Abstract
Aim: This real-world analysis evaluated docetaxel plus nintedanib in patients with advanced pulmonary adenocarcinoma after chemotherapy and immune checkpoint inhibitor failure, for whom treatment options are limited. Methods: Data were sourced retrospectively from seven German centers. Results: Of 93 patients, overall response rate was 41.4% (disease control rate: 75.9%). Of 57 patients given third-line docetaxel plus nintedanib, overall response rate was 50.0% (disease control rate: 82.7%). Median overall survival following third-line docetaxel plus nintedanib was 8.4 months. Adverse events were consistent with the known safety profile of docetaxel plus nintedanib. Conclusion: To date, this was the largest retrospective, real-world analysis of docetaxel plus nintedanib after chemotherapy–immunotherapy failure, indicating that docetaxel plus nintedanib offers meaningful clinical benefits in this setting.
Lay abstract
The standard of care for patients with lung adenocarcinoma has advanced with the introduction of immunotherapy in the first-line setting. However, limited clinical data are available to help guide treatment decisions after failure of chemotherapy and immunotherapy. Nintedanib is an oral antiangiogenic agent that is approved in the EU and other countries in combination with docetaxel for the treatment of patients with advanced/metastatic lung adenocarcinoma after first-line chemotherapy. This study is a retrospective, real-world analysis of docetaxel plus nintedanib in 93 patients with advanced lung adenocarcinoma who progressed on immunotherapy (either in sequence or in combination with chemotherapy). The results suggest that docetaxel plus nintedanib offers a meaningful clinical benefit in this setting. Safety findings were generally consistent with the known safety profile of docetaxel plus nintedanib.
The introduction of immune checkpoint inhibitors (ICIs) has radically altered the first-line treatment paradigm for advanced/metastatic non-small-cell lung cancer (NSCLC) without driver mutations, prolonging progression-free survival (PFS) and overall survival (OS) compared with previous standard-of-care options [Citation1–4]. ICIs are now recommended by international treatment guidelines in this disease subgroup [Citation5,Citation6]. Depending on PD-L1 expression level, ICIs can be administered as monotherapy or in combination with chemotherapy [Citation5,Citation6]. However, despite these achievements in the first-line setting, there is a continuing need for a clear strategy in the second-line setting and beyond [Citation7,Citation8].
Changes in the tumor microenvironment (TME) are growing in recognition as a mechanism of resistance to anti-PD-1/PD-L1 ICIs [Citation8]; therefore, targeting aspects of the TME may prove to be an effective therapeutic approach in the post-ICI setting [Citation7]. Vascular abnormalities in the TME arise as a result of disrupted expression of pro-angiogenic factors, such as VEGF, angiopoietin 2, FGF and PDGF [Citation8–10], and sustained angiogenesis is a well-described hallmark of cancer [Citation8]. VEGF also affects immunosuppression as abnormal vessels, and impaired perfusion can restrict the ability of cytotoxic drugs and immune cells to enter tumors [Citation8,Citation10].
Considering the evidence supporting a role for abnormal angiogenesis in creating an immunosuppressed TME, it follows that using antiangiogenic agents could restore a more immunosupportive environment [Citation8]. Antiangiogenic agents such as nintedanib or ramucirumab, in combination with docetaxel, are included in treatment guidelines as potential treatment options from the second-line setting onward after failure of first-line chemotherapy [Citation5,Citation6,Citation11–13]. The use of antiangiogenic agents is well established in several types of metastatic cancer [Citation14–16]. This hypothesis also supports the use of angiokinase inhibitors following failure of first-line combination chemotherapy-ICI regimens in patients with advanced NSCLC.
Nintedanib is a small molecule, oral, triple angiokinase inhibitor targeting the tyrosine kinase activities of VEGFR1-3, PDGFRα and β and FGFR1-3 [Citation17–19]. In the EU, nintedanib is approved in combination with docetaxel for the treatment of adult patients with locally advanced, metastatic or locally recurrent NSCLC of adenocarcinoma tumor histology after first-line chemotherapy [Citation17]. The combination of docetaxel plus nintedanib has been shown to be an effective treatment option for advanced pulmonary adenocarcinoma initially treated with one line of platinum-based therapy in a randomized clinical trial (LUME-Lung 1), as well as several noninterventional real-world studies [Citation7,Citation12,Citation13,Citation20–22].
Here, we present a retrospective, real-world analysis of docetaxel plus nintedanib in patients with advanced/metastatic pulmonary adenocarcinoma after progression on an immune checkpoint inhibitor (either in sequence or in combination with an approved chemotherapy regimen).
Materials & methods
Patients
This was a retrospective analysis using real-world data collected from seven hospital sites located in North Rhine-Westphalia, Germany: Bethanien Hospital Moers, Evang. Kliniken Essen-Mitte, Marien-Hospital Wesel, Onkologische Gemeinschaftspraxis and Tagesklinik, University Hospital RWTH Aachen, University Hospital Cologne and University Hospital Essen.
The analysis included patients with advanced/metastatic NSCLC of adenocarcinoma histology who had received treatment with docetaxel plus nintedanib between July 2016 and February 2020 after therapy with a platinum-containing schedule combined with or followed by ICI therapy.
Patients were identified by chart review. Data were collected between September 2019 and February 2020, with a cut-off date for analyses of March 2020.
Treatment
Treatments were administered according to each center's routine protocols. Patients received docetaxel 75 mg/m2 intravenously (iv.) on day 1 of each 21-day treatment cycle alongside nintedanib 200 mg (oral) twice daily on days 2–21 of each 21-day treatment cycle.
In cases where the standard 3-weekly dose schedule for docetaxel was not appropriate due to tolerability concerns, a modified weekly schedule (30 mg/mg2 iv.) was administered. Weekly docetaxel administration in combination with nintedanib is now supported by favorable safety and efficacy data from the recent SENECA trial [Citation23]. In these patients, nintedanib was administered on the day after docetaxel infusion. Individual dose reductions of nintedanib, when required, were carried out according to the approved label.
Assessments
Responses were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Adverse events were recorded during routine clinical visits and were categorized and graded according to the Common Terminology Criteria for Adverse Events version 4.0.
Data analysis & end points
Key outcomes included overall response rate (ORR) and disease control rate (DCR), which were based on investigator-assessed end points of complete response, partial response, stable disease and progressive disease.
OS and duration of treatment were measured from the start of docetaxel plus nintedanib treatment. Time to event data were analyzed using Kaplan-Meier techniques.
Adverse events recorded during clinical visits were retrospectively analyzed from the chart review.
Ethics
This retrospective data analysis was approved by the appropriate institutional ethics committee for each center. All patients received docetaxel plus nintedanib in accordance with the approved EMA label or in line with local best-practice guidelines (as described earlier).
Where possible, all patients provided informed, written consent at the time of data collection. Data relating to deceased patients were collected in accordance with German data protection laws. Confidentiality of patient data and omission of any identifying information was maintained throughout this investigation.
Results
Patient characteristics & treatment
A total of 93 patients were included in this retrospective analysis. Patient demographics, including a breakdown of previous therapies, are outlined in . Eastern Cooperative Oncology Group performance status (ECOG PS) was 0 in 43 patients (46.2%), 1 in 43 patients (46.2%) and 2 in 7 patients (7.5%).
Table 1. Patient characteristics.
The majority of patients included in this analysis (n = 57 [61.3%]) received docetaxel plus nintedanib as third-line therapy, following first-line chemotherapy and second-line ICIs. Other treatment sequences represented in this dataset included second-line docetaxel plus nintedanib after a first-line combination chemotherapy-ICI regimen (n = 10 [10.8%]), third-line docetaxel plus nintedanib after first-line pembrolizumab monotherapy and second-line chemotherapy (n = 6 [6.5%]), and second-line docetaxel plus nintedanib after first-line pembrolizumab monotherapy (n = 1 [1.1%]). An additional 19 patients (20.4%) received docetaxel plus nintedanib as fourth- or later-line treatment.
The majority of patients (n = 84 [90.3%]) commenced treatment with the recommended schedule of docetaxel (75 mg/m2 on day 1) and nintedanib (200 mg 2-0-2 [two capsules morning and evening] on days 2-21). Eight patients (8.6%) received docetaxel on a weekly schedule (30 mg/m2). One patient started with a reduced dose of docetaxel (60 mg/m2 on day 1), and eight patients (8.6%) started with a reduced dose of nintedanib (six patients at 100 mg 2-0-2, and two patients at 150 mg 2-0-2).
Efficacy
Of 93 total patients, responses were evaluable for 87 patients (93.5%). Response rates (RRs) were not evaluable for six patients (6.5%) due to early therapy discontinuation. ORR for all evaluable patients was 41.4% (n = 36), with a DCR of 75.9% (n = 66) ().
Table 2. Efficacy of docetaxel plus nintedanib post-immune checkpoint inhibitor.
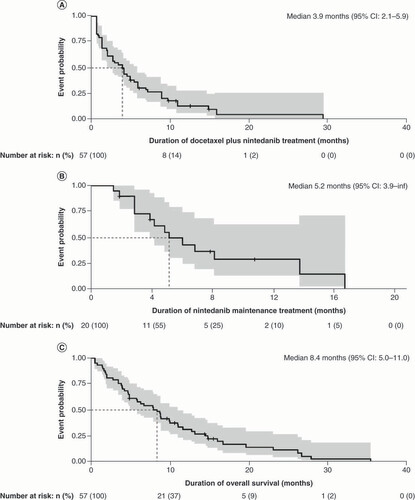
The highest RR was observed in patients who received third-line docetaxel plus nintedanib following first-line chemotherapy and second-line ICIs, with an ORR of 50.0% (n = 26) and a DCR of 82.7% (n = 43). Patients spent a median time of 3.9 months (5.7 treatment cycles) on third-line treatment with docetaxel plus nintedanib (95% CI: 2.1–5.9 months []). In total, 35.1% of these patients (n = 20) continued on nintedanib maintenance therapy for a median maintenance treatment duration of 5.2 months (95% CI: 3.9–infinity []). All discontinuations were due to tolerability or physician decision. Median OS for patients who received third-line treatment with docetaxel plus nintedanib was 8.4 months from the start of third-line treatment (95% CI: 5.0–11.0 []).
Kaplan-Meier curves illustrating the third-line treatment outcomes with docetaxel plus nintedanib following first-line chemotherapy and second-line immune checkpoint inhibitors, representing (A) treatment duration with docetaxel plus nintedanib (n = 57), (B) maintenance treatment with nintedanib (n = 20) and (C) overall survival following third-line docetaxel plus nintedanib treatment (n = 57).
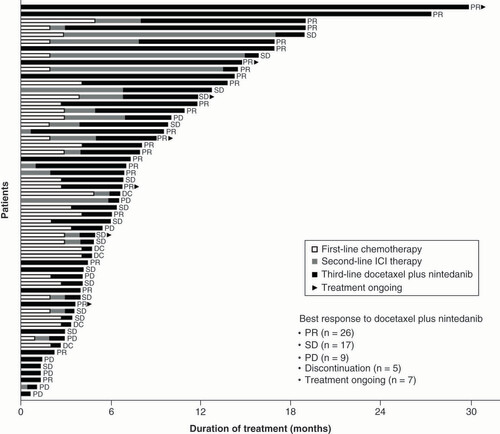
In 19 patients given docetaxel plus nintedanib as fourth- or later-line treatment, an ORR of 31.6% was reported (n = 6) with a DCR of 68.4% (n = 13). Of 10 evaluable patients who received second-line docetaxel plus nintedanib after first-line pembrolizumab-chemotherapy combination therapy, ORR was 40.0% (n = 4) and DCR was 50.0% (n = 5). Time on current and previous treatment varied by patient (). Analyses of treatment duration, maintenance therapy and OS for the full population (docetaxel plus nintedanib across all treatment lines) are presented in .
Data presented for 57 patients who received third-line docetaxel plus nintedanib. Treatment duration for first-line therapy was not available for 23 patients. Treatment duration for second-line therapy was not available for 33 patients.
DC: Discontinuation; ICI: Immune checkpoint inhibitor; PD: Progressive disease; PR: Partial response; SD: Stable disease.
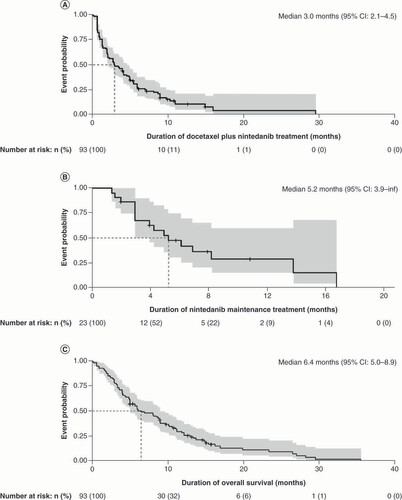
Kaplan-Meier curves illustrating the overall treatment outcomes with docetaxel plus nintedanib across all treatment lines included in the analysis, representing (A) duration of treatment with docetaxel plus nintedanib (n = 93), (B) maintenance treatment with nintedanib (n = 23) and (C) overall survival following treatment with docetaxel plus nintedanib (n = 93).
Safety
Safety findings were generally consistent with the known safety profile of docetaxel plus nintedanib in patients with advanced NSCLC. Across all 93 patients, the most frequently described adverse events were diarrhea (n = 17), skin reactions (which included hand-foot syndrome, dermatitis and rash; n = 7), and neuropathy (n = 6). Hand-foot syndrome is not typically observed with docetaxel treatment.
Serious adverse events reported in ≥2 patients were sepsis (n = 2; sepsis was fatal in one patient), large gastrointestinal (GI) perforation (n = 2) and epidermolysis/skin disorder/hand-foot syndrome (n = 2).
The nintedanib dose was reduced in 5/93 patients (5.4%) due to diarrhea, dehydration, hepatitis, reduced performance status and adverse event not specified (n = 1 each).
Treatment with docetaxel plus nintedanib was interrupted or discontinued due to adverse events in 15 of 93 patients (16.1%); two simultaneous events led to discontinuation in three patients. Adverse events leading to discontinuation included severe GI events (n = 9), fatigue (n = 2), sepsis (n = 1), myocardial infarction (n = 1), hemoptysis (n = 1), polyneuropathy (n = 1), epidermolysis (n = 1), pleural effusion (n = 1) and adverse event not specified (n = 1).
Discussion
To date, there have been no prospective, randomized controlled trials demonstrating the optimal treatment choice for patients with advanced NSCLC following failure of ICI treatment. Thus, analyses of real-world data from clinical practice are invaluable to help guide decision making in this setting. In the current study, a retrospective chart review in patients with advanced NSCLC of adenocarcinoma histology who progressed on prior ICI treatment (sequentially or in combination with chemotherapy) demonstrated the efficacy of docetaxel plus nintedanib. ORR was 41.4% across all treatment lines and was 50.0% in patients treated in the third-line setting. An encouraging DCR of 75.9% was observed across all treatment lines, and a DCR of 82.7% was observed in patients given docetaxel plus nintedanib as third-line therapy. In total, 35.1% of patients (n = 20) who received third-line docetaxel plus nintedanib continued to receive nintedanib as maintenance therapy for a median total maintenance treatment duration of 5.2 months, suggestive of a meaningful clinical benefit in these patients.
The patient population in the present study included a relatively high proportion of patients with ECOG PS ≥1 (53.8%), suggesting a population that reflects the real-world situation in clinical practice, with a worse health status compared with clinical trial populations.
The DCR findings presented here are consistent with available published data for docetaxel plus nintedanib in similar settings. For example, Corral et al. presented results from 11 patients in the Spanish compassionate use program. Patients were treated with docetaxel plus nintedanib after progression on platinum-based chemotherapy and subsequent ICI therapy, reporting a median PFS of 3.2 months (95% CI: 1.9–4.5), an ORR of 36% and a DCR of 82% [Citation22]. In a larger patient group, Grohé et al. assessed a cohort of 65 patients who received docetaxel plus nintedanib after failure of chemotherapy and ICI therapy as part of the prospective, noninterventional VARGADO study. A median PFS of 6.5 months (95% CI: 4.8–7.3; n = 55), an ORR of 50% and a DCR of 83% were reported in this patient group (n = 52) [Citation21]. Finally, in a subgroup analysis from the LUME-BioNIS biomarker study, 67 patients were treated with docetaxel plus nintedanib (third line or later: 57 patients; second line: 10 patients) for a mean treatment duration of 3.9 months (range: 0.2–25.5 months), similar to that reported here. A total of 55 patients provided tumor response data, reporting a similar DCR of 78.2% [Citation13,Citation20]. It can be seen that the third-line ORR and DCR presented in this current study, at 50.0% and 82.7%, respectively, are in line with the findings of these recent studies [Citation13,Citation20–22].
Corral et al. and Grohé et al. also included PFS as end points in their studies [Citation21,Citation22], whereas the current study assessed duration of time on treatment. In this respect, the data are not directly comparable. In the present analysis, the median duration of third-line treatment with docetaxel plus nintedanib was approximately 4 months, which is slightly longer than the median PFS reported by Corral et al. [Citation22]. Further, in approximately 40% of the third-line patients in our analysis, docetaxel plus nintedanib therapy was maintained for an additional 5.2 months, which, when considered alongside the initial treatment phase, exceeds the reported median PFS of Grohé et al. [Citation21].
To date, few data regarding OS have been reported for patients with advanced/metastatic NSCLC in the post-ICI setting. In the VARGADO study, among patients who received third-line docetaxel plus nintedanib, median OS from the start of third-line therapy was 12.2 months (and was 34.5 months from the start of first-line therapy) [Citation21]. In the LUME-BioNIS subgroup analysis of ICI-pretreated patients, docetaxel plus nintedanib treatment achieved a median OS of 8.8 months [Citation13]. In the current study, docetaxel plus nintedanib in the third-line setting achieved a median OS of approximately 8.4 months. Taken together, these data extend the real-world evidence base and address the continuing need for clinical data with docetaxel plus nintedanib in patients with advanced adenocarcinoma NSCLC in the post-ICI setting, especially in the absence of data from prospective, randomized controlled trials.
With regard to other possible third-line options in advanced adenocarcinoma NSCLC, a similar retrospective analysis was published for docetaxel plus ramucirumab (a VEGFR2 inhibitor) after nivolumab failure (n = 20; 16 with adenocarcinoma NSCLC) [Citation24]. In this limited patient group, an ORR of 60% and a DCR of 90% were reported, which is similar to that reported here for nintedanib [Citation24]. In another retrospective analysis of patients who received docetaxel plus ramucirumab (median treatment line: third line), median time to discontinuation was 3 months in the post-ICI treatment group [Citation25]. A more recent retrospective analysis investigated the efficacy of third-line docetaxel plus ramucirumab after failure on first-line chemotherapy and second-line ICI treatment (n = 67); ORR was 36%, DCR was 69%, median PFS was 6.8 months and median OS was 11.0 months after the start of third-line treatment. These data further highlight the potential for an antiangiogenic agent plus chemotherapy in this setting [Citation26].
The results of the current real-world analysis are encouraging when considered in line with existing data. However, the study has a number of limitations, including its noncomparative, retrospective design and the potential for selection bias. Bias in patient selection may have occurred due to the inclusion of patients who survived for long enough to receive third-line treatment. As such, comparisons with other studies may not be directly clinically relevant. Another limitation is that RECIST analyses were done for every patient by the treating oncologist with no central independent radiologic review board. Finally, due to the retrospective nature of the study, adverse events are likely to have been underreported. Nevertheless, the safety profile for docetaxel plus nintedanib reported here is broadly consistent with that reported in other studies [Citation7,Citation22].
Despite advances in ICI options for the treatment of NSCLC, innate or acquired treatment resistance continues to affect overall treatment outcomes [Citation8,Citation27]. Subsequent use of an antiangiogenic agent such as nintedanib may interrupt angiogenic processes in the TME, thus providing a window of opportunity in which the tumor is resensitized to therapy and allowing immune cells and oncologic therapies to be activated [Citation8,Citation28]. The results from the current retrospective analysis support the continued use of docetaxel plus nintedanib, showing that this combination may be effective in patients with advanced pulmonary adenocarcinoma who have failed on prior chemotherapy-ICI regimens.
Conclusion
The real-world, clinical practice data presented here provide the largest retrospective analysis to date of docetaxel plus nintedanib efficacy in patients with advanced non-squamous NSCLC of adenocarcinoma histology following chemotherapy and immunotherapy failure. There remains an evidence gap in the optimal treatment choice in patients with pulmonary adenocarcinoma who experience disease progression after chemotherapy-ICI treatment. Our data support the continued use of docetaxel plus nintedanib in these patients, suggesting that an antiangiogenic strategy may provide a meaningful clinical benefit in the post-chemotherapy-immunotherapy setting.
Although immune checkpoint inhibitors (ICIs) have altered the treatment paradigm for advanced/metastatic non-small-cell lung cancer, there remains an evidence gap in the optimal treatment choice for patients who experience disease progression on chemotherapy and ICI treatment.
This real-world analysis evaluated docetaxel plus nintedanib in patients with advanced pulmonary adenocarcinoma after chemotherapy-ICI failure.
Data were collected retrospectively from seven German centers.
In all evaluable patients (n = 87), overall response rate (ORR) was 41.4% (n = 36) with a disease control rate (DCR) of 75.9% (n = 66).
Among evaluable patients who received third-line docetaxel plus nintedanib following first-line chemotherapy and second-line ICIs (n = 52), ORR was 50.0% (n = 26) with a DCR of 82.7% (n = 43).
Patients spent a median time of 3.9 months on third-line docetaxel plus nintedanib (95% CI: 2.1–5.9 months), with a median OS from the start of third-line therapy of 8.4 months (95% CI: 5.0–11.0).
Safety findings were generally consistent with the known safety profile of docetaxel plus nintedanib.
The data suggest that docetaxel plus nintedanib offers a meaningful clinical benefit in patients with advanced pulmonary adenocarcinoma after chemotherapy-ICI failure.
Author contributions
All authors were responsible for study conception and design of the analysis. M Metzenmacher, F Rizzo, K Kambartel, J Panse, D Schaufler, M Scheffler, I Azeh, M Hoiczyk, AT Turki, DC Christoph were responsible for provision of study materials/patients. M Metzenmacher, F Rizzo, K Kambartel, J Panse, D Schaufler, M Scheffler, I Azeh, M Hoiczyk, AT Turki, DC Christoph, H Buchner were responsible for collection and assembly of data. All authors were responsible for data interpretation and analysis, and creation of the manuscript and final approval of the content.
Congress presentations
Results from this study have been previously presented at the European Society for Medical Oncology (ESMO) Virtual Congress, 19–21 September 2020 (Christoph DC, et al. Docetaxel/nintedanib as efficient treatment option after failure of immune checkpoint inhibition – real world evidence, Poster number 1374P).
Ethical conduct of research
This retrospective data analysis was approved by the appropriate institutional ethics committee for each center. All patients received docetaxel plus nintedanib in accordance with the approved EMA label, or in line with local best-practice guidelines (as described above). Where possible, all patients provided informed, written consent at the time of data collection. Data relating to deceased patients were collected in accordance with German data protection laws. Confidentiality of patient data and omission of any identifying information was maintained throughout this investigation.
Acknowledgments
The authors thank the patients and their families who provided data for this analysis.
Financial & competing interests disclosure
M Metzenmacher reports personal fees from Amgen, AstraZeneca, Boehringer Ingelheim, Takeda, Roche, Novartis, Merck Sharp & Dohme and Bristol Myers Squibb outside the submitted work. F Rizzo, K Kambartel, I Azeh and M Hoiczyk report no conflicts of interest. J Panse reports personal fees from Alexion, Bristol Myers Squibb, Boehringer Ingelheim, Grünenthal, Merck Sharp & Dohme, Novartis, Pfizer, Chugai, Roche, Apellis and Blueprint medicines outside the submitted work. D Schaufler reports personal fees and other from AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Merck Sharp & Dohme, Novartis, Roche; personal fees from Healthcare Consulting Cologne; other from AstraZeneca outside the submitted work. M Scheffler reports fees for advisory services from Boehringer Ingelheim, AMGEN, Pfizer, Roche and Takeda outside the submitted work. AT Turki reports consultancy for Merck Sharp & Dohme, CSL Behring and Jazz Pharmaceuticals, and nonfinancial support from Neovii Biotech, outside the submitted work. J Atz and C Hoffmann are employees of Boehringer Ingelheim. H Buchner reports fees from Boehringer Ingelheim for statistical analyses performed during the conduct of the study. DC Christoph reports fees from Boehringer Ingelheim during the conduct of the study; personal fees and nonfinancial support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, from Bristol Myers Squibb, Chugai, Merck Sharp & Dohme, Novartis, Pfizer, Roche and Takeda outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance during the preparation of this manuscript was supported financially by Boehringer Ingelheim and provided by Joanne Smith and Michael Fisher (Syneos Health, UK). Boehringer Ingelheim provided financial support for data analysis.
Additional information
Funding
References
- Reck M , Rodríguez-AbreuD, RobinsonAGet al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med.375(19), 1823–1833 (2016).
- Gandhi L , Rodríguez-AbreuD, GadgeelSet al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med.378(22), 2078–2092 (2018).
- Socinski MA , JotteRM, CappuzzoFet al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med.378(24), 2288–2301 (2018).
- Reck M , Rodríguez-AbreuD, RobinsonAGet al. Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J. Clin. Oncol.37(7), 537–546 (2019).
- Planchard D , PopatS, KerrKet al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.29(Suppl. 4), iv192–iv237 (2018).
- National Comprehensive Cancer Network . Non-small cell lung cancer (version 3) (2019). www.nccn.org
- Grohé C , GleiberW, HaasSet al. Nintedanib plus docetaxel after progression on immune checkpoint inhibitor therapy: Insights from VARGADO, a prospective study in patients with lung adenocarcinoma. Future Oncol.15(23), 2699–2706 (2019).
- Popat S , GrohéC, CorralJet al. Anti-angiogenic agents in the age of resistance to immune checkpoint inhibitors: do they have a role in non-oncogene-addicted non-small cell lung cancer? Lung Cancer 144, 76–84 (2020).
- Claesson-Welsh L . Blood vessels as targets in tumor therapy. Ups. J. Med. Sci.117(2), 178–186 (2012).
- Yang J , YanJ, LiuB. Targeting VEGF/VEGFR to modulate antitumor immunity. Front. Immunol.9, 978 (2018).
- Garon EB , CiuleanuT-E, ArrietaOet al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet384(9944), 665–673 (2014).
- Reck M , KaiserR, MellemgaardAet al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol.15(2), 143–155 (2014).
- Reck M , SyrigosK, MiliauskasSet al. Non-interventional LUME-BioNIS study of nintedanib plus docetaxel after chemotherapy in adenocarcinoma non-small cell lung cancer: A subgroup analysis in patients with prior immunotherapy. Lung Cancer148, 159–165 (2020).
- Manzo A , MontaninoA, CarillioGet al. Angiogenesis Inhibitors in NSCLC. Int. J. Mol. Sci.18(10), 2021 (2017).
- Seeber A , GunsiliusE, GastlG, PircherA. Anti-angiogenics: Their value in colorectal cancer therapy. Oncol. Res. Treat.41(4), 188–193 (2018).
- Nienhüser H , SchmidtT. Angiogenesis and anti-angiogenic therapy in gastric cancer. Int. J. Mol. Sci.19(1), 43 (2017).
- Vargatef (nintedanib) . Summary of product pharacteristics. (2020). www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002569/WC500179970.pdf
- Hilberg F , RothGJ, KrssakMet al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res.68(12), 4774–4782 (2008).
- Hilberg F , Tontsch-GruntU, BaumAet al. Triple angiokinase inhibitor nintedanib directly inhibits tumor cell growth and induces tumor shrinkage via blocking oncogenic receptor tyrosine kinases. J. Pharmacol. Exp. Ther.364(3), 494–503 (2018).
- Reck M , SyrigosK, MiliauskasSet al. Nintedanib + docetaxel after immunotherapy in adenocarcinoma non-small cell lung cancer: First results from the non-interventional LUME-BioNIS study. Ann. Oncol.30(Suppl. 11), xi16–xi32 (2019).
- Grohé C , BlauW, GleiberWet al. Efficacy and safety of nintedanib plus docetaxel in lung adenocarcinoma patients after failure of previous immune checkpoint inhibitor therapy: Updated results from the ongoing non-interventional study VARGADO (NCT02392455). Poster presented at the European Society for Medical Oncology (ESMO) Virtual Congress 1372P (September 19–21, 2020).
- Corral J , MajemM, Rodríguez-AbreuDet al. Efficacy of nintedanib and docetaxel in patients with advanced lung adenocarcinoma treated with first-line chemotherapy and second-line immunotherapy in the nintedanib NPU program. Clin. Transl. Oncol.21(9), 1270–1279 (2019).
- Capelletto E , MigliorinoMR, MorabitoAet al. Final results of the SENECA (SEcond line NintEdanib in non-small cell lung CAncer) trial. Lung Cancer134, 210–217 (2019).
- Shiono A , KairaK, MouriAet al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac. Cancer10(4), 775–781 (2019).
- Offin M , XuC, JainHet al. Efficacy of ramucirumab and docetaxel given either before or after immune checkpoint inhibitors in patients with lung cancers. J. Clin. Oncol.37(Suppl. 15), 9078–9078 (2019).
- Brueckl WM , ReckM, RittmeyerAet al. Efficacy of docetaxel plus ramucirumab as palliative third-line therapy following second-line immune-checkpoint-inhibitor treatment in patients with non-small-cell lung cancer stage IV. Clin. Med. Insights Oncol.14, 1179554920951358 (2020).
- Jenkins RW , BarbieDA, FlahertyKT. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer118(1), 9–16 (2018).
- Fukumura D , KloepperJ, AmoozgarZ, DudaDG, JainRK. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol.15(5), 325–340 (2018).
