panel of judges and give instructions on the free speech. The participant was then given 5 min to prepare this task, after which she was asked to provide another saliva sample (sample labeled ‘anticipation’) and prompted to rate her stress level on the VAS. This was followed by delivery of the free speech and participation in an arithmetic task in front of the panel of judges. After completion of these two tasks, a third saliva sample (sample labeled ‘arithmetic’) was requested and the participant prompted to rate her stress level on the VAS. Afterwards, the participant was asked to complete a series of questionnaires, such as the BFS and the socio-demographic questionnaire and to relax for a period of 1 h. During that time, the participant was requested to provide four additional saliva samples along with a VAS rating at 10, 20, 40 and 60 min after the end of the TSST. Once the last sample was collected, there was a debriefing period during which the TSST was explained and the participant invited to share her experience. Upon the departure of the participant, all saliva samples were transferred in Eppendorf tubes and stored in a freezer at -80°C for further analysis.
• Statistical analyses
t-tests were used to assess group differences in age and fatigue level, and as post hoc tests following standard mixed design analysis of variance (ANOVA) analyses on diurnal and reactive cortisol data, and the VAS scores related to subjective stress. The post hoc analyses were based on peak TSST and area under the curve values and trend analyses (cortisol reactivity data) and area under the curve in the case of the diurnal cortisol data. Correlation analyses were used to determine relationships between specific medical variables. Finally χ-square tests evaluated the frequency of individuals who reported their status as either pre- or post-menopausal.
Results
• Participants
Demographic characteristics
Twenty-two breast cancer survivors and 25 women without a history of breast cancer completed both parts of the study. One breast cancer survivor provided saliva samples for the diurnal analysis but was unable to take part in the TSST and therefore did not complete any of the questionnaires. The range and distribution of ages was similar in both groups with no significant difference in mean age (t = -0.700; p = 0.488). The proportion of women with a postmenopausal status was also similar in both groups with no significant difference (χ2 = 2.101; p = 0.147) as was the average number of hours of sleep; women in the control group had an average of 7.6 h (standard deviation [SD] = 1.09), while breast cancer survivors had an average of 7.2 h (SD = 0.83; t = 1.391; p = 0.172). The majority of participants were Caucasian, in a relationship, had completed an undergraduate degree, and in a white collar profession or retired. A complete list of the demographic characteristics of participants is shown in .
Medical characteristics
The participants’ medical characteristics are presented in . Two women in the breast cancer group had experienced a recurrence in breast cancer prior to the study; one woman reported one recurrence and the other, two recurrences. The information in is based on the most recent breast cancer diagnosis of the participants. The treatment history was quite diverse, including chemotherapy, radiation and hormone therapy, either alone or in some combination. There were no serious or untreated comorbid medical conditions. Most commonly reported was hypertension, followed by one or two cases of diabetes per group, and several individuals with osteoarthritis in the breast cancer participants. None of these, if treated, have an impact on reactive cortisol, to our knowledge. Finally, none of the research participants reported being treated with cortisone medication.
• Diurnal saliva sample collection
The times at which saliva samples were collected at home in both groups are presented in . All participants presented good compliance to collection instruction and no significant group differences were found at any time point.
• Diurnal cortisol
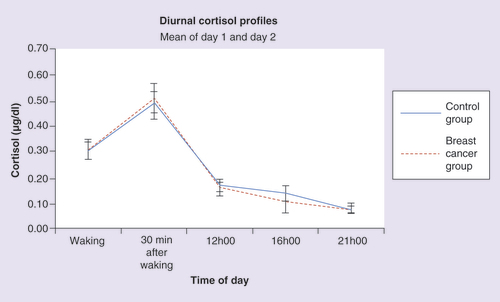
A mixed-design ANOVA was performed to assess group, time and group X time differences in mean cortisol concentrations over two consecutive days at five time points: waking, 30 min after waking, noon, 16h00 and 21h00. The between-subjects factor consisted of two levels (breast cancer survivor and control group), while time, the repeated factor, had five levels. The pattern observed in indicates that both breast cancer survivor and control groups followed a similar and typical pattern of diurnal cortisol secretion with peak concentrations at 30 min after awakening and gradual reduction of cortisol throughout the day. The analysis gave rise to a significant main effect of time (F = 51.427; p < 0.001) but no group (F = 0.005; p = 0.945) or interaction difference (F = 0.320; p = 0.721). The within-case area under the curve was calculated using trapezoidal integration. A t-test indicated no significant difference between groups in this measure (t = 0.116; p = 0.908).
• Cortisol reactivity
The patterns of cortisol reactivity were assessed via the TSST at seven time points (arrival, after the anticipation segment of the TSST, after the arithmetic segment of the TSST and 10, 20, 40 and 60 min after the end of the TSST. The results of the mixed-design ANOVA indicated a significant main effect of time (F = 3.282; p = 0.031) and group (F = 4.884; p = 0.033), but no significant interaction between the two factors (F = 1.703; p = 0.179). Removing the two breast cancer participants who experienced recurrences altered the probability of the group effect to a p-value of 0.052.
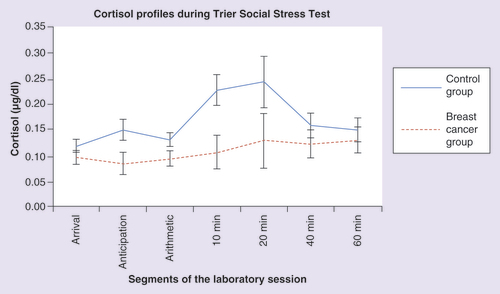
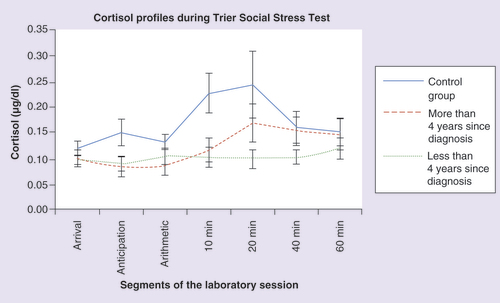
A test of within-subjects contrasts indicated a significant quadratic effect in control participants (F = 4.851; p = 0.040), but no such pattern in breast cancer survivors (F = 0.168; p = 0.687). We also calculated the within-case area under the curve using trapezoidal integration. A t-test indicated a significant group difference in the area under the curve (t = 2.251; p = 0.03). As can be seen in , women in the control group presented with a typical reactivity slope with peak concentrations at 10–20 min following the stressor and recovery to baseline levels. In comparison, women in the breast cancer survivor group showed a blunted cortisol response following the stressor with a relatively flat slope.
• Subjective levels of stress during TSST
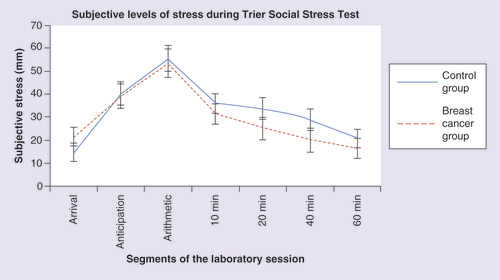
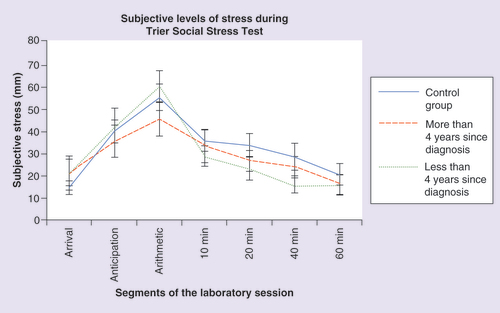
Subjective levels of stress were assessed during the TSST using the VAS (). Participants were asked to rate their level of stress exactly at the same time as saliva samples were collected. The results of the mixed-design ANOVA on these subjective responses indicated a significant main effect of time (F = 19.419; p < 0.001), but no group (F = 0.703; p = 0.406) or interaction (F = 0.810; p = 0.493) effects. A follow-up one-way ANOVA gave rise to no significant group difference in the area under the curve of all groups (F = 0.472; p = 0.627).
• Cortisol reactivity profiles & medical variables
Additional analyses were performed to evaluate the relationship between breast cancer characteristics and the cortisol profile observed during the TSST. Participants in the survivors group were organized into two groups based on the number of years since their breast cancer diagnosis – ‘less than 4 years’ and ‘more than 4 years.’ The number of years chosen was based on the statistical median of our sample (4 years). A mixed-design ANOVA was performed to assess group, time and group × time differences in cortisol concentrations during the TSST. The between subjects factor consisted of three levels (‘breast cancer survivor less than 4 years since diagnosis,’ ‘breast cancer survivors more than 4 years since diagnosis’ and ‘women in the control group’) while time, the repeated factor, had seven levels. The results indicated no significant main effect of time (F = 2.029; p = 0.123), group (F = 2.523; p = 0.094) or interaction (F = 1.061; p = 0.389) effects. A planned comparison of the peak value which occurred at 20 min following the TSST indicated a group difference that approached significance between women in the ‘more than 4 years since diagnosis’ and ‘less than 4 years since diagnosis’ groups (t = -1.809; p = 0.089). The difference in the area under the curve between the breast cancer survivors less than 4 years since diagnosis and 4 years or more since diagnosis was not significant (t = 0.945; p = 0.359). A test of within-subjects contrasts indicated a quadratic effect in breast cancer survivors who were diagnosed more than 4 years ago (F = 5.154; p = 0.057) while no such pattern was seen in breast cancer survivors diagnosed less than 4 years ago (F = 0.581; p = 0.465). These data are displayed in .
Subjective levels of stress were also evaluated to verify if the induced stress level by the TSST was perceived in a similar fashion in all groups on the VAS. The results of the mixed-design ANOVA on these subjective responses indicated a significant main effect of time (F = 16.868; p < 0.001) but no group (F = 0.351; p = 0.706) or interaction (F = 0.806; p = 0.573) effects. A t-test gave rise to no significant group difference in the area under the curve (t = 0.159; p = 0.875). The pattern seen in suggests that the women in all three groups experienced the expected psychological reaction to stress with higher levels perceived during the TSST and a gradual reduction following the end of the stressor.
To examine the role of cancer stage and reactive cortisol patterns as indexed by area under the curve, we subdivided the breast cancer participants into low (0 and 1) and high (2 and 3) cancer stages and determined if there were group differences on this measure; none was found (t = 0.391; p = 0.701).
We also evaluated the relationship between fatigue and the diurnal cortisol secretion pattern in both groups. First, using the cut-off score of ‘11 or more’ on the Bidimensional Fatigue Scale (BDF), the majority of the participants in both groups had a level of fatigue that would be considered significantly high: 88% of women without a history of cancer had a total fatigue score of 11 or more (mean = 12.63, SD = 3.32, range = 8–23) and 84% of breast cancer survivors had a total fatigue score of 11 or more (mean: 13.11; SD: 5.43; range: 1–24). A t-test indicated no difference between groups in the total level of fatigue (t = -0.310; p = 0.759) as well as in the level of physical fatigue (t = 0.174; p = 0.863) and mental fatigue (t = -0.446; p = 0.659). Follow-up correlational analysis revealed no relationship between total levels of fatigue and cortisol during the TSST in both breast cancer survivors (p-value ranged from 0.278 to 0.794) and women in the control group (p-values ranged from 0.123 to 0.727).
Finally, Pearson r correlations were performed to evaluate the relationship between time since diagnosis, the breast cancer stage at diagnosis and the total level of fatigue in breast cancer survivors. No significant correlation was found in any of the variables (p-values ranged from 0.297 to 0.462).
Discussion
The goal of this study was to evaluate if long-term breast cancer survivors are at risk of presenting with a dysregulated HPA axis functioning compared with women without a history of cancer in the similar age range. We assessed the functioning of the HPA axis by measuring the cortisol secretion patterns during the day as well as its reactivity profile following an acute stressor. Our results indicated that while the diurnal patterns of breast cancer survivors appeared typical and very similar to women in the control groups, their cortisol reactivity patterns were significantly altered, that is, characterized by a blunted response. Subjective levels of psychological stress monitored during the TSST protocol indicated that both breast cancer survivors and women in the control group reported feeling stressed during the stress protocol, especially during the free speech and arithmetic tasks. Women in both groups also reported a diminution of reported levels of psychological stress during the recovery period. These results suggest that breast cancer survivors appear to appraise psychological stress in a similar fashion to that of women without a history of cancer but have a diminished physiological stress response to that event. The hyporeactivity of the HPA axis has been observed in some breast cancer survivors [Citation3,Citation20] as well as in other individuals experiencing chronic stress [Citation27]. Among the plausible mechanisms that have been suggested, chronic inflammation related to the experience of cancer has been argued as one of the factors that could lead to dysregulation of the HPA axis in cancer survivors [Citation4,Citation19]. In their study, Bower et al. [Citation20] noted that fatigued breast cancer survivors had a flatter cortisol reactivity slope than did non-fatigued breast cancer survivors. These results are important since the majority of our participants were significantly fatigued based on the cut-off score of the BFS [Citation25]; however, this level applied to both survivors and control subjects. Another mechanism that has been proposed refers to the idea that the experience of cancer can be seen as a situation of chronic stress. Numerous studies have suggested that breast cancer survivors experience high levels of stress at the beginning of their diagnosis and that this distress may persist several years after diagnosis under the form of the fear of cancer recurrence [Citation28–31]. Hans Seyle was one of the first to suggest that the functioning of the stress system was optimal during punctual rather than chronic stress situations. Thus, chronic activation of the stress system was suggested to lead to its dysregulation. The concept of allostasis and allostatic load, coined by McEwen et al. [Citation32] can prove to be a good framework in the unravelling of the relationship between stress and cortisol patterns in chronically stressed individuals. It suggests that the HPA axis plays a crucial role in the maintenance of homeostasis under stressful situations. Thus, when there is a prolonged accumulation of stressors, chronic activation of the HPA axis may lead to its ‘wear and tear’ and potentially be associated with a reduced physiological response.
We also evaluated the role of medical variables such as time since diagnosis and cancer stage in altered cortisol reactivity patterns in breast cancer survivors. Albeit not significant, the overall profile suggests that cortisol reactivity increases or normalizes with the passage of time following the breast cancer diagnosis. No such pattern was observed when this analysis was applied to participants divided into low (0 and 1) and higher cancer stages (2 and 3). The relationship between medical characteristics and positive outcomes needs to be thoroughly established and should be examined more closely in terms of other health issues associated with chronic HPA axis dysregulation.
• Limitations
It is recognized that because convenience sampling was conducted, the participants in this study may not adequately represent all breast cancer survivors. They were recruited locally, generally from urban areas, and well-educated. Finally, it should be noted that our breast cancer participants presented with a variety of cancer treatments – chemotherapy, radiation, hormonal therapy or some combination of two or three of these treatments. The sample sizes of the subgroups were too small to consider further analysis. Future studies should investigate the influence of treatment on HPA axis regulation in breast cancer survivors. Ideally, physical examinations and clinical assessments would be included such as endocrine tests to rule out factors that may affect HPA axis functioning.
Conclusion
In summary, our results indicate normal diurnal cortisol patterns and stress experiences in breast cancer survivors but significant differences in acute stress reactivity. The cortisol patterns of breast cancer survivors following stress induction were characterized by relatively flat profiles compared with healthy individuals and these appeared to be somewhat associated with time since diagnosis.
Future perspective
The assessment of the HPA axis through salivary biomarkers represents a new avenue in the monitoring of breast cancer survivors’ health later on in their life. In other studies, we are exploring the role of different variables of psychological stress on the development of the flat patterns of cortisol seen in breast cancer survivors. Future lines of studies should include the assessment of cortisol patterns at different time points of the cancer trajectory in order to better understand stress-related physiological changes in this population over time.
Table 1. Demographic characteristics of the breast cancer and control groups.
Table 2. Medical characteristics of the breast cancer group.
Table 3. Average ± standard deviation collection time of diurnal saliva samples in each group.
Financial & competing interests disclosure
The authors are grateful for the Canadian Breast Cancer Research Alliance for funding of this project. The authors are thankful for the help and dedication of our research participants. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Acknowledgements
The authors are thankful for the help and dedication of our research participants.
Additional information
Funding
References
- Green Mcdonald P , O’ConnellM, LutgendorfSK. Psychoneuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodological innovations. Brain Behav. Immun.30(Suppl.), S1–S9 (2013).
- Abercrombie HC , Giese-DavisJ, SephtonS, EpelES, Turner-CobbJM, SpiegelD. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology29(8), 1082–1092 (2004).
- Spiegel D , Giese-DavisJ, TaylorCB, KraemerH. Stress sensitivity in metastatic breast cancer: analysis of hypothalamic–pituitary–adrenal axis function. Psychoneuroendocrinology31(10), 1231–1244 (2006).
- van der Pompe G , AntoniMH, HeijnenCJ. Elevated basal cortisol levels and attenuated ACTH and cortisol responses to a behavioral challenge in women with metastatic breast cancer. Psychoneuroendocrinology21(4), 361–374 (1996).
- Touitou Y , LéviF, BogdanA, BenavidesM, BailleulF, MissetJL. Rhythm alteration in patients with metastatic breast cancer and poor prognostic factors. J. Cancer Res. Clin. Oncol.121, 181–188 (1995).
- McEwen BS , WingfieldJC. The concept of allostasis in biology and biomedicine. Horm. Behav.43(1), 2–15 (2003).
- Sephton SE , SapolskyRM, KraemerHC, SpiegelD. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Natl Cancer Inst.92(12), 994–1000 (2000).
- Sephton SE , LushE, DedertEAet al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav. Immun.30(Suppl.), S163–S170 (2013).
- Fulford AJ , HarbuzMS. An introduction to the HPA axis. In: Handbook of Stress and the Brain Part 1: the Neurobiology of Stress.StecklerT, KalinNH, ReulJMHM ( Eds). Elsevier Science Ltd, Amsterdam, The Netherlands43–65 (2005).
- Kaltsas GA , ChrousosGP. The neuroendocrinology of stress. In: Handbook of Psychophysiology (3rd Edition).CacioppoJT, TassinaryLG, BerntsonG ( Eds). Cambridge University Press, NY, USA, 303–318 (2007).
- Stone AA , SchwartzJE, SmythJet al. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology26, 295–306 (2001).
- Foley P , KirschbaumC. Human hypothalamus–pituitary–adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci. Biobehav. Rev.35(1), 91–96 (2010).
- Kirschbaum C , PirkeKM, HellhammerDH. The “Trier Social Stress Test” – a tool for Investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology28, 76–81 (1993).
- Mormont MC , LéviF. Circadian-system alterations during cancer processes: a review. Int. J. Cancer70(2), 241–247 (1997).
- Giese-Davis J , SephtonSE, AbercrombieHC, DuranRE, SpiegelD. Repression and high anxiety are associated with aberrant diurnal cortisol rhythms in women with metastatic breast cancer. Health Psychol.23(6), 645–650 (2004).
- Vedhara K , TuinstraJ, MilesJN, SandermanR, RanchorAV. Psychosocial factors associated with indices of cortisol production in women with breast cancer and controls. Psychoneuroendocrinology31(3), 299–311 (2006).
- Carlson LE , CampbellTS, GarlandSN, GrossmanP. Associations among salivary cortisol, melatonin, catecholamines, sleep quality and stress in women with breast cancer and healthy controls. J. Behav. Med.30(1), 45–58 (2007).
- Porter LS , MishelM, NeelonV, BelyeaM, PisanoE, SooMS. Cortisol levels and responses to mammography screening in breast cancer survivors: a pilot study. Psychosom. Med.65(5), 842–848 (2003).
- Bower JE , GanzPA, DickersonSS, PetersenL, AzizN, FaheyJL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology30(1), 92–100 (2005).
- Bower JE , GanzPA, AzizN. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom. Med.67(2), 277–280 (2005).
- Dickerson SS , KemenyME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull.130(3), 355–391 (2004).
- Kudielka BM , HellhammerDH, KirschbaumC. Ten years of research with the Trier Social Stress Test-revisited. In: Social Neurosciences: Integrating Biological and Psychological Explanations of Social Behavior.Harmon-JonesE, WinkielmanP ( Eds). The Guildford Press, NY, USA, 56–83 (2007).
- Aitken RCB . Measurement of feelings using visual analog scales. Proc. R. Soc. Med.62(10), 989–993 (1969).
- Chalder T , BerelowitzG, PawlikowskaTet al. Development of a fatigue scale. J. Psychosom. Res.37(2), 147–153 (1993).
- Alexander S , MintonO, StonePC. Evaluation of screening instruments for cancer-related fatigue syndrome in breast cancer survivors. J. Clin. Oncol.27(8), 1197–1201 (2009).
- Salimetrics .Expanded range high sensitivity salivary cortisol enzyme immunoassay kit (2009).file:///C:/Users/mcout103/Downloads/salimetrics%20cortisol%20protocol.pdf.
- Miller GE , ChenE, ZhouES. If It goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychol. Bull.133, 25–45 (2007).
- Deimling GT , BowmanKF, SternsS, WagnerLJ, KahanaB. Cancer-related health worries and psychological distress among older adult, long-term cancer survivors. Psychooncology15(4), 306–320 (2006).
- Lim CC , DeviMK, AngE. Anxiety in women with breast cancer undergoing treatment: a systematic review. Int. J. Evid. Healthc.9(3), 215–235 (2011).
- Montgomery MM , McCroneSH. Psychological distress associated with the diagnostic phase for suspected breast cancer: systematic review. J. Adv. Nurs.66(11), 2372–2390 (2010).
- Vickberg SM . The Concerns About Recurrence Scale (CARS): a systematic measure of women’s fears about the possibility of breast cancer recurrence. Ann. Behav. Med.25(1), 16–24 (2003).
- McEwen BS . Protective and damaging effects of stress mediators. N. Engl. J. Med.338(3), 171–179 (1998).