ABSTRACT
Aims: We evaluated the efficacy and safety of erlotinib, and patient characteristics affecting progression-free survival (PFS), by analyzing data from two Phase II studies of first-line erlotinib in activating EGFR mutation-positive non-small-cell lung cancer. Methods: Data were combined from patients who received first-line erlotinib monotherapy in JO22903 (single-arm study; JapicCTI-101085) and JO25567 (randomized study; JapicCTI-111390). Results: Median PFS was 10.9 months in efficacy-evaluable patients (n = 177). Major adverse events were dermatologic; no new safety signals were observed. Baseline pleural/cardiac effusion notably affected PFS (yes median 8.0 months vs no median 15.3 months) as confirmed in multivariate analysis (hazard ratio: 0.38; 95% CI: 0.25–0.58). Conclusion: Efficacy and safety of erlotinib monotherapy were consistent with previous studies. Baseline pleural/pericardial effusion was associated with shorter PFS.
PFS: Progression-free survival.
ECOG PS: Eastern Cooperative Oncology Group performance status; HR: Hazard ratio; PFS: Progression-free survival.
HR: Hazard ratio; PFS: Progression-free survival.
Lung cancer is the most common cause of cancer-related deaths worldwide [Citation1]. In Japan, an estimated 94,855 cases of lung cancer were reported in 2012, with an estimated 75,120 deaths due to the disease [Citation2]. Recent advances in molecular translational research have led to breakthroughs in the treatment and diagnosis of lung cancer. One of the most researched genetic alterations in non-small-cell lung cancer (NSCLC) has been mutations of the EGFR gene. The EGFR family receptor tyrosine kinases are responsible for the regulation of developmental, metabolic and physiologic processes. EGFR-activating mutations occur more frequently in Asian-Pacific patients than in European patients, with observed mutation rates of 20–76% versus 6–41%, respectively [Citation3]. The development of EGFR tyrosine kinase inhibitors (TKIs) to target these mutations has led to significant progress in the treatment of NSCLC.
Current clinical guidelines recommend EGFR TKIs for the treatment of EGFR mutation-positive NSCLC [Citation4,Citation5]. Three EGFR TKIs, erlotinib, gefitinib and afatinib, are approved in the USA, EU and Japan for the first-line treatment of patients with this distinct subset of NSCLC. In seven randomized Phase III trials, these EGFR TKIs consistently showed significant progression-free survival (PFS) benefits compared with standard chemotherapy in patients with advanced NSCLC whose tumors harbored activating EGFR mutations [Citation6]. In Asian patients with activating EGFR mutation-positive NSCLC, first-line erlotinib, gefitinib and afatinib also showed improved clinical outcomes compared with chemotherapy [Citation7–9]. Furthermore, in the Phase II, single-arm, JO22903 study (JapicCTI-101085) in Japanese patients whose tumors harbored activating EGFR mutations, erlotinib monotherapy provided a median PFS (primary end point) of 11.8 months (95% CI: 9.7–15.3) [Citation10]. Similarly, the Phase II, JO25567 (JapicCTI-111390) randomized study demonstrated a median PFS (primary end point) of 16.0 months (95% CI: 13.9–18.1) versus 9.7 months (95% CI: 5.7–11.1), respectively, for erlotinib plus bevacizumab versus erlotinib alone; hazard ratio (HR): 0.54; 95% CI: 0.36–0.79; p = 0.0015 [Citation11].
Although EGFR TKI monotherapy has demonstrated good clinical efficacy in the first-line treatment of EGFR mutation-positive NSCLC, not all patients benefit to the same extent. The association between poor efficacy with EGFR TKIs and baseline characteristics of patients with activating EGFR mutation-positive NSCLC is still not well known. Identifying this link could be valuable for the development of more effective EGFR TKI treatment strategies and for designing future clinical trials in activating EGFR mutation-positive NSCLC. In addition, the pattern of tumor progression and metastasis, especially in the brain, could affect available treatment options and patient prognosis. Data from prospective studies on the pattern of disease progression following first-line EGFR TKI treatment are limited. For these reasons, we sought to evaluate the efficacy and safety of erlotinib more precisely by performing a combined analysis of the JO22903 and JO25567 study data, with particular focus on the sites of progression following first-line erlotinib monotherapy and on potential factors that could affect erlotinib efficacy.
Methods
Study design & patients
This combined analysis only included patients who were receiving first-line erlotinib monotherapy in study JO22903 or JO25567.
JO22903 (single arm [Citation10]) and JO25567 (randomized [Citation11]) were Phase II, multicenter, open-label studies conducted at 25 and 30 centers in Japan, respectively. Eligible patients were aged ≥20 years, had not received prior chemotherapy, and had advanced, untreated, metastatic stage IIIB/IV or recurrent NSCLC with Eastern Cooperative Oncology Group performance status (ECOG PS) 0 or 1. Patients with tumors harboring confirmed activating mutations of EGFR (exon 19 deletion or L858R point mutation in exon 21) with at least one measurable lesion according to Response Evaluation Criteria in Solid Tumors (RECIST; v.1.0 for JO22903 or v.1.1 for JO25567) were enrolled. Patients were excluded in JO25567 if they had EGFR T790M mutation, squamous cell carcinoma or brain metastases. Patients enrolled in JO22903 whose tumors harbored T790M mutations were excluded from this combined analysis in order to align the patient groups between the two studies.
In total, 103 patients were enrolled in JO22903 between April 2010 and October 2010, and 77 patients were enrolled in the erlotinib monotherapy arm of JO25567 between February 2011 and March 2012. In the combined analysis, 178 patients were included in the safety population, 101 from JO22903 and 77 from JO25567 (two patients from JO22903 who had EGFR T790M mutations were excluded). One patient from JO22903 was excluded from the efficacy population due to a major protocol violation, leaving 177 efficacy-evaluable patients. Both studies were carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol for each study was approved by the appropriate ethics committees and all patients gave informed consent for study participation.
Procedures
Eligible patients received oral erlotinib 150 mg/day until disease progression (PD) or unacceptable toxicity in JO22903 and the erlotinib monotherapy arm of JO25567. In JO25567, patients were randomly allocated in a 1:1 ratio to receive erlotinib (150 mg/day) plus bevacizumab (15 mg/kg) or erlotinib alone (150 mg/day) using a dynamic allocation method. Patients were screened for EGFR mutations in a local or central laboratory according to standard testing practice. Further study details have been reported in the primary manuscripts [Citation10,Citation11].
Assessments
Tumor response was assessed by an independent review committee using RECIST. Tumor response evaluation was scheduled every 6 weeks. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTC AE) v. 4.0. Safety was assessed according to the Medical Dictionary for Regulatory Activities (MedDRA; v.13.0 for JO22903 and v.14.0 for JO25567) preferred terms and tabulated by grade. At baseline, mandatory lung and abdominal scans (computerized tomography [CT]/MRI), brain scans (CT/MRI) and bone scans (bone scintigraphy, PET, CT or MRI) were performed.
Study end points
The primary end point in both studies was independent review committee-assessed PFS according to RECIST. Secondary end points included overall response rate (ORR), disease control rate (DCR), overall survival (OS) and safety.
Statistical analyses
Kaplan–Meier methodology was used to assess median PFS; 95% CI limit was calculated using the Greenwood method; HRs were estimated by Cox model. Individual patient data from JO22903 and the erlotinib monotherapy arm of JO25567 were combined. Univariate Cox regression analysis of PFS was conducted by baseline characteristics; age, EGFR mutation type, stage, gender, smoking status, ECOG PS, sum of the longest diameter of target lesions, number of affected organs and pleural/pericardial effusions (PPE). Multivariate Cox regression was used to estimate HRs for risk of PD associated with each baseline characteristic. All patients with activating EGFR mutations who received at least one dose of study treatment (excluding two patients with EGFR T790M mutations) were included in the safety population. Patients with major protocol violations were excluded from the efficacy population. Statistical analyses were performed using SAS v. 9.2.
Results
Patient population
Baseline characteristics for the combined population are shown in . There were some differences between the two populations in terms of the proportion of patients with ≥3 or <3 affected organs and the proportion of patients with PPE at baseline. The majority of patients were female (n = 118; 67%), aged <75 years (n = 148; 84%) and were nonsmokers (n = 102; 58%). Median age was 66 years (range: 36–86), 87 patients (49%) had an EGFR L858R mutation and 90 patients (51%) had an EGFR exon 19 deletion. A total of 69 patients (39%) had PPE and 20 patients (11%) had brain metastases at baseline.
Efficacy
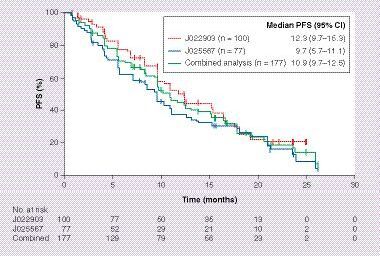
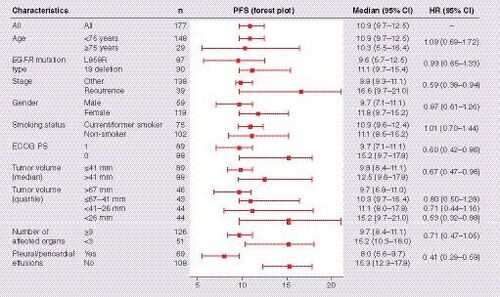
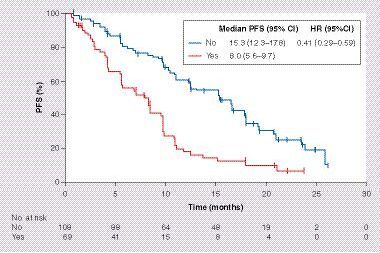
Median PFS with first-line erlotinib monotherapy was 10.9 months (95% CI: 9.7–12.5) for the combined analysis compared with 12.3 months (95% CI: 9.7–15.3) and 9.7 months (95% CI: 5.7–11.1) for the individual JO22903 and JO25567 study populations, respectively (). ORR was 73% (95% CI: 66–79) and DCR was 92% (95% CI: 87–96). Univariate Cox regression analysis for PFS is shown in . Despite many patient characteristics showing improvements in PFS, only ECOG PS (HR: 0.67; 95% CI: 0.45–0.99; p = 0.046) and PPE at baseline (HR: 0.38; 95% CI: 0.25–0.58; p < 0.0001) were significant risk factors for PFS in multivariate analysis ( & ). Specific EGFR mutations, being female, having smaller tumor diameter and fewer affected organs appeared to be associated with improved PFS in the multivariate analysis, but these associations were not statistically significant.
ORR and DCR in patients with PPE were 61 and 86%, respectively, versus 81 and 96%, respectively, in patients without PPE.
Common sites of progression are shown in . The majority of patients had PD in the lung (n = 101; 81%), lymph nodes (n = 31; 25%) and bone (n = 20; 16%). Progression of brain metastases was observed in six patients (4.8%) who had PD. Of these six patients, three had brain metastases at baseline and three patients did not (); two of the patients had carcinomatous meningitis at PD.
Safety
AEs reported in >20% of patients in the safety population, or AEs considered to be of special interest, are presented in . The most common AEs were rash (99% of patients at any grade, 19% at grade ≥3), and diarrhea (79% of patients at any grade, 1% at grade ≥3). Interstitial lung disease was reported in 5% (any grade) and 2% (grade ≥3) of patients.
Discussion
EGFR TKIs are the standard of care for the treatment of EGFR mutation-positive NSCLC and are recommended in clinical guidelines [Citation4,Citation5]. Erlotinib has shown significantly improved clinical outcomes compared with standard chemotherapy in the first-line treatment setting for EGFR mutation-positive NSCLC [Citation7,Citation12–13]. The Phase II JO22903 and JO25567 studies were prospectively designed to investigate erlotinib for the first-line treatment of Japanese patients with EGFR mutation-positive NSCLC [Citation10,Citation11].
In this combined analysis of JO22903 and JO25567, first-line erlotinib monotherapy had a favorable efficacy and safety profile in Japanese patients with EGFR mutation-positive NSCLC. This analysis revealed a median PFS of 10.9 months, which is in line with Phase III studies of first-line erlotinib in EGFR mutation-positive NSCLC; a median PFS of 13.1 and 11.0 months, respectively, was reported in the OPTIMAL trial [Citation7] and in the ENSURE trial [Citation13], both enrolling Chinese patients, compared with 10.4 months in the EURTAC trial [Citation12], which enrolled European patients. Also, first-line erlotinib monotherapy was well tolerated with an acceptable safety profile in patients with EGFR mutation-positive NSCLC. The safety profile of erlotinib was as expected, with rash and diarrhea being the most common AEs in this combined analysis, which were in line with previous Phase III studies of erlotinib [Citation6].
An advantage of this combined analysis is that both of the studies were conducted during the same time period, solely in Japanese patients, such that all patients were treated under almost identical medical conditions. As combined analyses are useful in terms of increased precision, due to a larger sample size, the results of this study provide further support for erlotinib monotherapy as an effective treatment option for first-line EGFR mutation-positive NSCLC.
It is well known that certain patient groups respond better to EGFR TKIs. These include female patients, nonsmokers and patients who have tumors with EGFR-activating mutations [Citation14]. However, the clinical factors that could affect the efficacy of EGFR TKIs are still unclear. In multivariate Cox regression analysis, PPE status was identified as an independent risk factor for PFS. Although there has been a wealth of data on EGFR TKIs in patients whose tumors harbor EGFR-activating mutations, few studies have reported data in patients with pleural effusions [Citation15]. In a study of 150 patients from Taiwan with pleural effusion, mean age 68.2 years (±13.7 years), who received EGFR TKIs as first-line treatment, median PFS was 9.6 months in 59 patients with L858R mutation or EGFR exon 19 deletion [Citation16]. Pleural effusion was associated with decreased survival in patients with distant metastases [Citation17]. Furthermore, in a retrospective study of 283 patients with lung adenocarcinoma from China, median age 59 years (range: 30–81), there was positive association between activating EGFR mutations and pleural effusion [Citation18]. A number of preclinical findings also support these clinical observations. VEGF expression contributes to EGFR TKI efficacy [Citation19] and has also been implicated as a critical cytokine in pleural effusion pathogenesis [Citation20]. Furthermore, mutant EGFR tumor has itself been shown to promote malignant pleural effusion formation via activation of the C-X-C motif chemokine ligand pathway [Citation21]. To our knowledge, the results of this combined analysis are the first to suggest that the absence of PPE may be a predictive factor of improved efficacy with erlotinib. These data suggest that more efficacious treatment strategies with EGFR TKIs are desired in patients with PPE who have activating EGFR mutations. It should be noted that due to the absence of a control arm in this combined analysis, any predictive factors could not be statistically evaluated against placebo. Therefore, as similar studies looking at erlotinib monotherapy for patients with PPE are limited, these findings should be examined more closely in prospective studies.
Although the pattern of tumor progression, particularly in the brain, following EGFR TKI treatment could affect subsequent treatment options and patient prognosis, data are still limited. In a retrospective Japanese study of 92 patients, the development of CNS metastases after acquired resistance to EGFR TKIs in patients with activating EGFR mutations was associated with poor prognosis, which suggested that controlling CNS metastases is important to improve OS [Citation22]. In previous reports, brain or leptomeningeal metastases as the initial PD site were present in >30% of patients who had an objective response to gefitinib (median age: 64 years [range: 38–87 years]) [Citation23]. Other studies have reported that brain metastases were present in 8% of patients as the initial site of PD following EGFR TKI therapy (gefitinib: n = 11; erlotinib: n = 90) [Citation24]. In our combined analysis, brain lesions were well controlled by erlotinib, with progression in the brain observed in only 4.8% of patients who had PD, which is consistent with previous reports [Citation24]. However, a limitation of these analyses is that the status of metastases at baseline may differ between studies, which could affect the pattern of progression. These results should be validated further in prospective studies.
Conclusion
Combined analyses of the Phase II JO22903 and JO25567 studies revealed that first-line erlotinib monotherapy demonstrates favorable efficacy and safety in Japanese patients with EGFR mutation-positive NSCLC. Exploratory analysis showed that erlotinib controls brain lesions well and that the absence of PPE was associated with a better PFS; these findings suggest that the efficacy of EGFR TKIs will be affected by disease status and some patients, such as those with PPE, may require more intensive therapy.
Table 1. Baseline characteristics.
Table 2. Multivariate Cox regression† for progression-free survival.
Table 3. Sites of progression in patients who had disease progression following EGFR tyrosine kinase inhibitor treatment (n = 125).
Table 4. Patient characteristics of six patients who had CNS progression.
Table 5. Adverse events with an incidence of >20% or considered adverse events of special interest.
Executive Summary
Erlotinib is a standard of care for the treatment of EGFR mutation-positive non-small-cell lung cancer (NSCLC) with significantly improved clinical outcomes compared with chemotherapy in the first-line treatment setting.
The Phase II JO22903 and JO25567 studies demonstrated that first-line erlotinib monotherapy is both effective and tolerable in Japanese patients with EGFR mutation-positive NSCLC.
A median progression-free survival of 10.9 months with erlotinib monotherapy was reported in this combined analysis of the JO22903 and JO25567 studies and was in line with previous Phase III studies of first-line erlotinib for EGFR mutation-positive NSCLC.
The absence of pleural/pericardial effusions was associated with a better progression-free survival.
Brain lesions were well controlled by erlotinib with progression in the brain observed in only 4.8% of patients who had progressive disease.
Erlotinib monotherapy had a tolerable and manageable safety profile.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved. The protocol for each study was approved by the appropriate ethics committees and all patients gave informed consent for study participation.
Acknowledgements
The authors would like to thank F Fernando, PhD, of Gardiner-Caldwell Communications for medical writing assistance, and all participating physicians and registered patients.
Financial & competing interests disclosure
S Atagi has received lecture fees and research funding from Chugai Pharmaceutical Co. Ltd; K Goto has received lecture fees and research funding from Chugai Pharmaceutical Co. Ltd. and has served on an advisory board for them; T Seto has received lecture fees or research funding from Astellas Pharma, AstraZeneca, Bayer Yakuhin, Chugai Pharmaceutical Co. Ltd, Daiichi Sankyo, Eisai, Eli Lilly Japan, Fuji Pharma, Hisamitsu Pharmaceutical, Kyowa Hakko Kirin, Mochida Pharmaceutical, Merck Serono, MSD, Nippon Boehringer Ingelheim, Nippon Kayaku, Novartis Pharma, Onol Pharmaceutical, Pfizer Japan, Roche Diagnostics, Sanofi, Showa Yakuhin Kako, Sumitomo Dainippon Pharma, Taiho Pharmaceutical, Takeda Pharmaceutical, Verastem, and Yakult Honsha; N Yamamoto has received lecture fees or research funding from AstraZeneca, Chugai Pharmaceutical Co. Ltd, and Nippon Boehringer Ingelheim; T Tamura has received lecture fees from Chugai Pharmaceutical Co. Ltd, and Nippon Boehringer Ingelheim; K Tajima and N Inagaki are employees of Chugai Pharmaceutical Co. Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance was provided by F Fernando, PhD, of Gardiner-Caldwell Communications and funded by Chugai Pharmaceutical Co. Ltd.
References
- Jemal A , BrayF, CenterMM, FerlayJ, WardE, FormanD. Global cancer statistics. CA Cancer J. Clin.61(2), 69–90 (2011).
- GLOBOCAN . Estimated cancer incidence, mortality and prevalence worldwide in 2012 (2015). http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- Midha A , DeardenS, McCormackR. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am. J. Cancer Res.5(9), 2892–2911 (2015).
- National Clinical Cancer Network . NCCN Clinical Practice Guidelines for non-small-cell lung cancer. V6.2015. www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- Reck M , PopatS, ReinmuthNet al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.25(Suppl. 3), iii27–iii39 (2014).
- Sgambato A , CasaluceF, MaionePet al. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non-small-cell lung cancer patients harboring EGFR mutation. Curr. Med. Chem.19(20), 3337–3352 (2012).
- Zhou C , WuYL, ChenGet al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, Phase 3 study. Lancet Oncol.12(8), 735–742 (2011).
- Mitsudomi T , MoritaS, YatabeYet al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised Phase 3 trial. Lancet Oncol.11(2), 121–128 (2010).
- Wu YL , ZhouC, HuCPet al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised Phase 3 trial. Lancet Oncol.15(2), 213–222 (2014).
- Goto K , NishioM, YamamotoNet al. A prospective, Phase II, open-label study (JO22903) of first-line erlotinib in Japanese patients with epidermal growth factor (EGFR) mutation-positive advanced non-small-cell lung cancer (NSCLC). Lung Cancer82(1), 109–114 (2013).
- Seto T , KatoT, NishioMet al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, Phase 2 study. Lancet Oncol.15(11), 1236–1244 (2014).
- Costa C , MolinaMA, DrozdowskyjAet al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized Phase III EURTAC trial. Clin. Cancer Res.20(7), 2001–2010 (2014).
- Wu YL , ZhouC, LiamCKet al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the Phase III, randomized, open-label, ENSURE study. Ann. Oncol.26(9), 1883–1889 (2015).
- Markman M . Genetics of non-small-cell lung cancer. Medscape, 2014. http://emedicine.medscape.com/article/1689988-overview.
- Froudarakis ME . Pleural effusion in lung cancer: more questions than answers. Respiration83(5), 367–376 (2012).
- Tsai TH , SuKY, WuSGet al. RNA is favourable for analysing EGFR mutations in malignant pleural effusion of lung cancer. Eur. Respir. J.39(3), 677–684 (2012).
- Morgensztern D , WagarS, SubramanianJ, TrinkausK, GovindanR. Prognostic impact of malignant pleural effusion at presentation in patients with metastatic non-small-cell lung cancer. J. Thorac. Oncol.7(10), 1485–1489 (2012).
- Zou J , BellaAE, ChenZet al. Frequency of EGFR mutations in lung adenocarcinoma with malignant pleural effusion: implication of cancer biological behaviour regulated by EGFR mutation. J. Int. Med. Res.42(5), 1110–1117 (2014).
- Naumov GN , NilssonMB, CasconeTet al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin. Cancer Res.15(10), 3484–3494 (2009).
- Bradshaw M , MansfieldA, PeikertT. The role of vascular endothelial growth factor in the pathogenesis, diagnosis and treatment of malignant pleural effusion. Curr. Oncol. Rep.15(3), 207–216 (2013).
- Tsai MF , ChangTH, WuSGet al. EGFR-L858R mutant enhances lung adenocarcinoma cell invasive ability and promotes malignant pleural effusion formation through activation of the CXCL12-CXCR4 pathway. Sci. Rep.5, 13574 (2015).
- Hata A , KatakamiN, YoshiokaHet al. Prognostic impact of central nervous system metastases after acquired resistance to EGFR-TKI: poorer prognosis associated with T790M-negative status and leptomeningeal metastases. Anticancer Res.35(2), 1025–1031 (2015).
- Omuro AM , KrisMG, MillerVAet al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer103(11), 2344–2348 (2005).
- Heon S , YeapBY, LindemanNIet al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small-cell lung cancer with EGFR mutations. Clin. Cancer Res.18(16), 4406–4414 (2012).
