Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive cancers, associated with poor survival outcomes. Lack of early diagnosis, resistance to conventional therapeutic treatments (including immunotherapy) and recurrence are some of the major hurdles in PDAC and contribute to its poor survival rate. While the risk of genetic predisposition to cancers is widely acknowledged and understood, recent advances in whole-genome and next-generation sequencing techniques have led to the acknowledgment of the role played by epigenetics, especially in PDAC. Epigenetic changes are heritable genetic modifications that influence gene expression without altering the DNA sequence. Epigenetic mechanisms (e.g., DNA methylation, post-translational modification of histone complexes and ncRNA) that result in reversible changes in gene expression are increasingly understood to be responsible for tumor initiation, development and even escape from immune surveillance. Our review seeks to highlight the various components of the epigenetic machinery that are known to be implicated in PDAC initiation and development and the feasibility of targeting these components to identify novel pharmacological strategies that could potentially lead to breakthroughs in PDAC treatment.
Plain language summary
Despite advances in detection and treatment, pancreatic cancer (pancreatic ductal adenocarcinoma [PDAC]) remains one of the most aggressive malignancies known to mankind, with one of the lowest 5-year survival rates (11%). Due to the lack of distinctive symptoms and the tumors’ aggressive ability to metastasize quickly, more than 50% of patients miss the opportunity to seek treatment at an early stage. While the role of genetics in cancer is well known, it is only recently that efforts have been made in identifying the role of heritable changes, regulated by epigenetic mechanisms, in the initiation and metastasis of cancer in individuals. Epigenetics refers to the phenomenon of alteration of gene expression through modifications of the chromatin landscape by DNA methylation, histone modifications and chromatin remodeling (due to ncRNA etc.) which is known to contribute to tumor heterogeneity. By identifying the epigenomic landscapes of patients’ tumors, we can identify the components of the epigenetic machinery that influence PDAC initiation and development. These proteins and enzymes could be excellent targets for developing novel pharmacological strategies that could potentially lead to breakthroughs in PDAC treatment.
Pancreatic ductal adenocarcinoma (PDAC) is a pernicious disease that often presents in an advanced state. The disease is associated with a poor prognosis and a 5-year survival rate of almost 11% [Citation1]. It is expected that by 2030, pancreatic cancer will be the second-deadliest malignancy in the USA [Citation2]. Whole-exome and whole-genome sequencing studies have reported PDAC to be a disease that is characterized by the presence of genetic aberrations [Citation3,Citation4]. Such studies have revealed that over 90% of PDAC cases have a gain-of-function KRAS mutation [Citation3,Citation4]. Noteworthy is the occurrence of subsequent deletion(s) or loss-of-function mutations in tumor suppressor genes, such as TP53, CDKN2A and SMAD4, which further exacerbates the disease [Citation4]. Numerous germline mutations have been identified to be associated with hereditary pancreatic cancer, including mutations in APC, CDKN2A, PRSS1, MSH2, MSH6, PMS2, PALB2, ATM, BRCA1, BRCA2, MLH1 and STK11 [Citation5,Citation6]. While the genetic basis of cancer is well appreciated, the enormous body of data produced in the past couple of decades has helped to substantiate the relevance of heritable changes, regulated by epigenetic mechanisms, in contributing to various stages of tumor development and malignancy, and therefore suggests that such changes may also prove to be pivotal for the evolution of tumors [Citation7,Citation8]. In 1942, Conrad Waddington used developmental connotation to describe epigenetics; he said that ‘between genotype and phenotype lies a whole complex of the development process’ and termed this the ‘epigenotype’ [Citation9].
Epigenetics is derived from a Greek word, epi, which means ‘over’ or ‘on top of’; thus ‘epigenetics’ is ‘on top of genetics’. It refers to the stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence [Citation10]. Epigenetic mechanisms are diverse, encompassing processes such as DNA methylation, histone post-translational modifications (PTMs), chromatin remodeling and post-transcriptional gene regulation through ncRNAs, to name a few. Such mechanisms regulate gene transcription, and while the apt functioning of these mechanisms is critical for normal development and tissue differentiation, changes in the epigenetic mechanisms can also serve as ‘drivers’ of tumorigenesis [Citation11] and escape from immune surveillance or drug resistance [Citation12]. Epigenetic mechanisms are reversible by nature and hence provide an opportunity for pharmaceutical intervention. The epigenetic status of PDAC is more relevant than that of other tumors because of the former’s low mutation burden [Citation11,Citation12]. This warrants a deeper understanding of the epigenetic landscape that drives PDAC. Several studies have highlighted the importance of genetic mutations as the main elements driving PDAC pathobiology. Herein, we focus on the major epigenetic mechanisms (DNA methylation, histone modifications, chromatin remodeling and ncRNAs) and how they are dysregulated in pancreatic cancer.
Genetic mutations in the epigenetic machinery as a contributor to PDAC
Although driver mutations in KRAS, TP53, CDKN2A and SMAD4 form the core of early PDAC progression, there is extensive genetic heterogeneity among PDAC patients, fostering a chain of less common genetic mutations that promote tumorigenesis [Citation3,Citation13]. For example, germline premature truncating variant mutations in genes involved in the DNA repair pathways (e.g., BRCA1, BRCA2, PALB2 and ATM), which have been predicted to result in loss of protein function, are found in about 10% of PDAC cases belonging to the familial pancreatic cancer category [Citation13,Citation14]. To add more, a subgroup of these PDAC patients has also been identified who have germline premature truncating variant mutations in regulators of the epigenome (e.g., in DNMT3A, TET2 and the polycomb group protein transcriptional regulator ASXL1) [Citation15]. This indicates that abnormal regulation of the epigenetic machinery has the potential to change the cellular gene expression profile, thereby predisposing individuals to PDAC ().
(A) PDAC is characterized by various mutations, including those involved in regulating epigenetic mechanisms. (B) Numerous epigenetic signatures could potentially be utilized for applications including early diagnosis and the study of tumor progression, and could be used as prognostic or therapeutic markers.
PDAC: Pancreatic ductal adenocarcinoma.
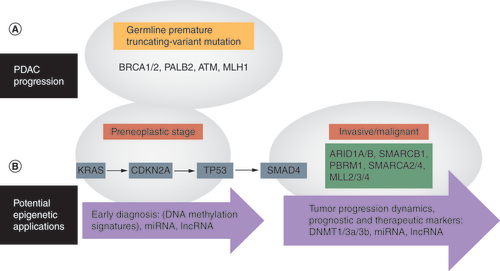
Sequencing efforts have not only substantiated the mutations in classical PDAC-associated oncogenes and tumor suppressor genes mentioned above but have also unraveled mutations in many chromatin-modifying enzymes and complexes [Citation13,Citation16,Citation17]. One of the most mutated genes is the ARID1A subunit belonging to the SWItch/sucrose non-fermentable (SWI/SNF) family of chromatin remodelers that utilize ATP to alter nucleosome topology and DNA accessibility to ultimately regulate gene transcription [Citation3,Citation15]. Multiplatform sequencing analyses have revealed 6% ARID1A mutations in human PDAC [Citation13]. ARID1A is known to function as a tumor suppressor gene in PDAC; its expression levels correlate with tumor stage and differentiation, but not with lymph node or distant metastasis [Citation18,Citation19]. Knockout of ARID1A leads to the formation of acinartoductal metaplasia and the subsequent formation of pancreatic intraepithelial neoplasia (PanIN) lesions [Citation19–21]. Interestingly, a recent study used genetically engineered mouse models of pancreatic cancer to show that Arid1a is an essential survival gene whose loss reduces cell growth and induces cell death [Citation22]. Furthermore, Arid1a loss in Ras-driven mouse models results in cellular growth constraint, leading to indolent low-grade cystic precursor lesions called intraductal papillary mucinous neoplasms [Citation22]. During tumorigenesis, however, mechanisms involving p53 loss or Myc upregulation result in a subset of Arid1a-deficient precursor cells progressing to adenocarcinomas [Citation22]. Other SWI/SNF subunits found to be mutated in PDAC include ARID1B, SMARCB1, PBRM1, SMARCA2 and SMARCA4 or BRG1 [Citation3,Citation15,Citation23]. BRG1 inactivating mutations and deletions have been found in pancreatic cancer cell lines [Citation24]. BRG1 is an ATP-dependent helicase and a core subunit of the SWI/SNF chromatin remodeling complex. BRG1 loss in conjunction with KRAS mutation results in the formation of neoplastic lesions resembling human intraductal papillary mucinous neoplasms and contributes to PDAC progression [Citation25]. Additionally, the authors of that study showed that BRG1 loss of function inhibited KRAS-mediated PanIN formation from adult acinar cells; however, it facilitated KRAS-dependent preneoplastic formation in adult pancreatic ductal cells, indicating that BRG1 is a determinant of context-dependent KRAS-mediated PDAC formation. This study highlights that chromatin remodeling may underlie the development of the distinct phenotypes observed in PDAC.
Transcriptomic studies in PDAC have found somatic mutations in genes encoding chromatin-modifying enzymes, notably KDM6A (MLL1) and MLL2/3/4 [Citation13,Citation15]. A recent mutagenic screen in PDAC tumors identified 100% of tumors to harbor one or more mutations in genes encoding chromatin-modifying enzymes [Citation26]. It was additionally found that such mutations coordinate with an oncogenic KRAS mutation to boost PDAC progression, implying that epigenomic alterations are significant contributors to PDAC progression [Citation25]. Ongoing efforts have allowed the appreciation of how mutations in epigenetic modifiers/regulators associate with and contribute to genomic structural evolution and hence tumor evolution, resulting in different PDAC transcriptional subtypes in human patients [Citation27,Citation28]. In one of these studies, Hayashi et al. performed genomic, transcriptomic and histological analysis of human PDAC in autopsy cohorts. Their in-depth investigation fostered the deduction of tumor evolutionary phylogenetic relationships within discrete areas of the primary tumors and metastases from the same patient [Citation28]. A central theme surfacing from such works is that individual PDAC tumors exist on a continuum of transcriptional phenotypes and that their progression from the initial stages is marked by structural genomic changes, aberrant regulation of epigenetic modifiers resulting in changes in the functional transcriptome, and increased aggressiveness with a proclivity to malignancy [Citation27,Citation28].
Adding to this evolutionary perspective is the evidence that driver mutations in primary tumors do not differ noticeably between primary tumors and metastases within the same patient [Citation29,Citation30], suggesting the involvement of epigenetic mechanisms during metastasis. Excitingly, the primary PDAC tumors and metastases in each patient mostly varied in their epigenetic mechanisms relating to the new tumor microenvironment (TME) or metastatic niche and its related metabolic necessity [Citation31]. Oliver et al. demonstrated distinct reprogramming of global chromatin modifications during the natural evolution of distant metastases. Genome-wide association studies unraveled changes linked to thousands of large chromatin domains throughout the genome that jointly specified malignant features, including the large chromatin K9-modified (LOCK) heterochromatin [Citation31]. These regions occupy >50% of the genome and correspond to selectable targets for large-scale epigenetic reorganization in PDAC. This study suggests that epigenetic deregulation within these regions propels tumor progression.
The evidence mentioned above implicates the role of epigenetic mechanisms, modifiers and chromatin remodeling events in the progression and metastatic spread of PDAC and underscores that mutations in the epigenetic machinery contribute to PDAC.
Significance of DNA methylation in PDAC progression
Historically speaking, DNA methylation is the most well appreciated of all the epigenetic mechanisms identified to date. The DNA methylation process involves the transfer of a methyl group from S-adenosyl-l-methionine to a specific site of DNA, mostly cytosine to form 5-methylcytosine. However, recent reports have indicated that methylation can also occur on adenine and guanine residues to form N6-methyladenine and 7-methylguanine, respectively [Citation32]. In the past 46 years, a great majority of studies have illustrated that altering the homeostasis of DNA methylation is fundamental to the evolution of human cancer, and many have led to the understanding that changes in the methylation patterns can help differentiate tumor cells from normal cells [Citation33]. Various regulators of DNA methylation – for example, DNA methyltransferases (writers), methyl-binding proteins (readers) and DNA demethylases (erasers) – have been found to contribute to aberrant methylation in PDAC development and progression (&).
Table 1. Alterations in epigenetic regulators and associated significance.
Table 2. List of epigenetic therapies in clinical trials.
It is seen that during normal development, about 60% of all gene promoters have CpG islands – stretches of CpG residues spanning gene promoters – the majority of which are protected from methylation [Citation33,Citation39,Citation40]. Lack of methylation at such promoters or other regulatory regions like enhancers is critical for the transcriptionally ‘open’ and ‘active’ status of the corresponding genes or chromatin region (e.g., in housekeeping genes like actin or tubulin); such chromatin is also termed euchromatin. Methylation of CpG residues at promoter or enhancer regions prevents the binding of transcription factors or activators and instead promotes the binding of repressor complexes, to foster a ‘closed’ and ‘inactive’ status of the corresponding genes or chromatin region. Such chromatin, when enriched by the presence of H3K9me3 marks and heterochromatin protein 1, is known as heterochromatin [Citation41]. Around 5–10% of CpG island genes are methylated in cancer and are known to directly facilitate carcinogenesis [Citation33]. Abnormal methylation patterns in PDAC have been well documented [Citation42]. With the advancement of next-generation sequencing (NGS), methods to study DNA methylation have also matured. Currently, methylation can be studied at single base-pair resolution through techniques like reduced representative bisulfite sequencing (RRBS) and whole-genome bisulfite sequencing [Citation42]. Using these methodologies, it has become possible to draw correlations between CpG methylation sites and disease outcomes. To give an example, tissues from a small cohort of normal and PDAC patient samples were used to perform RRBS and identified that CpG methylation at more than 17,000 sites correlated positively to survival response, while CpG methylation at a set of 3256 CpG residues correlated negatively with survival response [Citation43]. Furthermore/6, a survival analysis of multi-omics data recently reported 406 promoter CpG methylation loci that correlated with survival response [Citation44]. Sato and colleagues performed a drug screen to identify genes that became reactivated by epigenetic drugs in pancreatic cancer [Citation45]. They showed that TFPI2 was undetectable in PDAC tumor cells and lesions, which correlated to the aberrant hypermethylation of its promoter CpG island as compared with the normal pancreas, where it was abundantly expressed. Restoration of TFPI2 in pancreatic cancer cells resulted in suppression of tumor cell proliferation, migration and invasion, suggesting that epigenetic inactivation of TFPI2 is a unique mechanism that is leveraged by PDAC tumor cells to facilitate their aggressive phenotype [Citation45]. DNA methylation is used by PDAC tumor cells to modify the TME, causing gene expression changes which promote growth and proliferation. For example, direct interaction between PDAC cells and cancer-associated fibroblasts (CAFs) results in over 1500 genes becoming methylated and downregulated in CAFs, including SOCS1 [Citation46]. PDAC tumor cell-mediated SOCS1 suppression activated STAT3 signaling, thereby inducing IGF1 expression which inevitably promoted tumor growth in both the in vitro and in vivo settings. Xiao and colleagues demonstrated that treatment with 5-aza-2′-deoxycytidine (decitabine), an inhibitor of DNA methyltransferase activity, could reverse this process [Citation46]. The contributions of myofibroblastic CAFs and inflammatory CAFs in PDAC are well understood to produce phenotypes that promote cancer growth and immunosuppression, respectively [Citation47]. Coculture studies have demonstrated that methylation and downregulation of IL1A and IL1B in PDAC tumor cells results in the CAFs being locked into the myofibroblastic CAF phenotype, thereby directly promoting PDAC tumor growth [Citation48]. This also suggests that targeting IL-1 and TGF-β signaling pathways can be used to reprogram the PDAC TME.
Significance of DNA methylation writers, erasers&readers
Laird and colleagues reported that reducing DNA methyltransferase activity in mice resulted in fewer gastrointestinal tumors [Citation49]. DNMT1, also known as the maintenance methyltransferase enzyme, is overexpressed in PDAC, and it has been demonstrated that its expression increases with the gradual advancement of the transformation process from normal pancreatic tissue through precancerous lesion to PDAC, suggesting that high DNMT1 expression is associated with poor prognosis in patients [Citation50]. Multiple studies have reported that DNMT1 facilitates PDAC cell proliferation, invasion and migration as well as increasing the self-renewal of PDAC stem cells, suggesting that DNMT1 is a potential therapeutic target in pancreatic cancer [Citation51–53]. Currently, phase I/II clinical trials of the DNMT1 inhibitors decitabine and azacitidine are ongoing [Citation54,Citation55]. DNMT3a and DNMT3b are de novo methytransferases, responsible for establishing new methylation marks on the DNA and thereby regulating gene expression through methylation of regulatory regions [Citation33]. Like DNMT1, both DNMT3a and DNMT3b are writers of CpG methylation marks on DNA and their overexpression is correlated with poor prognosis in PDAC patients; thus they are new targets for therapy [Citation32,Citation56–58].
Ten–eleven translocation (TET) enzymes (erasers of methylation marks) catalyze the oxidation (or removal) of 5-methylcytosine from DNA to cytosine, through a series of intermediates, including 5-hydroxymethylcytosine (5hmc) [Citation59]. A critical balance of DNA methylation and hydroxymethylation has been shown to dictate major endodermal lineage intermediates during pancreatic differentiation of human embryonic stem cells [Citation60]. It was recently discovered that downregulation of TET1 and 5hmc is an early event in pancreatic tumorigenesis [Citation61]. Inactivating TP53 mutations are present in over 70% of PDAC patients; such mutations are often associated with metabolic reprogramming [Citation61]. Morris et al. showed that restoration of p53 in Kras-mutant murine pancreatic cancer cells led to the accumulation of α-ketoglutarate (α-KG), a metabolite that also serves as a cosubstrate for numerous chromatin-modifying enzymes, including TET enzymes [Citation62]. On the other hand, metabolites derived from glutamine oxidation – including malate, succinate and aspartate – progressively decreased after p53 restoration. Therefore, p53 restoration causes an increase in the α-KG/succinate ratio which alters the transcription status of cells by changing the activity of α-KG-dependent enzymes such as TET, thereby contributing to a more premalignant pattern of gene expression [Citation62]. This explains why normal pancreatic epithelial and premalignant PanIN precursors display low p53 mutation rates and high 5hmc staining. Malignant cells, on the other hand, carry p53 mutations and exhibit low 5hmc staining, as observed in other advanced malignancies [Citation63]. Morris et al. demonstrated decreased 5hmc levels during PDAC progression and observed that the reduced 5hmc levels coincided with the transition from benign to malignant progression that is often associated with the acquisition of a TP53 mutation [Citation62]. TET1 maintains pluripotency of embryonic stem cells; however, one study found that as the cells differentiate through endodermal intermediates into pancreatic epithelial cells, TET1 expression is steadily reduced and superseded by TET2 [Citation64]. Like p53, loss of SMAD4 expression was correlated with decreased TET2 expression in pancreatic cancer cells, which results in reduced levels of 5hmc and GATA6 (a pancreatic differentiation factor) [Citation64]. The authors discovered reduced levels of 5hmc in human PDAC tumors when compared with normal pancreas and also showed that epigenetic inactivation of GATA6 loci alters the epigenetic landscape in pancreatic cancer and contributes to the highly aggressive nature of PDAC [Citation64].
Wu and colleagues demonstrated that TET1 suppressed pancreatic cancer cell proliferation and metastasis both in vivo and in vitro [Citation65]. Mechanistically speaking, TET1 activated SFRP2, which is often silenced in various gastrointestinal cancers, by binding to and catalyzing the demethylation of the SFRP2 gene promoter, thereby preventing both the canonical and non-canonical Wnt signaling pathways, to eventually thwart epithelial–mesenchymal transition in pancreatic tumors [Citation65]. Bhagat et al. performed an epigenomic analysis of patient-derived and de novo generated PDAC CAFs and showed that a global loss of DNA methylation was correlated with overexpression of multiple inflammatory genes, including various chemokines and cellular receptors (e.g., CCL5, IL1A, CXCR4 and ICAM3) that are well appreciated for their central role in cell signaling pathways in cancer progression [Citation66]. They demonstrated that lactate produced by PDAC cells resulted in mesenchymal stem cell-mediated α-KG production. Because α-KG is a key cofactor of TET demethylase, this therefore activated TET, leading to reduced DNA methylation and increased 5hmc during de novo differentiation of mesenchymal stem cells to CAFs. Also, coinjection of PDAC cells (murine KPCs [K-rasLSL.G12D/+; Trp53R172H/+; Pdx-1-Cre]) with TET2-deficient mesenchymal stem cells reduced tumor growth in vivo. This mechanistic study highlighted the interaction between pancreatic tumor metabolism and epigenetic regulation of CAFs, wherein tumor cell-mediated lactate production associates with global epigenomic reprogramming observed during CAF formation, which caters to their tumor-promoting function [Citation66].
Methyl-binding domain (MBD)-containing proteins can recognize and bind to CpG methylation sites on the DNA [Citation67,Citation68]. MBD proteins ‘read’ methylation marks and coordinate with transcription activators and repressors to regulate gene expression [Citation69,Citation70]; they include MBD16, MeCP2 and SETDB1/2, among others [Citation32]. MeCP2 is the founding member of the MBD family of proteins that were recently identified to be upregulated in pancreatic cancer tissues [Citation71]. MeCP2 increases the proliferation of PDAC tumor cells in a TGF-β1-dependent manner and acts as a coactivator by promoting the binding of SMAD3 to the FURIN gene promoter to increase transcription of mesenchymal markers such as vimentin, N-cadherin and Snail, thereby increasing invasion and migration of pancreatic cancer cells [Citation71].
Methylation of RNA was recently identified and its implication in the pathogenesis of PDAC is still in its infancy [Citation32]. Nonetheless, there have been some reports of the role of RNA demethylases in inhibiting PDAC progression [Citation32,Citation72,Citation73].
Histone modifications&associated modifiers
Histone PTMs orchestrate several events within the nucleus, such as chromatin compaction, nucleosome positioning and providing access of the transcription machinery to the DNA [Citation71]. Many histone PTMs are known, yet those that are most frequently studied for regulating cellular gene transcription are acetylation, methylation, phosphorylation, ubiquitination and SUMOylating. Such PTMs are deposited by ‘writer’ enzymes onto the histone tails, which can be recognized and bound by ‘reader’ proteins, or they can be removed or hydrolyzed by ‘eraser’ enzymes. PTMs involving the processes of acetylation and methylation are well appreciated in this field of study.
Significance of lysine acetylation writers, erasers&readers
Acetylation is one of the most commonly studied histone PTMs and is catalyzed by histone lysine acetyltransferase (HAT) enzymes such as GNAT and p300 family enzymes. CBP and PCAF, among others, facilitate the transfer of an acetyl group from acetyl-CoA to the lysine residues present in histone or nonhistone proteins. These enzymes facilitate chromatin relaxation and/or increased accessibility for transcription factors or other members of the transcription machinery, thereby influencing gene expression [Citation74]. On the contrary, deacetylation is the enzymatic removal of acetyl groups using a water molecule, to restore the positive charge on the lysine residues; this results in increased attraction between the histone lysine residues and the DNA backbone, causing chromatin compaction and reduced accessibility to transcription factors. This process is mostly linked with gene silencing. In this way, while HATs are writers of acetylation marks on histones, histone deacetylases (HDACs) are erasers of the same. Histone lysine acetylation and deacetylation is a complex and dynamic process wherein both the enzyme groups could remain bound to gene regulatory regions through association with sequence-specific transcription factors, irrespective of the transcription status, and may increase or decrease transcription output depending on the cue [Citation74]. Recent genome-wide association studies in PDAC have revealed that increased histone 3 lysine 27 acetylation (H3K27ac) marks at the promoters of genes in LOCK regions are associated with increased expression of genes important for metastasis [Citation31]. In PDAC, there have been rather limited studies involving histone or nonhistone PTMs. p300 is known to mediate chemoresistance in pancreatic cancer [Citation75]; treatment of PDAC tumor cells with triptolide, a potent anticancer compound produced from the thunder god vine [Citation76], results in depletion of p300 which further causes inhibition of HIF1α transcriptional activity and reduced PDAC growth [Citation77]. A water-soluble compound of triptolide is currently in phase II clinical trial for the treatment of metastatic pancreatic cancer (NCT03117920)[Citation73]. We recently discovered that combined treatment with low doses of minnelide and standard-of-care chemotherapy produced a better response compared with conventional doses of standard-of-care chemotherapy in various preclinical mouse models of pancreatic cancer [Citation78]. HDAC inhibition is also a noteworthy strategy to target PDAC tumor cells. Clinical trials evaluating HDAC inhibition along with chemotherapeutic agents have demonstrated synergy in pancreatic cancer, although most studies were limited by low patient numbers [Citation77].
Hou and colleagues performed a gain-of-function screen of epigenetic regulators using an inducible KrasG12Dp53 null PDAC mouse model and discovered HDAC5 as the top regulator capable of enabling mutant Kras-independent tumor growth [Citation79]. HDAC5-driven tumors demonstrated a neutrophil-to-macrophage switch when compared with mutant Kras-driven tumors. The authors showed that HDAC5 bound to the promoter of Socs3 and suppressed its expression. SOCS3 is known to regulate the expression of the chemokine CCL2 [Citation80]. Hou et al. therefore showed that HDAC5 mediated Socs3 regulation, resulting in increased Ccl2 expression and leading to CCR2+ macrophage recruitment in the TME and supporting tumor recurrence in the absence of mutant Kras [Citation79]. HDAC3 is another deacetylase that is implicated in PDAC progression [Citation81]. Hu et al. showed that HDAC3 plays an important role in regulating the expression of the immune checkpoint molecule PD-L1 [Citation81]. The authors used a HDAC3-specific inhibitor and showed that the mRNA and protein levels of PD-L1 were reduced.
The pancreatic TME is characterized by the presence of a robust, fibroinflammatory stroma which cooperates with oncogenic signaling to influence PDAC initiation, progression and therapeutic outcomes. Sherman et al. showed that stromal fibroblasts induce epigenetic changes such as histone acetylation in the pancreatic cancer epigenome which cause transcriptional changes associated with accelerated growth, along with the anabolic changes of an altered metabolome [Citation82]. Interestingly, subsequent inhibition of the bromodomain and extra terminal (BET) family of epigenetic readers blocks stroma-mediated epigenetic changes in vitro and overall tumor growth in vivo. Shinke et al. showed that expression of HDAC1 is associated with metastasis in pancreatic cancer and that inhibition of HDAC1 suppressed epithelial–mesenchymal transition in pancreatic cancer cells by targeting SNAIL [Citation83]. Krauß et al. showed a similar role of HDAC2 in driving pancreatic cancer metastasis [Citation84].
Acetylation PTM marks on histones, or ‘acetylation histone codes’, can be recognized and translated to functional output via bromodomain-containing proteins [Citation85,Citation86]. In this way, bromodomain-containing proteins such as BRD2/BRD4 are categorized as readers. The BET proteins are overexpressed in PDACs and promote their growth and malignancy, and a combinatorial treatment strategy involving the usage of BET inhibitors and HDAC inhibitors was shown to be efficacious in pancreatic cancer [Citation87].
Significance of histone methylation (writers, readers&erasers)
Like HATs and HDACs, there are enzymes that either deposit or remove methyl marks from histone proteins: histone methyltransferases (HMTs) and histone demethylases (HDMs) [Citation88]. When a gene is actively transcribed – for example, in the case of a housekeeping gene – the chromatin is relaxed, and the regulatory regions are marked with characteristic histone PTMs. The active gene promoter is marked with H3K4 trimethylation (H3K4me3), H3K9ac and H3K27ac marks, while enhancer elements are marked with H3K4me1 and H3K27ac [Citation88]. On the other hand, a transcriptionally suppressed gene is also marked with characteristic histone PTMs. The gene promoters are enriched with H3K9me1/me2/me3 and H3K27me1/me2/me3 marks and have limited amounts of active marks, if any [Citation88,Citation89]. Disproportionate balance in the activity of such enzymes can facilitate random ‘opening’ and alteration of the chromatin architecture, causing abnormal transcription output and therefore malignant transformation and tumor progression in PDAC [Citation90]. Chromatin immunoprecipitation (ChIP) experiments and RNA sequencing analysis have revealed that reduction in the enrichment of histone methylation marks (H3K9me2 and H3K27me3) at the gene regulatory regions in LOCK-specified regions positively correlated with transcription of associated genes in distant metastasis [Citation31]. Zhang et al. recently discovered a novel pathway to target tumor suppressor gene silencing in solid tumors which involved the inhibition of CDK9 kinase [Citation91]. This resulted in chromatin relaxation together with the reduction of repressive H3K9me2 methylation marks and enrichment of active H3K4me2 methylation marks on gene regulatory regions. CDK9 is overexpressed in pancreatic cancer patients, and a combination of CDK9 inhibition with chemotherapeutic agents synergizes to target PDAC tumor cells [Citation92]. Pancreatic stromal cells also undergo metabolic reprogramming in the hypoxic TME to secrete 2-hydroxyglutarate (2HG). Our lab has shown that stromal cells produce the l isoform of 2HG as a result of promiscuous activity of lactate dehydrogenase A under a hypoxic microenvironment. Furthermore, stroma-secreted l-2HG deregulates histone methylation to suppress prodifferentiation genes and activate the expression of stemness genes in pancreatic cancer cells. We further showed that preventing l-2HG accumulation by the usage of a lactate dehydrogenase inhibitor promoted the infiltration of CD8+ T cells into the PDAC TME and sensitized the tumors to immunotherapy [Citation93]. To summarize, the tumor stroma provides important cues to pancreatic cancer cells to mediate epigenetic changes that play crucial roles in pancreatic tumorigenesis.
EZH2 is a histone lysine N-methyltransferase enzyme (an HMT) that forms the catalytic subunit of PRC2 and brings about the trimethylation of H3K27, causing gene silencing [Citation94]. High expression of EZH2 is noted in pancreatic cancer [Citation95]. In fact, EZH2 and BMI1, a member of the PRC1 complex, are key players in PDAC initiation and progression [Citation96,Citation97]. Both EZH2 and BMI1 are involved in PDAC cancer stem cell maintenance, thereby contributing to PDAC chemoresistance and relapse [Citation98,Citation99]. EZH2 deposits histone methylation marks to epigenetically silence NFATC1 expression during pancreatic regeneration, while mutant KRAS signaling in PDAC reverses the EZH2-contingent suppression and results in transcriptional activation of NFATC1, highlighting pancreatic cell plasticity [Citation100]. In one study in mice, partial loss of Ezh2 resulted in a more differentiated pancreatic tumor together with fewer liver metastases [Citation101]. Further, in the same study, restoring wild-type Ezh2 caused reduced Gata6 expression in Ezh2-deficient cells; GATA6 represents a key regulator of endodermal lineage differentiation and pancreatic development [Citation101].
KDM6A is an H3K27me3 demethylase (an HDM) that is mutated in about 18% of PDAC patients [Citation3,Citation15,Citation102]. KDM6A is known to counteract PRC2-mediated H3K27 trimethylation which is catalyzed by EZH2 methyltransferase to regulate developmental pathways [Citation103,Citation104]. Histone marks such as H3K4me1, H3K27ac or H3K27me3 can be found at active or poised enhancers; p300/CBP can mediate H3K27 acetylation of H3K4me1-defined enhancers and can convert a poised enhancer to an active enhancer [Citation105]. Groups of such enhancers form superenhancers which orchestrate lineage commitment and cell fate decisions and can become hijacked during tumorigenesis [Citation106]. Loss of KDM6A dysregulates the COMPASS (complex of proteins associated with Set1)-like complex and subsequently alters the enhancer chromatin conformation in pancreatic cancer [Citation102]. This study highlighted that PDAC tumor cells leverage KDM6A loss to alleviate enhancer suppression in a COMPASS-like complex-dependent manner to increase active H3K4 methylation marks, which promotes a gene expression profile that enhances the metastatic potential in vivo. In the same study, the authors identified KDM6A null pancreatic tumors to be highly sensitive to BET inhibitors.
Readers or sensors of histone lysine methylation marks exist that assist the HMTs and HDMs and function to locate and decipher such marks [Citation32]. Domains that recognize such marks include PHD, Chromo and Tudor [Citation107]. This realm of study remains underexplored in pancreatic cancer.
Mathison et al. utilized several NGS technologies to define an epigenomic signature driven by KrasG12D mutation in pancreatic cancer cells [Citation108]. The information from RRBS, ChIP-sequencing and the assay for transposase-accessible chromatin with high-throughput sequencing (ATAC-seq), revealed that active transcription start sites or flanking transcription start sites were characterized by the enrichment of H3K4me3 along with H3K27ac and H3K4me1. Interestingly, these regions when combined solely represent 1.3% of the whole genome, while 74% of the genome was devoid of these histone marks [Citation108]. Such evidence suggests the existence of an early KrasG12D-linked epigenomic landscape that promotes the remodeling of euchromatic and repressive chromatin domains at a global level, thereby accounting for the gene expression outcome. This pattern of histone-based chromatin reorganization is associated with gene promoter hypomethylation (as indicated by RRBS), increased accessibility to chromatin regions (as indicated by ATAC-seq) and concomitant increase in gene transcription (as indicated by RNA sequencing). In contrast, genes that lost H3K4me3, H3K27ac and H3K36me3 yet gained enrichment of H3K27me3 coupled with hypermethylation of gene promoters belong to networks that facilitate cells to acquire a pro-oncogenic phenotype [Citation108]. Nicolle and colleagues performed extensive multi-omic (genomic, epigenomic and metabolomic) profiling on PDAC xenografts from resectable and nonresectable patients. The authors suggested that the transformation stages of PDAC are characterized by genetic mutations, deletions and amplifications, while the response to treatments, clinical outcomes and tumor phenotypes are all regulated at the epigenetic level [Citation109]. This is supported by recent reports that illustrate no correlation among genetic alterations and treatment response to various drugs [Citation110,Citation111].
In efforts to facilitate prediction of PDAC progression and stratification of patient treatments, transcriptome profiling of resected tumors has been carried out. Recent advancement of technology involving molecular analysis has revealed that pancreatic tumors have multiple subtypes which harbor substantial molecular differences with distinctive biological and clinical behaviors [Citation112,Citation113]. RNA sequencing analysis of patient cohorts was first performed by Collisson et al., who defined three subtypes – classical, exocrine-like and quasi-mesenchymal – which exhibited variation in clinical outcomes and therapeutic responses [Citation114]. Exocrine-like PDAC cells closely resemble enzyme-secreting acinar cells in relation to their gene expression profile. The quasi-mesenchymal subtype was characterized by increased expression of mesenchymal genes, while the classical type showed KRAS dependency and exhibited an epithelial gene signature. Moffitt and colleagues applied a bioinformatic deconvolution of bulk transcriptomic data to differentiate the transcription patterns emerging from tumor, stroma and normal tissues, and identified two primary epithelial subtypes in pancreatic cancer: classical and basal-like [Citation115]. Using an integrative analysis comprising both genomic and transcriptomic data, Bailey et al. categorized PDAC into four subtypes: squamous or basal-like, pancreatic progenitor, immunogenic, and aberrantly differentiated endocrine exocrine [Citation3]. NGS efforts by The Cancer Genome Atlas and the International Cancer Genome Consortium supported the two-subtype classification model (i.e., basal-like and classical), suggesting that the immunogenic and exocrine-like/aberrantly differentiated endocrine exocrine subtypes were possibly a consequence of contamination [Citation13]. More recently, Lomberk and colleagues executed a multifactorial genome-wide association study comprising ChIP-sequencing, whole-genome methylation sequencing and RNA sequencing, which was utilized to illustrate the epigenetic landscape associated with the heterogeneity observed in patient-derived tumor xenografts; this study, too, recapitulated the two phenotypes observed in vivo, namely the classical and basal subtypes [Citation113].
To define the squamous subtype, Bailey and colleagues used cell lines derived from genetically engineered mouse models of PDAC (KrasG12D/+; Trp53fl/+; Tap63fl/fl KPC mice) based on the associations of TP53 mutation and increased TP63 expression in the squamous subtype [Citation3]. As compared with Tp53 null animals, mice with mutations in the DNA-binding domain had more aggressive disease and increased malignancy, primarily mediated via PDGFR-β [Citation116]. Analyzing the gene expression data from the same study highlighted that mutant Tp53 directly controls the transcriptional pathways linked to the squamous subtype, including that of PDGFRB [Citation3]. KrasG12D/+; Trp53fl/+; Tap63fl/fl mice were found to have aggressive metastatic PDAC than their KrasG12D/+; Trp53fl/+ counterparts, illustrating the role of Tap63 (an isoform of Tp63) in the squamous PDAC subtype.
PDAC transcription phenotype is epigenetically regulated
The basal or squamous pancreatic tumor subtype is endowed with reduced 5hmc levels, mutations in KDM6A and silencing of the transcription factors PDX1, GATA6 and HNF1B, indicating that epigenetic dysregulation is central to this subtype signature [Citation64]. Bailey and colleagues demonstrated that mutation in genes associated with chromatin modification such as KDM6A, MLL3 and MLL2 were observed in the basal or squamous PDAC subtype [Citation3]. Consequently, these tumors lack endodermal identity due to epigenetic silencing of the GATA6 and HNF4A cell fate determinants [Citation117,Citation118]. RNA-sequencing and ChIP-sequencing studies revealed EZH2 as a key repressor of differentiation programs in pancreatic cancer and identified the classical subtype-defining transcription factor GATA6 to be regulated by EZH2. Interestingly, genetic or pharmacological ablation of EZH2 increased GATA6 expression and activated a gene profile shift toward a less aggressive and more therapeutically vulnerable classical subtype [Citation101]. In contrast, rescuing EZH2 expression in EZH2-deficient PDAC cells caused attainment of their malignant properties, indicating that in the context of EZH2 inhibition, GATA6 expression is critical to counteract PDAC progression. Genome-wide association studies involving mapping of DNA modifications (5hmc and 5-methylcytosine) together with mutational profiling of resected PDAC patients revealed that the more aggressive basal-like subtype results from epigenetic inactivation of genetic loci including GATA6 [Citation64]. This subtype is characterized by low expression of TET2 5-methylcytosine hydroxylase, which results in greater loss of 5hmc [Citation64]. Loss of SMAD4 function, or low SMAD4 function, is linked to the basal PDAC subtype [Citation64,Citation115]. Eyres and colleagues discovered that SMAD4 supports TET2 expression in the pancreas and the classical PDAC subtype, while loss of SMAD4 levels is linked to reduction in 5hmc and GATA6 levels and a squamous-like PDAC gene signature [Citation64]. The authors demonstrated that variations in 5-methylcytosine and 5hmc can drive transcriptomic subtypes. Studies from human-derived organoids have demonstrated that GATA6 expression alone directly reversed the squamous subtype to the classical-progenitor subtype, together with the reversal of the WNT-independent growth phenotype [Citation47]. In pancreatic tumors, WNT derived from the stroma supports the PDAC stem cell compartment [Citation64]. However, loss of GATA6 expression makes tumors WNT-independent by producing WNT3A, WNT7B and WNT10A [Citation47]. Increased WNT levels are associated with higher squamous PDAC tumor subtype aggressiveness, characterized by greater dissemination and metastasis, as the tumor no longer requires stromal-derived WNT signals.
Aberrant expression of ΔNp63, the shorter isoform of the TP63 transcription factor, also contributes to the basal phenotype of PDAC tumors [Citation119]. Various groups have reported that the outcome phenotypes are established through epigenetic mechanisms rather than genetic events in pancreatic cancer [Citation109,Citation113,Citation120]. It is noteworthy that in the basal or squamous subtype, the DNA methylation patterns of several effectors and inhibitors belonging to the WNT pathway are altered, while in the classical subtype, DNA hypomethylation results in overexpression of molecular transporters such as SLC1A1 and NPC1L1 [Citation120]. The classical subtype is known to be linked to genes related to pancreatic morphogenesis (e.g., PDX1, GATA6) and metabolic processes (e.g., HKDC1, FBP1) [Citation113]. Abnormal expression of p63 reorganizes the PDAC enhancer landscape, causing upregulation of the squamous-like transcription program and thereby contributing to tumor growth and metastasis in vivo [Citation118]. In the same study, the authors discovered that ΔNp63 fosters enrichment of H3K27ac marks at the enhancer region of squamous subtype genes. This study provided evidence that epigenetic mechanisms regulate this transcriptional program and contribute to aggressive PDAC phenotypes [Citation118].
Andricovich and colleagues discovered that KDM6A loss in PDAC can directly induce a squamous PDAC subtype signature by activating the gene expression of specific transcription factors involving MYC, p63, RUNX3 and ZEB1 expression [Citation101]. The authors demonstrated that KDM6A loss allows for increased occupancy and activation of enhancers by histone lysine methyltransferases type 2 (KMT2) of squamous differentiation-promoting genes, as manifested by the enrichment of H3K4me1 and KMT2D occupancies at these loci. Lomberk et al. applied a multiparametric integrated approach on genomic and epigenomic datasets and demonstrated that superenhancers better represent the heterogeneity of the various PDAC subtypes [Citation113].
Noncoding RNAs
As the name suggests, ncRNAs are nontranslated RNAs; they are classified into various types based on their respective lengths [Citation117]. Commonly studied ncRNAs include lncRNAs and miRNAs, both of which regulate gene expression at the transcription, post-transcription and chromatin levels. Various other types of ncRNAs have been described elsewhere [Citation118,Citation119] and include (but are not limited to) circRNAs, siRNAs, eRNAs and piRNA. ncRNAs can act as both oncogenic drivers and tumor suppressors in PDAC [Citation120]. Herein, we will focus mainly on miRNAs and lncRNAs.
Significance of miRNAs in PDAC
miRNAs are evolutionarily conserved, single-stranded ncRNAs, 18–24 nucleotides long, that regulate gene expression at the post-transcriptional level by binding to the complementary sequences of their target cognate mRNAs at the 3′ untranslated region, causing translation inhibition [Citation121]. It is estimated that over 1000 miRNAs regulate about 30% of all protein-coding genes [Citation122–126]. In PDAC tumor cells, miRNAs can act as oncogenes or tumor suppressor genes [Citation126,Citation127].
Abnormal miRNA expression plays a prominent role in the initiation, proliferation, epithelial–mesenchymal transition and chemoresistance associated with PDAC tumor cells, as well as their formation of distant metastases [Citation128]. We have demonstrated that ectopic expression of miR-142-3p inhibits the proliferation of PDAC tumor cells [Citation129]. It has been observed that abnormal expression of miRNAs is caused due to the amplification or deletion of genomic regions associated with miRNA expression [Citation130]. Several miRNAs are known to be abnormally expressed in PDAC; these include, but are not limited to, miR-21, miR-34, miR-21, miR-155, miR-200, miR-221 and miR-222 [Citation130–132]. Hong and colleagues investigated the feasibility of profiling miRNAs to identify biomarkers by using small specimens obtained via fine-needle aspirations of PDAC tumors [Citation133]. They validated their microarray results with qPCR analysis, which revealed the five most upregulated miRNAs to be miR-21, miR-27a, miRNA-146a, miRNA-196a and miRNA200a, and showed the expression of miR-20a, miR-96 and miR-217 to be downregulated in a statistically significant manner in almost all the PDAC tissues analyzed [Citation134]. This indicated that while some miRNAs can have oncogenic associations, others can be tumor suppressive by nature. Mortoglou et al., in a comprehensive manner, classified miRNA involvement in PDAC progression with the ‘cancer hallmarks’ described by Hanahan and Weinberg [Citation118,Citation134].
Significance of lncRNAs in PDAC
lncRNAs are >200 nucleotides in length; due to their inherent ability to interact with DNA, RNA and proteins, they have emerged as a unique class of RNA species that can serve as a scaffold, guide and decoy, thereby regulating transcription, chromatin remodeling, histone modification, metabolic processes, cellular transport, cell cycle control, apoptosis, cell differentiation and tissue development [Citation118,Citation135,Citation136]. Kung and colleagues organized the diverse lncRNAs based on the respective genomic locations where these RNA species are transcribed, using well-characterized and curated protein-coding genes [Citation137]. In this way, lncRNAs could be classified into five broad groups: pseudogenes that have lost their protein-coding potential owing to a frameshift or other mutations; stand-alone lncRNAs that are located away from protein-coding genes; natural antisense transcripts, whose location is on the opposite strand to the curated transcription units; long intronic ncRNAs that are transcribed from the intronic regions of the curated genes; and divergent transcripts, promoter-associated transcripts and enhancer RNAs, whose corresponding lncRNAs arise at the vicinity of the transcription start site from both sense and antisense directions.
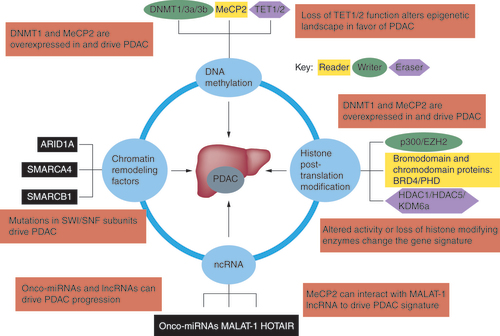
Various studies have shown that lncRNAs play a major role in the etiology of PDAC [Citation138]. The lncRNA NUTF2P3-001 has been demonstrated to be overexpressed in PDAC tumor cells and contributes to the proliferation and invasion of pancreatic cancer cells [Citation139]. HOTAIR is a HOX antisense intergenic RNA whose expression is elevated in pancreatic cancer [Citation140]. In that study the authors reported that reducing HOTAIR levels through a knockdown approach revealed decreased cell proliferation, abnormal cell-cycle progression and apoptosis. ChIP experiments uncovered that HOTAIR-mediated gene suppression was both PRC2-dependent and -independent. Finally in vivo studies demonstrated that HOTAIR knockdown in PDAC cells prevented tumor growth in xenograft models [Citation140]. We performed ChIP-sequencing and discovered that MeCP2, which promotes pancreatic cancer (as described above), binds to various RNA species including lncRNAs (MALAT1 and NEAT1), suggesting that MeCP2 can associate with lncRNAs to promote growth in solid tumors () [Citation141]. We recently showed that GAS5, another lncRNA, acts as a molecular ‘switch’ for regulating growth arrest and quiescence observed in CD133+ tumor-initiating PDAC cells and is responsible for the hostile biology linked to this disease [Citation142].
Mutation or abnormal functioning of writers, readers and erasers associated with the processes of DNA methylation and histone post-translational modifications can cause PDAC. Additionally, onco-miRNA and other ncRNAs can promote the disease. Finally, mutations in chromatin remodelers can further exacerbate PDAC.
PDAC: Pancreatic ductal adenocarcinoma.
Epigenetic changes associated with PDAC risk factors
Cigarette smoking and tobacco usage is one of the well-recognized risk factors for pancreatic cancer; around 25% of pancreatic cancer patients can be attributed to cigarette or tobacco smoking [Citation143]. Various reports have linked tobacco use to increases in promoter-specific DNA methylation, which causes predisposition to various diseases, including cancer [Citation144,Citation145]. Genome-wide DNA methylation analysis has shown changes in CpG sites following tobacco exposure [Citation146,Citation147]. Apart from DNA methylation, tobacco exposure or smoking also affects histone modifications [Citation148]. Additionally, smoking also modulates the expression of miRNAs such as miR-0223, miR-340, miR-34b and miR-21 [Citation149]. High alcohol consumption is also widely recognized as a major risk factor for pancreatic cancer [Citation150]. Chronic consumption of alcohol can cause pancreatic injury or pancreatitis, which can predispose to pancreatic cancer. Zhao et al. showed that chronic alcohol exposure in rats caused pancreatic injury and changes in inflammatory cytokine (IL-1α, IL-10, NF-κB, TNF-α and TGF-β) DNA methylation in pancreatic tissue [Citation150].
Using epigenetic signatures as diagnostic assays for PDAC
To date, there are no specific cost-effective screening tests available to detect early-stage pancreatic cancer in people who have no symptoms of the disease. Most diagnoses occur at an advanced stage of the disease, when the tumor can no longer be removed with surgery and has spread from the pancreas to other parts of the body. Early detection and treatment strategies can greatly reduce the high mortality associated with the disease. Tumor markers that have been employed for this include carcinoembryonic antigen, carbohydrate antigen 19-9 (CA19-9) and carbohydrate antigen 242 [Citation151]. However, given the low specificity and sensitivity of CA19-9 during the early stage of PDAC, there is a pressing need to discover novel biomarkers to differentiate PDAC patients from their healthy counterparts [Citation151]. Diagnostic tools relevant for PDAC have explored the potential of combining multiple candidate genes into a combined biomarker approach. Biological fluids such as blood and pancreatic juice have been utilized to differentially detect preneoplastic or PDAC states by analyzing the methylation patterns of various genes in aggregate. Examination of a panel of 42 pancreatic cancers led to the identification of 11 genes that were abnormally methylated in PDAC but not in normal pancreatic ductal epithelial cells [Citation152]. Three out of the 11 genes (CLDN5, NPTX2and SARP2) were explored as aggregate biomarkers for further analysis in a larger panel of specimens. Aberrant methylation of one of these genes was discovered in 75% of pancreatic juice samples and 100% of primary pancreatic cancer specimens. Given these promising results, biological fluids were evaluated from larger cohorts in various other studies [Citation153].
The common nature of abnormal DNA methylation patterns in cancer and the stability of cell-free DNA (cfDNA) in body fluids are engaging features for diagnostic development. To date, however, there are no liquid biopsy methodologies that can function to distinguish PDAC and pancreatitis patients with sufficient sensitivity and specificity. Recent efforts to enhance a cfDNA methylation-based strategy have included the development of a sensitive immunoprecipitation-based method to evaluate the methylome of small amounts of circulating cfDNA and to establish the capability to reveal large-scale DNA methylation changes enriched for cancer-specific patterns [Citation154]. Compared with previous cfDNA profiling methods, this approach had much higher sensitivity and could identify and differentiate different tumor types, including PDAC, based on their cfDNA methylation patterns; this would be especially valuable in clinical studies where the primary location of the disease is not known. In their study, Shen et al. substantiated the assay in a separate group of 199 samples, including 47 PDAC cancer patients [Citation154]. This assay demonstrated decent clinical utility as it performed similarly well with early- and late-phase PDAC patients.
Thus far, it remains a challenging task to integrate the changes in methylation patterns within tumors with the larger landscape of diagnostic protocols for pancreatic cancer. The idea of combining cfDNA along with circulating serum proteins in a single assay for PDAC has begun to receive traction [Citation155]. This strategy was recently extended to the Johns Hopkins CancerSEEK panel, inclusive of 61 PCR amplicons and eight protein analytes [Citation156]. The panel was prospectively tested in 812 controls and 1005 cancer patients, with a specificity of >99% and sensitivity of 69% in pancreatic cancer patients. This test was remarkable in that it could identify the primary location of the disease. Combining mutational and protein biomarker testing (such as those in the CancerSEEK panel) with cfDNA methylation testing has the potential to greatly enhance the sensitivity and specificity of the test and provides a promising avenue to usher in the area of early-stage pancreatic cancer diagnosis. Guler et al. used 5hmc-based biomarkers in circulating cfDNA as molecular diagnostic tests to detect pancreatic cancer at an early stage of the disease [Citation157]. 5hmc-based early detection in plasma samples has shown great promise in relation to various cancers, including lung cancer, colon cancer, gastric cancer, hepatocellular carcinoma and pancreatic cancer [Citation158,Citation159]. These early detection studies from plasma solely rely on detecting the 5hmc profile of circulating tumor DNA (ctDNA), which can be difficult at early stages because of the small amount of ctDNA present at early stages of the disease. Guler et al. used hydromethylation profiling of all the circulating DNA present in plasma, which included the signals from tumor cells as well as other cells such as immune cells, making it easier to detect at early stage of the disease [Citation157].
Stable miRNAs have been detected in tissues and body fluids including blood, urine and breast milk [Citation160], and this has allowed specific miRNAs to be associated with disease stage, aggressiveness and survival responses [Citation161,Citation162]. This also becomes relevant when there is a dearth of biopsies from primary PDAC tumors. Such scenarios have revealed a significant correlation between plasma miR-221 and clinicopathological outcomes from 47 pancreatic carcinoma patients [Citation163]. Plasma miR-221 concentrations were higher in PDAC patients than in benign and control cases, in a statistically significant manner. In the same study, Kawaguchi and colleagues reported that the plasma miR-221 levels were significantly decreased in postoperative specimens. Additionally, significant correlations were observed between PDAC patients with high plasma miRNA concentrations and those with distant metastasis and nonresectable tumors [Citation164]. Therefore, plasma miRNA levels (e.g., of miR-221) can serve as a useful biomarker for PDAC diagnosis, following tumor dynamics and predicting metastatic events, and may potentially assist in discerning clinical decisions in PDAC therapy. Certain miRNAs are elevated in tissues such as miR-301 and miR-376a, while overexpression of miR-23a and miR-23b has been detected in the saliva of PDAC patients [Citation165,Citation166]. Some of the miRNAs identified as biomarkers have been demonstrated to show an accuracy of 83.6% compared with about 56% using CA19-9 [Citation118,Citation166–168].
Multiple recent reports have shown that lncRNAs correlate with the clinicopathological features and prognosis of PDAC patients and may thus serve as novel biomarkers. The term ‘onco-lncRNA’ has been associated with HOTAIR given its elevated expression in PDAC tumors. It was discovered that HOTAIR is a negative prognostic factor and correlates with lymph node metastasis and poor overall survival [Citation140]. Using post-survival analysis of pancreatic cancer patients, one study found that low expression of HOTAIR in patients correlated with significantly longer survival times than in those with high expression, which illustrates the relevance of this lncRNA in predicting prognosis [Citation169]. MALAT1 is another lncRNA whose expression is significantly higher in PDAC tissues compared with adjacent normal tissues [Citation170]. In that study, the authors supported this observation through the mRNA expression results from various PDAC cell lines. Further, MALAT1 expression correlated with tumor stage, tumor size and the depth of invasion. They also showed that tumor location and MALAT1 overexpression were independent predictors of survival response in PDAC, suggesting that MALAT1 could serve as a target for gene therapy and diagnosis for pancreatic cancer. A different study corroborated the findings on MALAT1 by Liu and colleagues [Citation171]. In summary, the research and applications of lncRNAs as a target for PDAC remain in their infancy, yet this area offers huge promise.
Limitations of identifying a diagnostic marker for early detection
While epigenetic signatures offer valuable tools in diagnostics, nonetheless several challenges remain in terms of the early detection of the disease:
A challenge associated with the detection of methylated DNA biomarkers is the background methylated DNA (noise) from rapidly proliferating tissues like the duodenum [Citation172].
An important limitation is seen in the case of reliably detecting low levels of ctDNA [Citation173]. Given that the detection of ctDNA is more specific than that of other markers such as elevated serum CA19-9, its usage is still very limited owing to the low sensitivity. However, it is thought that soon this will improve.
Identifying ‘sporadic’ high-risk individuals who lack any germline mutations is an important challenge. Such high-risk individuals can benefit from a surveillance program aimed at discerning risk rather than the early detection of asymptomatic cancer [Citation174]. Such programs will require the provision of genetic and environmental history (e.g., smoking, alcohol use). Individuals who meet the set ‘threshold risk’ can be placed on a longitudinal surveillance regimen and closely monitored.
While numerous challenges remain, early detection in PDAC is no longer a far-fetched dream.
Therapeutic targeting of epigenetic mechanisms in PDAC
In the previous sections we have discussed the contributions of various epigenetic mechanisms toward PDAC progression and diagnosis of the disease. There is much excitement concerning the reversal of epigenetic abnormalities as a basis for cancer therapy [Citation34]. Multiple clinical trials have recently opened and started actively recruiting pancreatic cancer patients (). These include epigenetic therapy in combination with standard-of-care chemotherapy or with immunotherapy. With increasing understanding of epigenetic mechanisms in PDAC, the list will improve.
It is worth mentioning that therapeutic targeting of epigenetic mechanisms is a challenging task, as numerous problems need to be overcome for a productive epigenetic drug design. This includes target enzyme isoform selectivity, substrate specificity (e.g., HATs, HDACs, HMTs and KDMs ([K]-specific demethylase) all have both histone and nonhistone targets), design of regimens allowing epigenetic drugs to be used in combination, or the formulation of drugs that have dual targets. Another challenge involves the fact that most, if not all, epigenetic enzymes function in multimeric complexes, which makes the translation from in vitro potency to in vivo efficacy difficult. Epigenetic therapy thus far has had great success in the treatment of hematological malignancies [Citation7] and as observed above, many such therapies are currently in clinical trials for PDAC.
Conclusion
Pancreatic cancer is a debilitating and insidious disease that imposes a burden on society. Given the complex nature of the disease, our understanding has been rather limited. Advances in NGS and genome-wide association studies have shown the importance of epigenetics in the initiation, progression and evolution of PDAC. This review not only provides a background of the major epigenetic mechanisms but also highlights how the epigenetic machinery is modified or ‘hijacked’ in pancreatic cancer. Writers of epigenetic marks such as the DNA methyltransferases, HATs and HMTs deposit key information on the chromatin which is ‘decoded’ and translated to actionable information by the reader proteins such as MBD-containing proteins, bromodomain proteins and chromodomain-containing proteins. Eraser proteins such as TET enzymes, HDACs and HDMs remove such marks from the chromatin depending on the environmental signal. Chromatin remodeling is also an important epigenetic mechanism that regulates gene expression. In PDAC, the activity of such enzymes is aberrantly regulated and mediates the pathogenesis of the disease. It is observed that the epigenome is modified not only in the PDAC tumor cells but also in the TME (e.g., in CAFs). Chronologically speaking, ncRNAs are the newest members of the epigenetic mechanisms that regulate gene expression and are equally relevant in PDAC pathobiology. Our increasing knowledge of these processes has provided us with a lucid perspective on how best to diagnose, stratify risks and employ therapeutic regimens for PDAC patients. DNA methylation signatures, methylated cfDNAs, lncRNAs, miRNAs and so on are key components of the epigenetic machinery that have been applied for disease diagnosis. Selected mutations in chromatin remodelers and epigenetic enzymes may also serve as predictive biomarkers for more targeted therapy. PDAC has high intratumoral heterogeneity; thus, capitalizing and building on this knowledge of epigenetic foundations will bring us closer to our aim of diagnosing and treating PDAC patients at the early stages.
Future perspective
Despite advances in understanding the pathobiology of PDAC, a ‘one size fits all’ approach to the treatment for PDAC is unlikely to be very beneficial given the intra- and inter-individual tumor heterogeneity dictated at the gene transcription level. We believe that the best way to counter this variability would be to target the epigenetic machinery that dictates these variations at the molecular level in every tumor. To this end, we think that targeting the epigenetic regulators and modulators with effective pharmaceutical or even immunotherapeutic approaches would be a game-changer in our fight against this aggressive malignancy. One major limitation in employing such epigenetic reprogramming of PDAC tumors is the risk of pleiotropic effects, given that some components of the epigenetic machinery have opposite effects in other cellular compartments. The recent advances in single-cell sequencing technologies which offer multi-omics information from the genome and transcriptome could be valuable in identifying the precise roles of the myriad players in the epigenetic regulation of PDAC tumors. Altogether, we are confident that by manipulating the epigenetic machinery, either alone or as a part of combination treatment strategy, we can reprogram the aggressive PDAC tumor profile to a benign or easily detectable and treatable state that could benefit patients soon.
Epigenetics (Greek: epi, meaning ‘over’ or ‘on top of’) refers to the stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence.
Abnormal regulation of the epigenetic machinery has the potential to change the cellular gene expression profile.
Such changes may predispose individuals to tumors, including pancreatic ductal adenocarcinoma (PDAC). For instance, germline loss-of-function mutations in DNA repair genes (e.g., BRCA1, BRCA2, PALB2 and ATM) are found in about 10% of PDAC patients who have familial pancreatic cancer. Somatic mutations in chromatin-modifying enzymes such as KDM6a and MLL2/3/4 are among the notable ones in nonfamilial PDAC.
Epigenetic modifiers – writers, erasers and readers – alter the genome through DNA methylation, changes in chromatin marks, or post-translational chromatin modifications. Maintenance methyltransferase enzymes like DNMT1, DNMT3a and DNMT3b are writers of CpG methylation marks on DNA and correlate with poor prognosis in PDAC patients and are new targets for therapy.
TET enzymes (erasers of methylation marks) like TET1 suppressed pancreatic cancer cell proliferation and metastasis in both in vivo and in vitro studies.
Methyl-binding domain proteins ‘read’ methylation marks and coordinate with transcription activators and repressors to regulate gene expression, and some (e.g., MBD16, MeCP2, SETDB1/2) have been reported to be upregulated in pancreatic cancer.
Histone modifications, particularly histone acetylation, are associated with poor prognosis and increased stroma.
NSD1 and SETD2, two lysine methyltransferases, are mutated in 8–10% of cases of PDAC.
Many clinical studies are investigating the efficiency of DNA methyltransferase and histone deacetylase inhibitors or their combination.
ncRNAs are nontranslated RNAs which can act as both oncogenic drivers and tumor suppressors in PDAC.
In PDAC tumor cells, miRNAs can act as oncogenes or tumor suppressor genes. Abnormal miRNA expression plays a prominent role in the initiation, proliferation, epithelial–mesenchymal transition, chemoresistance and formation of distant metastases associated with PDAC tumor cells.
The lncRNAs NUTF2P3-001 and HOTAIR are known to be overexpressed in PDAC.
Efforts are ongoing to harness epigenetic signatures from body fluids and assess their utility as biomarkers to identify PDAC in early stages.
Preclinical studies have succeeded in targeting the enzymes responsible for aberrant epigenetic markers. However, more clinical evidence is needed to validate these approaches for PDAC treatment.
S Lavania is the corresponding author and responsible for writing, overall organization and editing of the manuscript. All authors contributed equally to the content of the manuscript.
Acknowledgments
The authors would like to acknowledge A Saluja, Professor of Surgery at the University of Miami, for his guidance and support.
Financial&competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- National Cancer Institute: Surveillance, Epidemiology, and End Results Program . Cancer stat facts: pancreatic cancer. https://seer.cancer.gov/statfacts/html/pancreas.html
- Rahib L , SmithBD , AizenbergR , RosenzweigAB , FleshmanJM , MatrisianLM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res.74(11), 2913–2921 (2014).
- Bailey P , ChangDK , NonesKet al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature531(7592), 47–52 (2016).
- Roberts NJ , NorrisAL , PetersenGMet al. Whole genome sequencing defines the genetic heterogeneity of familial pancreatic cancer. Cancer Discov.6(2), 166–175 (2016).
- Vincent A , HermanJ , SchulickR , HrubanRH , GogginsM. Pancreatic cancer. Lancet378(9791), 607–620 (2011).
- Solomon S , DasS , BrandR , WhitcombDC. Inherited pancreatic cancer syndromes. Cancer J.18(6), 485–491 (2012).
- Baylin SB , JonesPA. A decade of exploring the cancer epigenome – biological and translational implications. Nat. Rev. Cancer11(10), 726–734 (2011).
- Bonasio R , TuS , ReinbergD. Molecular signals of epigenetic states. Science330(6004), 612–616 (2010).
- Waddington CH . The epigenotype 1942. Int. J. Epidemiol.41(1), 10–13 (2012).
- Berger SL , KouzaridesT , ShiekhattarR , ShilatifardA. An operational definition of epigenetics. Genes Dev.23(7), 781–783 (2009).
- Chatterjee A , RodgerEJ , EcclesMR. Epigenetic drivers of tumourigenesis and cancer metastasis. Semin. Cancer Biol.51, 149–159 (2018).
- Ciernikova S , EarlJ , GarciaBermejo ML , StevurkovaV , CarratoA , SmolkovaB. Epigenetic landscape in pancreatic ductal adenocarcinoma: on the way to overcoming drug resistance?Int. J. Mol. Sci.21(11), 4091 (2020).
- Cancer Genome Atlas Research Network . Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell32(2), 185–203 (2017).
- St Pierre R , KadochC. Mammalian SWI/SNF complexes in cancer: emerging therapeutic opportunities. Curr. Opin. Genet. Dev.42, 56–67 (2017).
- Waddell N , PajicM , PatchAMet al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature518(7540), 495–501 (2015).
- Kadoch C , CrabtreeGR. Mammalian SWI/SNF chromatin remodeling complexes and cancer: mechanistic insights gained from human genomics. Sci. Adv.1(5), e1500447 (2015).
- Kadoch C , HargreavesDC , HodgesCet al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet.45(6), 592–601 (2013).
- Zhang L , WangC , YuSet al. Loss of ARID1A expression correlates with tumor differentiation and tumor progression stage in pancreatic ductal adenocarcinoma. Technol. Cancer Res. Treat.17, 1533034618754475 (2018).
- Xu S , TangC. The role of ARID1A in tumors: tumor initiation or tumor suppression?Front. Oncol.11, 745187 (2021).
- Livshits G , Alonso-CurbeloD , MorrisJPTet al. Arid1a restrains Kras-dependent changes in acinar cell identity. Elife7, e352162018).
- Wang SC , NassourI , XiaoSet al. SWI/SNF component ARID1A restrains pancreatic neoplasia formation. Gut68(7), 1259–1270 (2019).
- Ferri-Borgogno S , BaruiS , McGeeAMet al. Paradoxical role of AT-rich interactive domain 1A in restraining pancreatic carcinogenesis. Cancers (Basel)12(9), 2695 (2020).
- Hessmann E , JohnsenSA , SivekeJT , EllenriederV. Epigenetic treatment of pancreatic cancer: is there a therapeutic perspective on the horizon?Gut66(1), 168–179 (2017).
- Jones S , ZhangX , ParsonsDWet al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science321(5897), 1801–1806 (2008).
- von Figura G , FukudaA , RoyNet al. The chromatin regulator Brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nat. Cell Biol.16(3), 255–267 (2014).
- Mann KM , WardJM , YewCCet al. Sleeping Beauty mutagenesis reveals cooperating mutations and pathways in pancreatic adenocarcinoma. Proc. Natl Acad. Sci. USA109(16), 5934–5941 (2012).
- Chan-Seng-Yue M , KimJC , WilsonGWet al. Author correction: transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet.52(4), 463 (2020).
- Hayashi A , FanJ , ChenRet al. A unifying paradigm for transcriptional heterogeneity and squamous features in pancreatic ductal adenocarcinoma. Nat. Cancer1(1), 59–74 (2020).
- Reiter JG , Makohon-MooreAP , GeroldJMet al. Minimal functional driver gene heterogeneity among untreated metastases. Science361(6406), 1033–1037 (2018).
- Makohon-Moore AP , ZhangM , ReiterJGet al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat. Genet.49(3), 358–366 (2017).
- McDonald OG , LiX , SaundersTet al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat. Genet.49(3), 367–376 (2017).
- Zhao Y , YangM , WangSet al. An overview of epigenetic methylation in pancreatic cancer progression. Front. Oncol.12, 854773 (2022).
- Baylin SB , JonesPA. Epigenetic determinants of cancer. Cold Spring Harb. Perspect. Biol.8(9), a019505 (2016).
- Kelly TK , DeCarvalho DD , JonesPA. Epigenetic modifications as therapeutic targets. Nat. Biotechnol.28(10), 1069–1078 (2010).
- Yi Z , WeiS , JinLet al. KDM6A regulates cell plasticity and pancreatic cancer progression by noncanonical activin pathway. Cell. Mol. Gastroenterol. Hepatol.13(2), 643–667 (2022).
- Bazzichetto C , LuchiniC , ConciatoriFet al. Morphologic and molecular landscape of pancreatic cancer variants as the basis of new therapeutic strategies for precision oncology. Int. J. Mol. Sci.21(22), 8841 (2020).
- Yang Q , ShenR , XuHet al. Comprehensive analyses of PBRM1 in multiple cancer types and its association with clinical response to immunotherapy and immune infiltrates. Ann. Transl. Med.9(6), 465 (2021).
- Versemann L , HessmannE , UlisseM. Epigenetic Therapeutic Strategies to Target Molecular and Cellular Heterogeneity in Pancreatic Cancer. Visceral Medicine38(1), 11–19 (2022).
- Shain AH , GiacominiCP , MatsukumaKet al. Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer. Proc. Natl Acad. Sci. USA109(5), E252–E259 (2012).
- Jones PA , BaylinSB. The epigenomics of cancer. Cell128(4), 683–692 (2007).
- Mathieu O , PicardG , TourmenteS. Methylation of a euchromatin-heterochromatin transition region inArabidopsis thalianachromosome 5 left arm. Chromosome Res.10(6), 455–466 (2002).
- Wang SS , XuJ , JiKY , HwangCI. Epigenetic alterations in pancreatic cancer metastasis. Biomolecules11(8), 1082 (2021).
- Thompson MJ , RubbiL , DawsonDW , DonahueTR , PellegriniM. Pancreatic cancer patient survival correlates with DNA methylation of pancreas development genes. PLOS ONE10(6), e0128814 (2015).
- Mishra NK , SouthekalS , GudaC. Survival analysis of multi-omics data identifies potential prognostic markers of pancreatic ductal adenocarcinoma. Front. Genet.10, 624 (2019).
- Sato N , ParkerAR , FukushimaNet al. Epigenetic inactivation of TFPI-2 as a common mechanism associated with growth and invasion of pancreatic ductal adenocarcinoma. Oncogene24(5), 850–858 (2005).
- Xiao Q , ZhouD , RuckiAAet al. Cancer-associated fibroblasts in pancreatic cancer are reprogrammed by tumor-induced alterations in genomic DNA methylation. Cancer Res.76(18), 5395–5404 (2016).
- Ohlund D , Handly-SantanaA , BiffiGet al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med.214(3), 579–596 (2017).
- Pan X , ZhengL. Epigenetics in modulating immune functions of stromal and immune cells in the tumor microenvironment. Cell. Mol. Immunol.17(9), 940–953 (2020).
- Laird PW , Jackson-GrusbyL , FazeliAet al. Suppression of intestinal neoplasia by DNA hypomethylation. Cell81(2), 197–205 (1995).
- Zhang JJ , ZhuY , ZhuYet al. Association of increased DNA methyltransferase expression with carcinogenesis and poor prognosis in pancreatic ductal adenocarcinoma. Clin. Transl. Oncol.14(2), 116–124 (2012).
- Hong L , SunG , PengLet al. The interaction between miR 148a and DNMT1 suppresses cell migration and invasion by reactivating tumor suppressor genes in pancreatic cancer. Oncol. Rep.40(5), 2916–2925 (2018).
- Xie VK , LiZ , YanYet al. DNA-methyltransferase 1 induces dedifferentiation of pancreatic cancer cells through silencing of Kruppel-like factor 4 expression. Clin. Cancer Res.23(18), 5585–5597 (2017).
- Zagorac S , AlcalaS , FernandezBayon Get al. DNMT1 inhibition reprograms pancreatic cancer stem cells via upregulation of the miR-17-92 cluster. Cancer Res.76(15), 4546–4558 (2016).
- Clinicaltrials.gov . NCT01845805. https://clinicaltrials.gov/ct2/show/NCT01845805
- Clinicaltrials.gov . NCT02959164. https://clinicaltrials.gov/ct2/show/NCT02959164
- Jing W , SongN , LiuYet al. DNA methyltransferase 3a modulates chemosensitivity to gemcitabine and oxaliplatin via CHK1 and AKT in p53 deficient pancreatic cancer cells. Mol. Med. Rep.17(1), 117–124 (2018).
- Jing W , SongN , LiuYPet al. DNMT3a promotes proliferation by activating the STAT3 signaling pathway and depressing apoptosis in pancreatic cancer. Cancer Manag. Res.11, 6379–6396 (2019).
- Wang LH , HuangJ , WuCRet al. Downregulation of miR 29b targets DNMT3b to suppress cellular apoptosis and enhance proliferation in pancreatic cancer. Mol. Med. Rep.17(2), 2113–2120 (2018).
- Ito S , ShenL , DaiQet al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science333(6047), 1300–1303 (2011).
- Li J , WuX , ZhouYet al. Decoding the dynamic DNA methylation and hydroxymethylation landscapes in endodermal lineage intermediates during pancreatic differentiation of hESC. Nucleic Acids Res.46(6), 2883–2900 (2018).
- Fujikura K , AlruwaiiZI , HaffnerMCet al. Downregulation of 5-hydroxymethylcytosine is an early event in pancreatic tumorigenesis. J. Pathol.254(3), 279–288 (2021).
- Morris JPT , YashinskieJJ , KocheRet al. α-Ketoglutarate links p53 to cell fate during tumour suppression. Nature573(7775), 595–599 (2019).
- Cimmino L , AifantisI. Alternative roles for oxidized mCs and TETs. Curr. Opin. Genet. Dev.42, 1–7 (2017).
- Eyres M , LanfrediniS , XuHet al. TET2 drives 5hmc marking of GATA6 and epigenetically defines pancreatic ductal adenocarcinoma transcriptional subtypes. Gastroenterology161(2), 653–668 e616 (2021).
- Wu J , LiH , ShiMet al. TET1-mediated DNA hydroxymethylation activates inhibitors of the Wnt/β-catenin signaling pathway to suppress EMT in pancreatic tumor cells. J. Exp. Clin. Cancer Res.38(1), 348 (2019).
- Bhagat TD , Von AhrensD , DawlatyMet al. Lactate-mediated epigenetic reprogramming regulates formation of human pancreatic cancer-associated fibroblasts. Elife8, e50663 (2019).
- Hendrich B , BirdA. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol.18(11), 6538–6547 (1998).
- Pandey S , PruittK. Functional assessment of MeCP2 in Rett syndrome and cancers of breast, colon, and prostate. Biochem. Cell Biol.95(3), 368–378 (2017).
- Pandey S , SimmonsGEJr , Malyarchuk , S , CalhounTN , PruittK. A novel MeCP2 acetylation site regulates interaction with ATRX and HDAC1. Genes Cancer6(9-10), 408–421 (2015).
- Jorgensen HF , BirdA. MeCP2 and other methyl-CpG binding proteins. Ment. Retard. Dev. Disabil. Res. Rev.8(2), 87–93 (2002).
- Wang H , LiJ , HeJet al. Methyl-CpG-binding protein 2 drives the Furin/TGF-beta1/Smad axis to promote epithelial–mesenchymal transition in pancreatic cancer cells. Oncogenesis9(8), 76 (2020).
- Guo X , LiK , JiangWet al. RNA demethylase ALKBH5 prevents pancreatic cancer progression by posttranscriptional activation of PER1 in an m6A-YTHDF2-dependent manner. Mol. Cancer19(1), 91 (2020).
- Tang B , YangY , KangMet al. m 6 A demethylase ALKBH5 inhibits pancreatic cancer tumorigenesis by decreasing WIF-1 RNA methylation and mediating Wnt signaling. Mol. Cancer19(1), 3 (2020).
- Marmorstein R , TrievelRC. Histone modifying enzymes: structures, mechanisms, and specificities. Biochim. Biophys. Acta1789(1), 58–68 (2009).
- Ono H , BassonMD , ItoH. P300 inhibition enhances gemcitabine-induced apoptosis of pancreatic cancer. Oncotarget7(32), 51301–51310 (2016).
- Chugh R , SangwanV , PatilSPet al. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci. Transl. Med.4(156), 156ra139 (2012).
- McGinn O , GuptaVK , DauerPet al. Inhibition of hypoxic response decreases stemness and reduces tumorigenic signaling due to impaired assembly of HIF1 transcription complex in pancreatic cancer. Sci. Rep.7(1), 7872 (2017).
- Modi S , GiriB , GuptaVKet al. Minnelide synergizes with conventional chemotherapy by targeting both cancer and associated stroma components in pancreatic cancer. Cancer Lett.537, 215591 (2022).
- Hou P , KapoorA , ZhangQet al. Tumor microenvironment remodeling enables bypass of oncogenic KRAS dependency in pancreatic cancer. Cancer Discov.10(7), 1058–1077 (2020).
- Qin H , NiyongereSA , LeeSJ , BakerBJ , BenvenisteEN. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J. Immunol.181(5), 3167–3176 (2008).
- Hu G , HeN , CaiCet al. HDAC3 modulates cancer immunity via increasing PD-L1 expression in pancreatic cancer. Pancreatology19(2), 383–389 (2019).
- Sherman MH , YuRT , TsengTWet al. Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc. Natl Acad. Sci. USA114(5), 1129–1134 (2017).
- Shinke G , YamadaD , EguchiHet al. Role of histone deacetylase 1 in distant metastasis of pancreatic ductal cancer. Cancer Sci.109(8), 2520–2531 (2018).
- Krauß L , UrbanBC , HastreiterSet al. HDAC2 facilitates pancreatic cancer metastasis. Cancer Res.82(4), 695–707 (2022).
- Strahl BD , AllisCD. The language of covalent histone modifications. Nature403(6765), 41–45 (2000).
- Jang MK , MochizukiK , ZhouM , JeongHS , BradyJN , OzatoK. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell19(4), 523–534 (2005).
- Mazur PK , HernerA , MelloSSet al. Combined inhibition of BET family proteins and histone deacetylases as a potential epigenetics-based therapy for pancreatic ductal adenocarcinoma. Nat. Med.21(10), 1163–1171 (2015).
- Hyun K , JeonJ , ParkK , KimJ. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med.49(4), e324 (2017).
- Raynal NJ , SiJ , TabyRFet al. DNA methylation does not stably lock gene expression but instead serves as a molecular mark for gene silencing memory. Cancer Res.72(5), 1170–1181 (2012).
- Schneider G , KrämerOH , SchmidRM , SaurD. Acetylation as a transcriptional control mechanism – HDACs and HATs in pancreatic ductal adenocarcinoma. J. Gastrointest. Cancer42(2), 85–92 (2011).
- Zhang H , PandeyS , TraversMet al. Targeting CDK9 reactivates epigenetically silenced genes in cancer. Cell175(5), 1244–1258.e1226 (2018).
- Kretz AL , SchaumM , RichterJet al. CDK9 is a prognostic marker and therapeutic target in pancreatic cancer. Tumour Biol.39(2), 1010428317694304 (2017).
- Gupta VK , SharmaNS , DurdenBet al. Hypoxia-driven oncometabolite L-2HG maintains stemness–differentiation balance and facilitates immune evasion in pancreatic cancer. Cancer Res.81(15), 4001–4013 (2021).
- Cao R , WangL , WangHet al. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science298(5595), 1039–1043 (2002).
- Jin X , YangC , FanPet al. CDK5/FBW7-dependent ubiquitination and degradation of EZH2 inhibits pancreatic cancer cell migration and invasion. J. Biol. Chem.292(15), 6269–6280 (2017).
- Bednar F , SchofieldHK , CollinsMAet al. Bmi1 is required for the initiation of pancreatic cancer through an Ink4a-independent mechanism. Carcinogenesis36(7), 730–738 (2015).
- van Vlerken LE , KieferCM , MorehouseCet al. EZH2 is required for breast and pancreatic cancer stem cell maintenance and can be used as a functional cancer stem cell reporter. Stem Cells Transl. Med.2(1), 43–52 (2013).
- Proctor E , WaghrayM , LeeCJet al. Bmi1 enhances tumorigenicity and cancer stem cell function in pancreatic adenocarcinoma. PLOS ONE8(2), e55820 (2013).
- Chen NM , NeesseA , DyckMLet al. Context-dependent epigenetic regulation of nuclear factor of activated T cells 1 in pancreatic plasticity. Gastroenterology152(6), 1507–1520 e1515 (2017).
- Patil S , SteuberB , KoppW et al. EZH2 regulates pancreatic cancer subtype identity and tumor progression via transcriptional repression of GATA6 . Cancer Res.80(21), 4620–4632 (2020).
- Andricovich J , PerkailS , KaiY , CasasantaN , PengW , TzatsosA. Loss ofKDM6Aactivates super-enhancers to induce gender-specific squamous-like pancreatic cancer and confers sensitivity to BET inhibitors. Cancer Cell33(3), 512–526 e518 (2018).
- Welstead GG , CreyghtonMP , BilodeauSet al. X-linked H3K27me3 demethylase Utx is required for embryonic development in a sex-specific manner. Proc. Natl Acad. Sci. USA109(32), 13004–13009 (2012).
- Lan F , BaylissPE , RinnJL et al. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature449(7163), 689–694 (2007).
- Creyghton MP , ChengAW , WelsteadGGet al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA107(50), 21931–21936 (2010).
- Zhang X , ChoiPS , FrancisJMet al. Identification of focally amplified lineage-specific super-enhancers in human epithelial cancers. Nat. Genet.48(2), 176–182 (2016).
- Yun M , WuJ , WorkmanJL , LiB. Readers of histone modifications. Cell Res.21(4), 564–578 (2011).
- Mathison AJ , KerkettaR , de AssuncaoTMet al. Kras(G12D) induces changes in chromatin territories that differentially impact early nuclear reprogramming in pancreatic cells. Genome Biol.22(1), 289 (2021).
- Nicolle R , BlumY , MarisaLet al. Pancreatic adenocarcinoma therapeutic targets revealed by tumor–stroma cross-talk analyses in patient-derived xenografts. Cell Rep.21(9), 2458–2470 (2017).
- Knudsen ES , BalajiU , MannakeeBet al. Pancreatic cancer cell lines as patient-derived avatars: genetic characterisation and functional utility. Gut67(3), 508–520 (2018).
- Witkiewicz AK , BalajiU , EslingerCet al. Integrated patient-derived models delineate individualized therapeutic vulnerabilities of pancreatic cancer. Cell Rep.16(7), 2017–2031 (2016).
- Collisson EA , BaileyP , ChangDK , BiankinAV. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol.16(4), 207–220 (2019).
- Lomberk G , BlumY , NicolleRet al. Distinct epigenetic landscapes underlie the pathobiology of pancreatic cancer subtypes. Nat. Commun.9(1), 1978 (2018).
- Collisson EA , SadanandamA , OlsonPet al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med.17(4), 500–503 (2011).
- Moffitt RA , MarayatiR , FlateELet al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet.47(10), 1168–1178 (2015).
- Weissmueller S , ManchadoE , SaborowskiMet al. Mutant p53 drives pancreatic cancer metastasis through cell autonomous PDGF receptor β signaling. Cell157(2), 382–394 (2014).
- Diaferia GR , BalestrieriC , ProsperiniEet al. Dissection of transcriptional and cis -regulatory control of differentiation in human pancreatic cancer. EMBO J.35(6), 595–617 (2016).
- Somerville TDD , XuY , MiyabayashiKet al. TP63-mediated enhancer reprogramming drives the squamous subtype of pancreatic ductal adenocarcinoma. Cell Rep.25(7), 1741–1755 e1747 (2018).
- Kloesch B , IonaszV , PaliwalSet al. A GATA6 -centred gene regulatory network involving HNFs and DeltaNp63 controls plasticity and immune escape in pancreatic cancer. Gut71(4), 766–777 (2022).
- Lomberk G , DusettiN , IovannaJ , UrrutiaR. Emerging epigenomic landscapes of pancreatic cancer in the era of precision medicine. Nat. Commun.10(1), 3875 (2019).
- Matsui M , CoreyDR. Non-coding RNAs as drug targets. Nat. Rev. Drug. Discov.16(3), 167–179 (2017).
- Mortoglou M , TabinZK , ArisanED , KocherHM , Uysal-OnganerP. Non-coding RNAs in pancreatic ductal adenocarcinoma: new approaches for better diagnosis and therapy. Transl. Oncol.14(7), 101090 (2021).
- Cech TR , SteitzJA. The noncoding RNA revolution – trashing old rules to forge new ones. Cell157(1), 77–94 (2014).
- Yu J , LiA , HongSM , HrubanRH , GogginsM. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin. Cancer Res.18(4), 981–992 (2012).
- Bartel DP . MicroRNAs: genomics, biogenesis, mechanism, and function. Cell116(2), 281–297 (2004).
- Carthew RW , SontheimerEJ. Origins and mechanisms of miRNAs and siRNAs. Cell136(4), 642–655 (2009).
- Borchert GM , LanierW , DavidsonBL. RNA polymerase III transcribes human microRNAs. Nat. Struct. Mol. Biol.13(12), 1097–1101 (2006).
- Pillai RS , BhattacharyyaSN , FilipowiczW. Repression of protein synthesis by miRNAs: how many mechanisms?Trends Cell Biol.17(3), 118–126 (2007).
- Berezikov E , PlasterkRH. Camels and zebrafish, viruses and cancer: a microRNA update. Hum. Mol. Genet.14(2), R183–R190 (2005).
- Hussain SP . Pancreatic cancer: current progress and future challenges. Int. J. Biol. Sci.12(3), 270–272 (2016).
- Gilles ME , HaoL , HuangLet al. Personalized RNA medicine for pancreatic cancer. Clin. Cancer Res.24(7), 1734–1747 (2018).
- MacKenzie TN , MujumdarN , BanerjeeSet al. Triptolide induces the expression of miR-142-3p: a negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol. Cancer Ther.12(7), 1266–1275 (2013).
- Calin GA , SevignaniC , DumitruCDet al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl Acad. Sci. USA101(9), 2999–3004 (2004).
- Volinia S , CalinGA , LiuCGet al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl Acad. Sci. USA103(7), 2257–2261 (2006).
- Hampton T . MicroRNAs linked to pancreatic cancer. JAMA297(9), 937 (2007).
- Szafranska AE , DavisonTS , JohnJet al. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene26(30), 4442–4452 (2007).
- Hong TH , ParkIY. MicroRNA expression profiling of diagnostic needle aspirates from surgical pancreatic cancer specimens. Ann. Surg. Treat Res.87(6), 290–297 (2014).
- Hanahan D , WeinbergRA. Hallmarks of cancer: the next generation. Cell144(5), 646–674 (2011).
- Bhat SA , AhmadSM , MumtazPTet al. Long non-coding RNAs: mechanism of action and functional utility. Noncoding RNA Res.1(1), 43–50 (2016).
- Chen X , YanCC , ZhangX , YouZH. Long non-coding RNAs and complex diseases: from experimental results to computational models. Brief. Bioinform.18(4), 558–576 (2017).
- Kung JT , ColognoriD , LeeJT. Long noncoding RNAs: past, present, and future. Genetics193(3), 651–669 (2013).
- Muller S , RaulefsS , BrunsPet al. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol. Cancer14, 94 (2015).
- Li X , DengSJ , ZhuSet al. Hypoxia-induced lncRNA- NUTF2P3-001 contributes to tumorigenesis of pancreatic cancer by derepressing the miR-3923/ KRAS pathway. Oncotarget7(5), 6000–6014 (2016).
- Kim K , JutooruI , ChadalapakaGet al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene32(13), 1616–1625 (2013).
- Castro-Piedras I , VartakD , SharmaMet al. Identification of novel MeCP2 cancer-associated target genes and post-translational modifications. Front. Oncol.10, 576362 (2020).
- Sharma NS , GnamlinP , DurdenBet al. Long non-coding RNA GAS5 acts as proliferation ‘brakes’ in CD133 + cells responsible for tumor recurrence. Oncogenesis8(12), 68 (2019).
- Raimondi S , MaisonneuveP , LowenfelsAB. Epidemiology of pancreatic cancer: an overview. Nat. Rev. Gastroenterol. Hepatol.6(12), 699–708 (2009).
- Herman JG , UmarA , PolyakKet al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc. Natl Acad. Sci. USA95(12), 6870–6875 (1998).
- Ottini L , RizzoloP , SiniscalchiEet al. Gene promoter methylation and DNA repair capacity in monozygotic twins with discordant smoking habits. Mutat. Res. Genet. Toxicol. Environ. Mutagen.779, 57–64 (2015).
- Besingi W , JohanssonA. Smoke-related DNA methylation changes in the etiology of human disease. Hum. Mol. Genet.23(9), 2290–2297 (2014).
- Jin T , HaoJ , FanD. Nicotine induces aberrant hypermethylation of tumor suppressor genes in pancreatic epithelial ductal cells. Biochem. Biophys. Res. Commun.499(4), 934–940 (2018).
- Hussain M , RaoM , HumphriesAEet al. Tobacco smoke induces polycomb-mediated repression of Dickkopf-1 in lung cancer cells. Cancer Res.69(8), 3570–3578 (2009).
- Vrijens K , BollatiV , NawrotTS. MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ. Health Perspect.123(5), 399–411 (2015).
- Zhao X , ZhuS , LiJ , LongD , WanM , TangW. Epigenetic changes in inflammatory genes and the protective effect of cooked rhubarb on pancreatic tissue of rats with chronic alcohol exposure. Biomed. Pharmacother.146, 112587 (2022).
- Ni XG , BaiXF , MaoYLet al. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur. J. Surg. Oncol.31(2), 164–169 (2005).
- Sato N , FukushimaN , MaitraAet al. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res.63(13), 3735–3742 (2003).
- Natale F , VivoM , FalcoG , AngrisanoT. Deciphering DNA methylation signatures of pancreatic cancer and pancreatitis. Clin. Epigenetics11(1), 132 (2019).
- Shen SY , SinghaniaR , FehringerGet al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature563(7732), 579–583 (2018).
- Cohen JD , JavedAA , ThoburnCet al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc. Natl Acad. Sci. USA114(38), 10202–10207 (2017).
- Cohen JD , LiL , WangYet al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science359(6378), 926–930 (2018).
- Guler GD , NingY , KuCJet al. Detection of early stage pancreatic cancer using 5-hydroxymethylcytosine signatures in circulating cell free DNA. Nat. Commun.11(1), 5270 (2020).
- Song CX , YinS , MaLet al. 5-Hydroxymethylcytosine signatures in cell-free DNA provide information about tumor types and stages. Cell Res.27(10), 1231–1242 (2017).
- Li W , ZhangX , LuXet al. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic biomarkers for human cancers. Cell Res.27(10), 1243–1257 (2017).
- Turchinovich A , WeizL , LangheinzA , BurwinkelB. Characterization of extracellular circulating microRNA. Nucleic Acids Res.39(16), 7223–7233 (2011).
- Wang H , PengR , WangJ , QinZ , XueL. Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage. Clin. Epigenetics10, 59 (2018).
- Yonemori K , SekiN , IdichiTet al. The microRNA expression signature of pancreatic ductal adenocarcinoma by RNA sequencing: anti-tumour functions of the microRNA-216 cluster. Oncotarget8(41), 70097–70115 (2017).
- Kawaguchi T , KomatsuS , IchikawaDet al. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br. J. Cancer108(2), 361–369 (2013).
- Lee EJ , GusevY , JiangJ et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int. J. Cancer120(5), 1046–1054 (2007).
- Kunovsky L , TesarikovaP , KalaZet al. The use of biomarkers in early diagnostics of pancreatic cancer. Can. J. Gastroenterol. Hepatol.2018, 5389820 (2018).
- Yu Y , TongY , ZhongA , WangY , LuR , GuoL. Identification of serum microRNA-25 as a novel biomarker for pancreatic cancer. Medicine (Baltimore)99(52), e23863 (2020).
- Yang SZ , XuF , ZhouT , ZhaoX , McDonaldJM , ChenY. The long non-coding RNAHOTAIRenhances pancreatic cancer resistance to TNF-related apoptosis-inducing ligand. J. Biol. Chem.292(25), 10390–10397 (2017).
- Liu JH , ChenG , DangYW , LiCJ , LuoDZ. Expression and prognostic significance of lncRNAMALAT1in pancreatic cancer tissues. Asian Pac. J. Cancer Prev.15(7), 2971–2977 (2014).
- Pang EJ , YangR , FuXB , LiuYF. Overexpression of long non-coding RNAMALAT1is correlated with clinical progression and unfavorable prognosis in pancreatic cancer. Tumour Biol.36(4), 2403–2407 (2015).
- Young MR , WagnerPD , GhoshSet al. Validation of biomarkers for early detection of pancreatic cancer: summary of the Alliance of Pancreatic Cancer Consortia for Biomarkers for Early Detection workshop. Pancreas47(2), 135–141 (2018).
- Singhi AD , KoayEJ , ChariST , MaitraA. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology156(7), 2024–2040 (2019).
