Abstract
Background: Biological aging may be a robust biomarker of dementia or cognitive performance. This systematic review synthesized the evidence for an association between epigenetic aging and dementia, mild cognitive impairment and cognitive function. Methods: A systematic search was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Results: 30 eligible articles were included. There was no strong evidence that accelerated epigenetic aging was associated with dementia/mild cognitive impairment (n = 7). There was some evidence of an association with poorer cognition (n = 20), particularly with GrimAge acceleration, but this was inconsistent and varied across cognitive domains. A meta-analysis was not performed due to high study heterogeneity. Conclusion: There is insufficient evidence to indicate that current epigenetic aging clocks can be clinically useful biomarkers of dementia or cognitive aging.
Plain language summary
As individuals get older, changes in cognitive performance are common, including some degree of cognitive decline. Dementia is a symptom characterized by significant decline in cognitive function that affects daily living, and age is the biggest risk factor. Researchers have now identified ways to estimate a person’s biological age from a blood sample (referred to as ‘epigenetic aging’), and this measure is thought to better estimate an individual’s rate of aging than his or her chronological age. This study brought together all of the previous studies, 30 in total, that have investigated links between biological aging and cognitive performance, as well as dementia risk. Synthesizing all of this evidence, the authors found no strong evidence that the individuals with dementia had accelerated aging. However, there was some evidence, although inconsistent, indicating that accelerated aging was associated with worse cognitive performance.
Tweetable abstract
Although age is the strongest risk factor for dementia, there is no strong evidence that individuals with dementia have accelerated biological aging (based on existing epigenetic clocks).
Globally each year, there are nearly 10 million new cases of dementia, and dementia is becoming the seventh leading cause of death [Citation1]. Dementia is characterized by decline in cognition that is significant enough to cause impairments in daily functioning [Citation2]. The growing burden of dementia worldwide has posed significant medical, social and economic challenges to public health.
It is well acknowledged that aging is the greatest risk factor for dementia [Citation3], with rates increasing exponentially after 80 years of age. However, dementia is not synonymous with aging. The identification of individuals at risk of dementia at the presymptomatic stage is of great value for intervention and prevention, as well as improvement of quality of life and extension of healthspan. Considerable between-person variation has been constantly observed in the rate of aging, as well as the risk of age-associated diseases. This indicates that chronological age alone does not sufficiently capture individual differences in the aging process. Therefore, there has been strong interest in utilizing early biomarkers of aging, which have the potential to accurately predict the risk of age-related conditions, diseases and mortality. Emerging evidence suggests that epigenetic age measured using the epigenetic clock is a promising biomarker of biological aging across heterogeneous cells, tissues and organs [Citation4]. Changes in epigenetic patterns may also be an underlying mechanism that drives human aging [Citation5].
A number of epigenetic clock algorithms have been developed and applied in the literature. The first generation of epigenetic clocks was developed by identifying a combination of DNA methylation sites across the genome, which were highly correlated with chronological age [Citation4,Citation6]. Individuals with older epigenetic age compared with their actual chronological age were said to have accelerated aging. Accelerated aging was subsequently found to be an accurate predictor of various age-related diseases, such as cancer [Citation7], diabetes [Citation8] and all-cause mortality risk [Citation9,Citation10]. Accelerated aging has also been shown to be a marker of longevity [Citation11]. Several years later, the second generation of epigenetic clocks was developed. These clocks were developed specifically to reflect age-related health or disease phenotypes and incorporated proteomic markers that reflect clinical measures of health risk [Citation12–15].
The development of these epigenetic clocks has allowed researchers to investigate the role of biological aging in the risk of dementia. However, whether accelerated epigenetic aging is associated with worse cognitive performance, or is an early indicator of dementia risk, remains unclear. The first study to examine this, among 289 Caucasians, reported that individuals with Parkinson’s disease were significantly more likely to have accelerated epigenetic aging [Citation16]. A number of subsequent studies also investigated the association between epigenetic aging and dementia, across different population groups (with varying chronological ages and health statuses). While some identified findings similar to those of the initial work of Horvath, with accelerated epigenetic age significantly associated with dementia or cognitive performance [Citation17–19], some middle-to-large-sized cohort studies reported insignificant results [Citation20–22]. To date, there has been no systematic review on this topic.
Hence, this systematic review aims to identify and synthesize the evidence to determine whether accelerated epigenetic age, measured in peripheral tissue, is associated with dementia and cognitive function in adulthood.
Method
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISM) guidelines (www.prisma-statement.org/) were followed in conducting this systematic review [Citation23]. The systematic review protocol was registered with the International Prospective Register of Ongoing Systematic Reviews [Citation24], registration number CRD42022320557. After registration of the protocol, and based on the relatively small number of studies investigating dementia as an outcome, a decision was made to expand the review to also include studies examining cognitive performance (detailed further below). As this was not preregistered, extra caution should be taken in interpreting the findings from these studies.
Inclusion criteria
This review considered all types of quantitatively measured and population-based studies, including prospective cohort studies, case–control studies and cross-sectional studies. Randomized controlled trials were not applicable to address the study question. Reviews were not included. Measures of the outcome included 1) dementia or any subtype of dementia, such as Alzheimer’s disease, assessed according to Diagnostic and Statistical Manual of Mental Disorders criteria or based on clinical records or ICD-10 Classification of Mental and Behavioural Disorders codes and 2) major neurocognitive disorder, such as memory or cognitive decline, and mild cognitive impairment (MCI). Studies assessing cognitive performance were also eligible for inclusion, even if performance did not meet the formal criteria for cognitive impairment. Studies were included if they measured epigenetic age in peripheral tissue. Only studies of adults (with participants aged at least 18 years) that had been published in English were eligible.
Epigenetic clock
The authors’ previous systematic review [Citation25] focused on only the first generation of epigenetic clocks, commonly referred to as the Horvath [Citation4] and Hannum [Citation6] clocks, and their derivatives intrinsic epigenetic age acceleration (IEAA [Citation26]) and extrinsic epigenetic age acceleration (EEAA [Citation9]). However, recent publications have reported the development of new, reliable methods of calculating epigenetic biomarkers of aging [Citation12,Citation14,Citation15]. Therefore, the scope of this review was broadened to include all epigenetic clocks that had been generated using a validated algorithm. This included, but was not limited to, Horvath, Hannum, GrimAge, PhenoAge and DunedinPACE.
Both Horvath’s and Hannum’s epigenetic clocks, as the first generation of DNA methylation-based biomarkers, were developed for their correlation with chronological age and were thought to be surrogate markers of biological age. While Horvath’s epigenetic clock performs well across different cell types and tissues by measuring DNA methylation at 353 CpG sites [Citation4], Hannum’s epigenetic clock is blood-specific with DNA methylation data measured from 71 CpG sites in leukocytes [Citation6]. The second generation of epigenetic clocks, by contrast, involve phenotypic or behavioral surrogate markers that reflect differences in healthspan and all-cause mortality in the algorithm. For example, Levine et al. developed a PhenoAge estimator by regressing phenotypic measures of mortality risk on 513 CpG sites [Citation14], while Lu et al. incorporated surrogate markers of smoking pack-years and seven plasma proteins before regressing time-to-death on these surrogate biomarkers in the development of GrimAge [Citation15]. The recently developed DunedinPACE estimator was calculated by a two-stage approach. The authors first modeled the ‘pace of aging’ based on longitudinal within-individual changes in organ system functioning indicators and then distilled this measurement of change into a single-time-point DNA methylation blood test [Citation12]. Age acceleration was calculated as the residuals by regressing epigenetic age on the chronological age, with a positive value representing individuals who are biologically older than their actual age.
Search strategy
The authors conducted a systematic search of MEDLINE, Embase and PsycINFO to identify relevant articles using appropriate search terms: (epigenetic clock or epigenetic ag* or methylation ag*or [methyl* and (biological ag* or Horvath* or Hannum* or PhenoAge* or GrimAge* or Dunedin*)]) AND (dement* or Alzheimer* or MCI or cogniti*). The reference list of retrieved articles was also searched. Studies published in English up until 25 March 2022 were eligible for inclusion.
All records retrieved by the literature search were screened independently by at least two reviewers (A Zhou, Z Wu, AZZ Phyo, D Torres, S Vishanwath). The titles and abstracts were first screened for eligibility. Full texts of potentially eligible studies were then reviewed to determine final eligibility. All disagreements were resolved by discussion or referring to a third reviewer.
Data extraction
A predesigned data extraction form was used specifically for this review, and data were extracted independently by at least two authors for each of the studies. Discrepancies were resolved through discussion or, if needed, consultation with a third author. The following information was retrieved from each study, if available: study design, country, participants (including sample size, group size, age, sex, ethnicity), the platform for DNA methylation analysis and the sample type used, the epigenetic clock that was measured and the correlation with chronological age, the associations identified (including a measure of effect size and significance) and the covariates included in the adjusted analysis.
Assessment of methodological quality
Two authors independently assessed the risk of bias for studies deemed eligible for inclusion. The Joanna Briggs Institute Critical Appraisal Checklist for case–control, cross-sectional or cohort studies was used as appropriate [Citation27]. The relevant criteria were rated as unclear, low risk or high risk of bias for each study. The authors assessed the risk of bias to help evaluate the quality of evidence for each study, but not to exclude any studies from the review.
Data synthesis
Heterogeneity was assessed regarding the study population characteristics, types of epigenetic clocks examined and measures of outcome. Due to the large methodological heterogeneity of eligible studies, meta-analysis was not performed. Instead, the results for dementia and cognition outcomes were grouped in separate tables and summarized qualitatively with effect sizes where available. Estimates of any statistically significant effect size, such as correlations, odds ratio, and beta values from linear or logistic regression, were reported where available.
Results
Search results
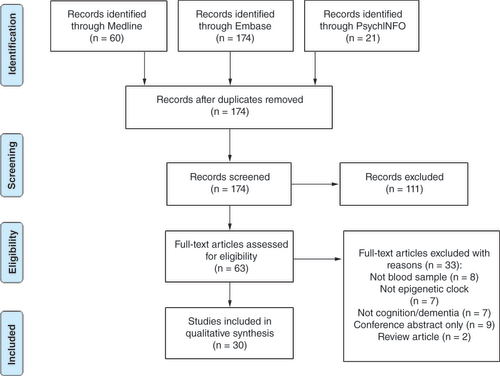
The database search yielded 255 articles in total. After removing 81 duplicates, the authors screened the titles and abstracts of 174 articles; 111 records were excluded after initial screening, leaving 63 articles for full-text assessment (). Of these, seven studies were excluded because epigenetic clocks were not measured, eight were excluded because they did not use peripheral tissues to calculate epigenetic clocks and seven were excluded because they did not measure the outcome of interest, such as dementia or cognitive performance. A further nine articles were excluded because they were conference abstracts only and two were review articles.
Characteristics of included studies
The authors finally included 30 studies in this systematic review, with ten focused on dementia [Citation14,Citation16,Citation17,Citation19–21,Citation28–31], 19 focused on cognitive performance [Citation12,Citation13,Citation22,Citation32–47] and one examining both [Citation18]. Approximately 20,610 participants were involved, and most studies had been undertaken in North America or the UK. For each study, the authors report characteristics of the study design and population, as well as information regarding the outcomes measured and the main findings ( & ).
Table 1. Studies investigating the association between accelerated epigenetic aging and neurodegenerative disorders.
Table 2. Studies investigating the association between accelerated epigenetic aging and cognitive function in adulthood.
Study design & participants
Four studies used a case–control design with a total of 990 cases and 650 controls identified, including participants with HIV [Citation31] and Parkinson’s disease [Citation14,Citation16,Citation17]. All four studies focused on dementia, while three studies on Parkinson’s disease used data from the same cohort – the PEG study [Citation14,Citation16,Citation17].
There were 14 prospective cohort studies (n = 8596 participants) with follow-up times ranging from 3 [Citation21] to 20 years [Citation12,Citation39]. Most studies had a sample size between 200 and 800 participants; the smallest study included 52 [Citation18] and the largest 1115 [Citation47]. The mean age of participants in each study ranged from 38 [Citation34] to 79.1 years [Citation20]. The majority of the studies included both male and female participants; however, one cohort recruited only women from the WHIMS study [Citation28], and one used data from only the male participants of the VET registry [Citation46].
A total of 9563 participants were included in the remaining studies, which conducted cross-sectional analyses. The studies varied in sample size between 29 [Citation35,Citation43] and 4535 [Citation38]. While most studies focused on middle-aged and older populations, one involved young adults aged 25–30 years [Citation45].
Risk of bias assessment
The results of the risk of bias assessment for case–control, cross-sectional and cohort designs are summarized in Supplementary Tables 1–3, respectively. Most cohort studies had a low risk of bias based on all criteria, with only two articles [Citation22,Citation28] giving inadequate information about whether the follow-up was complete. Almost all case–control studies had a low risk of bias, except for one [Citation14], where it was unclear if appropriate statistical analysis had been used, as well as strategies to deal with confounding factors. Seventy percent of the cross-sectional studies were rated as low risk of bias; however, there were two studies [Citation14,Citation45] that did not deal with confounding factors, and one of those had insufficient information about the statistical method and objective criteria used for the measurement of conditions [Citation14]. Two other articles did not provide sufficiently clear information on the measurement of outcome [Citation19] or exposure [Citation29].
Summary of outcomes
Overall, 11 different epigenetic clocks were investigated, including Horvath’s age acceleration (AAH), Hannum’s age acceleration (AAHa), the AAH-AAHa consensus model [Citation48], intrinsic epigenetic age acceleration (IEAA), extrinsic epigenetic age acceleration (EEAA), Weidner’s clock [Citation49], PhenoAge acceleration (PhenoAA), GrimAge acceleration (GrimAA), DunedinPoAm, DunedinPACE and epigenetic mitotic aging (epiTOC) [Citation17]. Generally, the first generation of epigenetic clocks correlated better with chronological age; the correlation coefficients ranged from 0.62 [Citation38] to 0.94 [Citation41].
Neurodegenerative disorders
A total of 12 studies (n = 4255 participants) from 11 articles [Citation14,Citation16–21,Citation28–31] investigated whether dementia was associated with accelerated epigenetic age ().
Parkinson’s disease
Three studies [Citation14,Citation16,Citation17] focused on Parkinson’s disease and reported significant results. Horvath and Ritz [Citation16] were the first to report that Parkinson’s disease was associated with increased age acceleration based on the first-generation epigenetic clocks (AAH β = 0.036, p = 0.04, and EEAA β = 0.031, p = 0.03, respectively), although the effect size was small. Levine et al. [Citation14] applied a different clock, PhenoAge, to the same set of participants and also reported a significant association (p = 0.03); however, the effect size was not reported. Paul et al. [Citation17] used an additional measure of epigenetic age, epiTOC, on a slightly larger sample from the same cohort (n = 807) and reported a twofold increased risk of Parkinson’s disease in individuals with accelerated epigenetic aging (odds ratio: 2.11; p < 0.001).
Dementia and/or MCI
By contrast, the findings were largely null from seven studies [Citation14,Citation18,Citation20,Citation21,Citation28–30] that looked at incident dementia and/or MCI. These included four studies that used both first-generation (IEAA, EEAA) and second-generation (PhenoAge, GrimAge) epigenetic clocks [Citation20,Citation21,Citation28,Citation29]. One small study (n = 52) [Citation18] reported that Horvath’s epigenetic age was a significant predictor of incident dementia but no significant association when accelerated aging (the difference between Horvath’s epigenetic age and chronological age) was examined. Another, larger study (n = 604) [Citation14] found increased PhenoAge in patients with probable Alzheimer’s disease and/or frontotemporal dementia but accelerated aging was not examined. Only one study [Citation19] examined the age of onset of amyotrophic lateral sclerosis and frontotemporal dementia, rather than disease status per se, as well as the disease duration, and reported a significant reverse association.
Cognition
20 studies [Citation12,Citation13,Citation18,Citation22,Citation32–47] examined the association between at least one epigenetic clock and cognitive performance (). While some studies looked at multiple aspects of cognitive performance [Citation12,Citation13,Citation22,Citation35–40,Citation42–47] or assessed overall cognition [Citation22,Citation36,Citation37,Citation39,Citation42–44], others focused on a single domain of cognitive function [Citation18,Citation32–34,Citation41].
Seven studies [Citation22,Citation36,Citation37,Citation39,Citation42–44] reported overall cognition either by calculating the composite score based on different subdomains of cognition [Citation22,Citation39,Citation42] or by using a global measure, such as the Mini-Mental State Examination [Citation36,Citation37], the Montreal Cognitive Assessment [Citation44] or the Korean version of the modified Consortium to Establish a Registry for Alzheimer’s Disease Assessment Battery [Citation43].
However, there was a lack of consistent findings across studies. Four studies [Citation22,Citation36,Citation37,Citation42] involving 2284 participants in total reported no association, including with epigenetic clocks such as AAH [Citation22,Citation37], AAHa [Citation22], IEAA [Citation37], PhenoAA [Citation36] and GrimAA [Citation42]. By contrast, significant results with a similar direction were reported by three studies [Citation39,Citation43,Citation44] involving a total of 981 participants. Among these, two examined correlation coefficients that ranged from -0.21 PhenoAA and AAH [Citation44] to -0.30 GrimAA [Citation39], suggesting that increased accelerated age was associated with worse cognition. One study [Citation43] investigated the difference in biological age acceleration between high and low performers, based on median score, and found a stronger relationship, with Cohen’s d equal to 0.82 (IEAA) and 0.78 (EEAA), respectively.
Cognitive domains
A number of subdomains of cognition were investigated [Citation12,Citation13,Citation18,Citation22,Citation32–47]; these included episodic memory, working memory, processing speed, attention, executive function and verbal fluency. Most studies looked at more than one epigenetic clock algorithm and found varying results across these measures. Full details are provided in Supplementary Table 4. Some of the main findings are presented below.
Nine studies [Citation18,Citation35,Citation37,Citation38,Citation40,Citation42,Citation43,Citation46,Citation47] looked at episodic memory. Degerman et al. [Citation18] showed that individuals who had maintained memory were at least 2.7 years younger in AAH compared with the average or declining memory group. Two additional studies of AAH also found negative associations with episodic memory [Citation35,Citation46], but three others did not [Citation37,Citation38,Citation40]. Another four studies [Citation37,Citation40,Citation42,Citation47] also identified findings in the same direction, but with different epigenetic clocks; the most consistent was with GrimAA [Citation40,Citation42,Citation47]. However, it is worth noting that insignificant results were also reported in a number of studies [Citation37,Citation38,Citation40,Citation43,Citation46,Citation47], including all studies that examined AAHa [Citation37,Citation38,Citation40,Citation46] and the two studies that examined PhenoAA [Citation40,Citation47].
There were seven studies [Citation12,Citation13,Citation33,Citation35,Citation43–45] on working memory, all of which examined AAH, but only two of those reported significant associations [Citation35,Citation44]. One study [Citation43] reported insignificant results with all five epigenetic clocks (AAH, IEAA, EEAA, GrimAA and PhenoAA) measured in that study, and these null findings were generally replicated in at least one additional study. Two longitudinal studies [Citation12,Citation13] used data from the Dunedin study and reported significant negative associations with the DunedinPoAm clock (β = -0.17; p < 0.001) [Citation13] and the related DunedinPACE (β = -0.23; p < 0.001) [Citation12], but no subsequent studies have yet attempted to replicate these findings.
A total of 11 studies [Citation12,Citation13,Citation35–38,Citation40,Citation42,Citation44,Citation45,Citation47] measured processing speed. However, nearly half of them reported null findings [Citation35,Citation36,Citation38,Citation44,Citation45]. The most consistent finding was with GrimAA, with age acceleration associated with slower processing speed in three of the five studies that used this measure [Citation12,Citation40,Citation42]. In contrast, seven of the eight studies of AAH [Citation13,Citation35,Citation37,Citation38,Citation40,Citation44,Citation45] and all four studies of AAHa [Citation12,Citation13,Citation38,Citation40] reported null findings.
Six studies [Citation37,Citation43–47] investigated biological aging and executive function, and most identified significant associations. For example, three studies [Citation43–45] reported Pearson correlation coefficients that ranged from -0.19 with PhenoAA [Citation44] to -0.42 with AAH [Citation43], indicating that biological age acceleration was associated with decline in executive function. Likewise, both Vaccarino et al. and Zheng et al. found that age acceleration in AAH (β = 0.08; p < 0.05) [Citation46], IEAAH (β = 0.08; p < 0.05) [Citation46] and GrimAA (β = 0.23; p = 0.005) [Citation47] was associated with worse executive function. However, a number of null associations were also reported, including all five studies that looked at IEAA [Citation37,Citation43–45,Citation47] and five of the six studies that investigated EEAA [Citation37,Citation43,Citation44,Citation46,Citation47].
Among the six studies [Citation36–38,Citation43–45] that looked at verbal fluency, two studies with a total of 4596 participants showed that accelerated epigenetic aging measured by AAH [Citation45], AAHa [Citation38] or EEAA [Citation45] was associated with worse verbal fluency. No-significant relationships were identified for IEAA [Citation37,Citation43–45], GrimAA [Citation44] and PhenoAA [Citation36,Citation44].
Discussion
This study, the first systematic review to examine the extent to which epigenetic aging in blood is associated with the risk of dementia or related outcomes, included 30 eligible studies with over 20,000 participants. There was substantial heterogeneity in the included studies, especially in terms of the epigenetic clocks that were used (n = 11 variations) and the specific outcomes that were examined (ranging from all-cause dementia to HIV-neurocognitive disorder and from verbal fluency to reasoning). There were 11 studies that investigated whether individuals with neurodegenerative disorder have accelerated epigenetic aging, which extends the earlier work of the present study’s authors, which included only two studies [Citation25] and demonstrates the contemporary nature of the field. However, the majority reported no significant association, including six studies of all-cause dementia in later life [Citation18,Citation20,Citation21,Citation28–30]. On the other hand, all three studies of Parkinson’s disease [Citation14,Citation16,Citation17], using different epigenetic clocks, reported significantly accelerated epigenetic aging. However, these studies all used data from the same (PEG) cohort, which means that independent replication in other cohorts is needed.
20 studies investigated epigenetic aging and cognitive performance across a range of different domains, but the findings across studies were highly variable. Although at least one significant finding was reported for each epigenetic aging clock, none of them was consistently associated with a specific cognitive domain or with composite measures or global cognitive function. These findings indicate that there is currently insufficient evidence that epigenetic aging, based on existing epigenetic clocks, is a biomarker of cognitive performance or the risk of dementia.
Dementia is a clinical syndrome characterized by substantial decline in cognitive functioning, resulting in significant impairments in social or occupational functioning [Citation50]. Alzheimer’s disease is the most common neuropathology resulting in dementia, affecting nearly a third of people over age 85 [Citation51]. Age is the primary risk factor for cognitive decline and dementia, and this has driven interest to better understand the role of aging in dementia risk [Citation52]. An accurate measure of biological, rather than chronological, aging has great potential to be an early biomarker of cognitive decline with aging and the risk of dementia. This is becoming increasingly important, given the rapidly aging global population and the increasing number of individuals with incident dementia [Citation1]. Identifying individuals with accelerated biological aging could permit personalized, targeted interventions to help prevent or delay the onset of dementia.
One of the most promising and extensively investigated biomarkers of aging is based on DNA methylation [Citation53]. With aging, there are changes in the patterns of DNA methylation, with a global decline in DNA methylation after adulthood [Citation54] but hypermethylation in specific gene regions and increasing intra- and interindividual variability in DNA methylation patterns [Citation55]. A number of metrics have been developed that estimate biological age based on DNA methylation at specific CpG sites. These epigenetic clocks have been shown to be some of the strongest and most robust biomarkers of aging [Citation56]. The difference between an individual’s chronological age and biological age, based on these metrics, is known as accelerated or decelerated epigenetic aging. Accelerated epigenetic aging (higher biological than chronological age) has been observed to be negatively associated with a range of health outcomes [Citation25] and, in turn, is potentially modifiable [Citation57]. Therefore, there has been increasing interest in whether this blood epigenetic measure is a good predictor of dementia and cognitive decline.
Almost all studies in this review measured epigenetic aging using more than one epigenetic clock algorithm, but they rarely reported consistent associations. This is not entirely surprising. Although all the epigenetic clocks are based on DNA methylation, they were originally developed for slightly different purposes and have been shown to measure different aspects of the aging process. The first-generation epigenetic clocks [Citation4,Citation6] and their derivatives that considered cell composition [Citation9,Citation26] were developed for their correlation with chronological age. They were considered robust predictors of longevity and mortality risk. Most studies in this review reported high correlations between chronological age and epigenetic age based on Horvath’s and Hannum’s epigenetic clocks. On the other hand, the second-generation epigenetic clocks, emerging only 3 or 4 years ago [Citation14,Citation15], were developed against age-related physiological measures and disease. Instead of focusing on only the correlation with chronological age, they also incorporated specific proteomic as well as phenotypic measures and considered health-related behaviors such as smoking status in the algorithm. They may thus hold the best promise to assess not only health status but also the influence of environmental and behavioral factors during the aging process. Indeed, GrimAA was consistently associated with episodic memory (three of three studies reported a significant negative association), and a number of studies also found it was associated with worse processing speed (three of five studies). Findings regarding other cognitive domains, however, were inconsistent. A very recent study [Citation58] on cognition in bipolar disorder patients (published after the present study’s searches) supports the findings of the present study, with a significant negative association between GrimAA and short-term memory, as well as problem-solving ability.
Furthermore, the newest epigenetic clocks, DunedinPoAm [Citation13] and DunedinPACE [Citation12], were distinguished from the previous second generation of epigenetic clocks. DunedinPACE, an updated version of DunedinPoAm, was developed based on changes in 19 biomarkers over 20 years using a single birth cohort’s data that excluded the confounding effects of potential exposures that may differ across generations, with individuals aged between 26 and 45 years [Citation12]. Although this clock was trained on a relatively younger sample, it was the first to focus on young to middle-aged adults and their within-individual changes. In fact, there is evidence that early life factors may play a role in epigenetic aging and health outcomes in later life. For example, one of the studies included in this review [Citation36] reported a significant association between PhenoAA and cognitive function, but this was no longer significant after adjusting for IQ at 11 years. Another study [Citation42], based on the same cohort but using a different epigenetic clock, GrimAA, also found a similar trend, with the association between GrimAA and cognitive function attenuated by an average of 41.1% after adjustment for age 11 IQ.
There have been some promising early findings with the DunedinPACE measure in terms of cognitive performance. Consistent associations have been reported across a whole range of cognitive domains, including working memory, processing speed and fluid intelligence, and with increasing effect size compared with the former estimator, DunedinPoAm. In addition, a very recent study showed evidence that DunedinPACE outperformed the first- and second-generation epigenetic clocks in terms of correlation with cognitive aging and the risk of dementia [Citation59]. Therefore, DunedinPACE may hold the best potential for its capacity to predict cognitive aging and risk of neurodegenerative disease. However, due to its recent development, there is a lack of independent studies that have examined this measure, and replication of this clock’s performance in other populations is needed.
To date, no epigenetic clock has been specifically developed as a predictor of cognitive aging or dementia risk, although studies of blood epigenetic biomarkers of dementia exist [Citation60,Citation61]. Efforts are warranted to continue exploiting epigenetic biomarkers, especially those with high sensitivity to cognitive aging, as well as the risk of dementia.
Strengths & limitations
This study comprehensively examined the evidence for an association between epigenetic aging and dementia and related outcomes, including MCI and cognitive performance. The strengths of this systematic review are that it was preregistered on the International Prospective Register of Ongoing Systematic Reviews (CRD42022320557) [Citation24] and was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (www.prisma-statement.org). This means that the systematic search was transparent, rigorous and reproducible. The authors used defined inclusion and exclusion criteria, and at least two authors independently screened the studies for eligibility and extracted relevant data, which has ensured that any bias in the presentation and inclusion of studies and data has been reduced. Two authors also independently assessed the risk of bias for the included studies using standardized checklists and found that there was generally a low risk of bias from eligible studies. A total of 11 epigenetic clocks, including the most commonly studied first-generation clocks and recently developed second-generation clocks, allowed the investigation of all currently available epigenetic clocks using peripheral tissues in this review.
However, there are also limitations to this review. First, after registering the review with a focus on only dementia and MCI, the authors expanded it to include cognitive performance. This outcome was not prespecified and therefore findings related to these outcomes cannot be considered as transparent or rigorous. Second, given the vast heterogeneity between the studies, in terms of the different subtypes of dementia and cognitive domains examined or even specific cognitive tests that assess similar domains, as well as the variability in epigenetic aging measures, the authors were not able to undertake a meta-analysis. Thus, this study presents only a qualitative summary of the findings rather than an overall effect estimate. The majority of the studies were undertaken in White or Caucasian populations, and thus it remains unclear whether these findings can be generalized to other ethnic/racial groups. Indeed, one of the studies that included participants that were both African–American and European–American found significant associations in the former group only [Citation38], indicating that this may be a factor that modifies the associations. In addition, although most studies included sex as a covariate of adjustment, only five studies performed sex-stratified analysis [Citation17,Citation21,Citation37,Citation40,Citation47], with only one largely identifying stronger associations among men [Citation37]. Given the sex difference in risk of dementia [Citation26] and cognitive decline [Citation62], as well as the epigenetic aging [Citation26], exploring the sex-specific associations between epigenetic clocks and cognitive performance as well as dementia may contribute to a better understanding of the relationship between biological and cognitive aging. Positive publication bias also remains an issue, whereby studies with significant findings are more likely to be published and thus included in this review. The possibility that such insignificant results exist would provide more weight toward a null association between epigenetic aging and cognition/dementia.
Conclusion
There is insufficient evidence to indicate that epigenetic aging, based on existing epigenetic clocks, is a biomarker of the risk of dementia or cognitive performance more broadly. However, the number of studies that have looked specifically at late-onset dementia, or Alzheimer’s disease, is low, and further large studies in multiethnic populations, as well as sex-stratified analyses, are required.
Future perspective
An epigenetic aging biomarker that accurately predicts dementia risk would hold great promise for early identification of risk and prevention. However, to date, the findings provide no strong support that epigenetic aging measured in blood, and using existing epigenetic clocks, is associated with dementia. Additional studies are required to determine whether newer epigenetic clocks specifically developed to predict age-related disease have the potential to be more accurate biomarkers of cognitive aging. Furthermore, better understanding the extent to which aging is a causal factor driving the risk of Alzheimer’s disease and related dementia could open up new opportunities for targeted interventions to slow aging and in turn reduce the burden of dementia.
A systematic search was conducted in MEDLINE, Embase and PsycINFO to identify articles meeting the eligibility criteria, according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Data were extracted from eligible studies and the risk of bias was assessed using Joanna Briggs Institute critical appraisal checklists.
The evidence for an association between epigenetic aging and cognitive performance in adulthood and dementia risk was synthesized qualitatively.
30 eligible articles with more than 20,000 participants were included.
Six studies of late-life dementia reported no strong evidence of an association with epigenetic aging.
Twenty studies investigated epigenetic aging and cognitive performance across a range of domains, including episodic memory, working memory, executive function, attention, verbal fluency and processing speed.
Most studies reported at least one significant association, but these differed depending on the cognitive domain and epigenetic clock measured, and overall there was a lack of consistent findings across studies.
Due to high heterogeneity across studies, a meta-analysis was not performed.
Based on existing epigenetic clocks, accelerated epigenetic aging is not associated with the risk of dementia; nor is it a good biomarker of cognitive performance.
However, newer epigenetic clocks specifically developed to predict age-related disease have the potential to be more accurate biomarkers of cognitive aging.
Author contributions
A Zhou and J Ryan made a substantial contribution to the conception and design of the work, interpretation of the data and drafting of the work. Z Wu, AZZ Phyo, D Torres and S Vishwanath were involved in the acquisition and analysis of the data and critical revision of the work for important intellectual content. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/epi-2022-0209
Financial & competing interests disclosure
Z Wu and AZZ Phyo are funded by a Research Training Program scholarship, awarded by Monash University and the Australian government. S Vishwanath is funded by a Monash University–King’s College PhD scholarship. J Ryan is funded by an NHMRC Dementia Research Leader Fellowship (APP1135727). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Dementia Key Facts. WHO, Geneva, Switzerland (2022).
- Abraha I , RimlandJM , TrottaFMet al. Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series. BMJ Open7(3), e012759 (2017).
- Hickman RA , FaustinA , WisniewskiT. Alzheimer disease and its growing epidemic: risk factors, biomarkers, and the urgent need for therapeutics. Neurol. Clin.34(4), 941–953 (2016).
- Horvath S . DNA methylation age of human tissues and cell types. Genome Biol.14(10), 3156 (2013).
- Slieker RC , Van ItersonM , LuijkRet al. Age-related accrual of methylomic variability is linked to fundamental ageing mechanisms. Genome Biol.17(1), 191 (2016).
- Hannum G , GuinneyJ , ZhaoLet al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell49(2), 359–367 (2013).
- Dugué PA , BassettJK , JooJEet al. DNA methylation-based biological aging and cancer risk and survival: pooled analysis of seven prospective studies. Int. J. Cancer142(8), 1611–1619 (2018).
- Grant CD , JafariN , HouLet al. A longitudinal study of DNA methylation as a potential mediator of age-related diabetes risk. Geroscience39(5–6), 475–489 (2017).
- Chen BH , MarioniRE , ColicinoEet al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY)8(9), 1844–1865 (2016).
- Marioni RE , ShahS , McRaeAFet al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol.16(1), 25 (2015).
- Horvath S , PirazziniC , BacaliniMGet al. Decreased epigenetic age of PBMCs from Italian semi-supercentenarians and their offspring. Aging (Albany NY)7(12), 1159–1170 (2015).
- Belsky DW , CaspiA , CorcoranDLet al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife11, e73420 (2022).
- Belsky DW , CaspiA , ArseneaultLet al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. eLIFEe54870, 9 (2020).
- Levine ME , LuAT , QuachAet al. An epigenetic biomarker of aging for lifespan and healthspan. Aging10(4), 573–591 (2018).
- Lu AT , QuachA , WilsonJGet al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY)11(2), 303–327 (2019).
- Horvath S , RitzBR. Increased epigenetic age and granulocyte counts in the blood of Parkinson’s disease patients. Aging7(12), 1130–1142 (2015).
- Paul KC , BinderAM , HorvathSet al. Accelerated hematopoietic mitotic aging measured by DNA methylation, blood cell lineage, and Parkinson’s disease. BMC Genomics22(1), 696 (2021).
- Degerman S , JosefssonM , NordinAdolfsson Aet al. Maintained memory in aging is associated with young epigenetic age. Neurobiol. Aging55, 167–171 (2017).
- Zhang M , TartagliaM , MorenoDet al. DNA methylation age acceleration is associated with disease duration and age at onset in C9orf72 patients. Amyotroph. Lateral Scler. Frontotemporal Degener.18(Suppl. 2), S50–S51 (2017).
- Sibbett RA , AltschulDM , MarioniRE , DearyIJ , StarrJM , RussTC. DNA methylation-based measures of accelerated biological ageing and the risk of dementia in the oldest-old: a study of the Lothian Birth Cohort 1921. BMC Psychiatry20(1), 91 (2020).
- Fransquet PD , LacazeP , SafferyRet al. Accelerated epigenetic aging in peripheral blood does not predict dementia risk. Curr Alzheimer Res.18(5), 443–451 (2021).
- Starnawska A , TanQ , LenartAet al. Blood DNA methylation age is not associated with cognitive functioning in middle-aged monozygotic twins. Neurobiol. Aging50, 60–63 (2017).
- Moher D , ShamseerL , ClarkeMet al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev.4(1), 1 (2015).
- Booth A , ClarkeM , GhersiD , MoherD , PetticrewM , StewartL. An international registry of systematic-review protocols. Lancet377(9760), 108–109 (2011).
- Fransquet PD , WrigglesworthJ , WoodsRL , ErnstME , RyanJ. The epigenetic clock as a predictor of disease and mortality risk: a systematic review and meta-analysis. Clin. Epigenet.11(1), 62 (2019).
- Horvath S , GurvenM , LevineMEet al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol.17(1), 171 (2016).
- The Joanna Briggs Institute . The Joanna Briggs Institute Reviewer’s Manual 2014.The Joanna Briggs Institute, Adelaide, Australia (2014).
- Shadyab AH , McEvoyLK , HorvathSet al. Association of epigenetic age acceleration with incident mild cognitive impairment and dementia among older women. J. Gerontol. A Biol. Sci. Med. Sci. doi:10.1093/gerona/glab245 (2021 (Epub ahead of print).
- Inkster AM , Duarte-GutermanP , AlbertAY , BarhaCK , GaleaLAM , RobinsonWP. Are sex differences in cognitive impairment reflected in epigenetic age acceleration metrics?Neurobiol. Aging109, 192–194 (2022).
- Chouliaras L , PishvaE , HaapakoskiRet al. Peripheral DNA methylation, cognitive decline and brain aging: pilot findings from the Whitehall II imaging study. Epigenomics10(5), 585–595 (2018).
- Lew BJ , SchantellMD , O’NeillJet al. Reductions in gray matter linked to epigenetic HIV-associated accelerated aging. Cereb. Cortex31(8), 3752–3763 (2021).
- Marioni RE , ShahS , McRaeAFet al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int. J. Epidemiol.44(4), 1388–1396 (2015).
- Wolf EJ , LogueMW , HayesJPet al. Accelerated DNA methylation age: associations with PTSD and neural integrity. Psychoneuroendocrinology63, 155–162 (2016).
- Belsky DW , MoffittTE , CohenAAet al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am. J. Epidemiol. 187(6), 1220–1230 (2018).
- Cruz-Almeida Y , SinhaP , RaniA , HuoZ , FillingimRB , FosterT. Epigenetic aging is associated with clinical and experimental pain in community-dwelling older adults. Mol. Pain15, 1744806919871819 (2019).
- Stevenson AJ , McCartneyDL , HillaryRFet al. Childhood intelligence attenuates the association between biological ageing and health outcomes in later life. Transl. Psychiatry9(1), 323 (2019).
- Beydoun MA , ShakedD , TajuddinSM , WeissJ , EvansMK , ZondermanAB. Accelerated epigenetic age and cognitive decline among urban-dwelling adults. Neurology94(6), e613–e625 (2020).
- Bressler J , MarioniRE , WalkerRMet al. Epigenetic age acceleration and cognitive function in African American adults in midlife: the Atherosclerosis Risk in Communities study. J. Gerontol. A Biol. Sci. Med. Sci.75(3), 473–480 (2020).
- Li X , PlonerA , WangYet al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife9, e51507 (2020).
- Maddock J , Castillo-FernandezJ , WongAet al. DNA methylation age and physical and cognitive aging. J. Gerontol. A Biol. Sci. Med. Sci.75(3), 504–511 (2020).
- Wiesman AI , RezichMT , O’NeillJet al. Epigenetic markers of aging predict the neural oscillations serving selective attention. Cereb. Cortex30(3), 1234–1243 (2020).
- Hillary RF , StevensonAJ , CoxSRet al. An epigenetic predictor of death captures multi-modal measures of brain health. Mol. Psychiatry26(8), 3806–3816 (2021).
- Park J , WonCW , SaliganLN , KimYJ , KimY , LukkahataiN. Accelerated epigenetic age in normal cognitive aging of Korean community-dwelling older adults. Biol. Res. Nurs.23(3), 464–470 (2021).
- Shiau S , ArpadiSM , ShenYet al. Epigenetic aging biomarkers associated with cognitive impairment in older African American adults with human immunodeficiency virus (HIV). Clin. Infect. Dis.73(11), 1982–1991 (2021).
- Shiau S , CantosA , RamonCVet al. Epigenetic age in young African American adults with perinatally acquired HIV. J. Acquir. Immune Defic. Syndr.87(4), 1102–1109 (2021).
- Vaccarino V , HuangM , WangZet al. Epigenetic age acceleration and cognitive decline: a twin study. J. Gerontol. A Biol. Sci. Med. Sci.76(10), 1854–1863 (2021).
- Zheng Y , HabesM , GonzalesMet al. Mid-life epigenetic age, neuroimaging brain age, and cognitive function: coronary artery risk development in young adults (CARDIA) study. Aging14(4), 1691–1712 (2022).
- Gross AM , JaegerPA , KreisbergJFet al. Methylome-wide analysis of chronic HIV infection reveals five-year increase in Biological age and epigenetic targeting of HLA. Mol. Cell62(2), 157–168 (2016).
- Weidner CI , LinQ , KochCMet al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol.15(2), R24 (2014).
- Livingston G , HuntleyJ , SommerladAet al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet396(10248), 413–446 (2020).
- Jack CR Jr , BennettDA , BlennowKet al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement.14(4), 535–562 (2018).
- Mattson MP , ArumugamTV. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell. Metab.27(6), 1176–1199 (2018).
- Fraga MF , BallestarE , PazMFet al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl Acad. Sci. USA102(30), 10604–10609 (2005).
- Jones MJ , GoodmanSJ , KoborMS. DNA methylation and healthy human aging. Aging Cell14(6), 924–932 (2015).
- Johansson A , EnrothS , GyllenstenU. Continuous aging of the human DNA methylome throughout the human lifespan. PLOS ONE8(6), e67378 (2013).
- López-Otín C , BlascoMA , PartridgeL , SerranoM , KroemerG. The hallmarks of aging. Cell153(6), 1194–1217 (2013).
- Ryan J , WrigglesworthJ , LoongJ , FransquetPD , WoodsRL. A systematic review and meta-analysis of environmental, lifestyle, and health factors associated with DNA methylation age. J. Gerontol. A Biol. Sci. Med. Sci.75(3), 481–494 (2020).
- Lima CNC , SuchtingR , ScainiGet al. Epigenetic GrimAge acceleration and cognitive impairment in bipolar disorder. Eur. Neuropsychopharmacol.62, 10–21 (2022).
- Sugden KA-O , CaspiAA-O , ElliottMA-Oet al. Association of pace of aging measured by blood-based DNA methylation with age-related cognitive impairment and dementia. Neurology. doi:10.1212/WNL.0000000000200898 (2022) ( Epub ahead of print).
- Fransquet PD , LacazeP , SafferyRet al. Blood DNA methylation signatures to detect dementia prior to overt clinical symptoms. Alzheimers Dement. (Amst.)12(1), e12056 (2020).
- Pérez RF , Alba-LinaresJJ , TejedorJRet al. Blood DNA methylation patterns in older adults with evolving dementia. J. Gerontol. A Biol. Sci. Med. Sci. doi:10.1093/gerona/glac068 (2022) (Epub ahead of print).
- McCarrey AC , AnY , Kitner-TrioloMH , FerrucciL , ResnickSM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol. Aging31(2), 166–175 (2016).