Abstract
Aim: To determine the incidence of chemotherapy-induced febrile neutropenia (FN) and related outcomes after same-day pegfilgrastim in lung cancer. Materials & methods: This single-center, retrospective study evaluated electronic health records of patients with lung cancer treated between 2013–2018. The main end points were incidence of FN and grade 3/4 neutropenia after the first and across all chemotherapy cycles. Results: A total of 114 patients received same-day pegfilgrastim in 384 cycles. The incidence of FN and grade 3/4 neutropenia was 2.3 and 25% after the first chemotherapy cycle and 1.6 and 10.4% across all cycles, respectively. Conclusion: Same-day prophylactic pegfilgrastim in patients with lung cancer may be a suitable option, owing to its low incidence of FN and related outcomes.
Plain language summary
Chemotherapy is intended to kill fast-growing cancer cells but can also kill immune cells that are needed to prevent infections. When too many immune cells are killed by chemotherapy, patients with cancer may experience febrile neutropenia, a serious condition that can lead to death in the most severe cases. To prevent this side effect of chemotherapy, a protein that helps certain immune cells to grow (pegfilgrastim) is administered on the day after chemotherapy. To avoid a second clinic visit the day after receiving chemotherapy, it has become common to administer pegfilgrastim on the same day as chemotherapy. Whether this approach is as effective and safe as next-day administration is currently unclear. This study from the University of Arizona Cancer Center showed that in patients with lung cancer who receive chemotherapy, administration of pegfilgrastim on the same day as chemotherapy is a safe, effective method to prevent febrile neutropenia.
Lung cancer is the leading cause of cancer-related deaths worldwide [Citation1]. It is the second most common cancer in both men and women in the USA and is associated with the highest rate of mortality among cancer types [Citation2]. For 2021, the American Cancer Society estimated the occurrence of 235,760 new cases of lung cancer and 131,880 deaths related to lung cancer in the USA [Citation3]. Non-small-cell lung cancer (NSCLC) is the predominant form of lung cancer and accounts for 84% of all diagnoses, followed by small-cell lung cancer (SCLC), which accounts for 13% of all diagnoses [Citation3].
Standard-of-care treatment for patients with metastatic lung cancer typically consists of an immune checkpoint inhibitor in combination with a platinum-based myelosuppressive chemotherapy doublet [Citation4,Citation5]. A common side effect of myelosuppressive chemotherapy is the development of febrile neutropenia (FN; absolute neutrophil count [ANC] of <500 neutrophils/μl with a single oral temperature of ≥38.3°C or a temperature of ≥38.0°C for >1 h) [Citation6]. FN, a serious and sometimes lethal condition that can result in chemotherapy dose delays or reductions or in hospitalizations, is associated with increased treatment costs [Citation7,Citation8]. Patients with lung cancer who receive treatment with a platinum-based chemotherapy doublet are generally at an intermediate risk (i.e., 10–20%) of developing FN [Citation6].
G-CSFs are currently recommended as prophylaxis for FN in patients undergoing myelosuppressive chemotherapy, because GCS-Fs reduce the risk of chemotherapy-induced neutropenia or FN by stimulating production and maturation of neutrophils in the bone marrow [Citation6]. Pegfilgrastim and its biosimilars are commonly used pegylated G-CSF agents with a long half-life (15–80 h after subcutaneous injection), which permits single dosing once per chemotherapy cycle [Citation6,Citation9,Citation10]. The National Cancer Comprehensive Network (NCCN) Guidelines for hematopoietic growth factors and the prescribing information for pegfilgrastim recommend administration at ≥24 h after myelosuppressive chemotherapy [Citation6,Citation9–11] because of concerns that same-day administration may potentially exacerbate neutropenia by stimulating neutrophil progenitor cells to rapidly divide, making them more susceptible to the cytotoxic effects of chemotherapy [Citation12]. However, next-day administration of pegfilgrastim requires patients to either self-administer pegfilgrastim or return to a clinic for administration; thereby, increasing treatment burden [Citation11,Citation12]. To address this problem, an on-body injector (OBI) is available for originator pegfilgrastim – but not its biosimilars – that delivers pegfilgrastim at approximately 27 h after application to the patient’s body. However, OBI failure and malfunction rates of 1.7–6.9% have been reported [Citation13–15]; this may increase the risk of developing neutropenia or FN in patients relying on this device.
Same-day administration of pegfilgrastim may offer patients and caregivers convenience by eliminating patient noncompliance or failure of the delivery device, thus reducing medication expense and office visit copay and potentially avoiding unnecessary COVID-19 exposure during the current pandemic. In 2015, the American Society of Clinical Oncology published an update on the use of white blood cell growth factors that endorsed the use of pegfilgrastim on the same day as chemotherapy when it is the only feasible means of G-CSF administration for patients [Citation16]. However, limited data are currently available on the incidence of neutropenia or FN with same-day versus next-day prophylactic pegfilgrastim in patients receiving chemotherapy [Citation16–18]. Furthermore, the interpretation of these data are limited by small numbers of patients and the inclusion of numerous chemotherapy regimens due to varying tumor types [Citation10,Citation17]. Data are even more scarce for patients with lung cancer [Citation10,Citation19]. Therefore, the optimal timing of administration of prophylactic pegfilgrastim following chemotherapy in patients with lung cancer warrants further investigation. Herein, we report results from a retrospective study that evaluated the incidence of FN, grade 3/4 neutropenia and associated chemotherapy dose delays or hospitalizations in patients with lung cancer who received same-day pegfilgrastim.
Materials & methods
Study design & patients
This retrospective study was conducted using the electronic health records of patients with lung cancer who were treated at the University of Arizona Cancer Center between 1 November 2013 and 31 August 2018. Eligible patients were identified using the International Classification of Diseases-9 and International Classification of Diseases-10 codes corresponding with confirmed diagnoses of lung cancer. Study inclusion further required patients to be aged ≥18 years and to have received pegfilgrastim on the same day as chemotherapy, with or without anti-PD1 or PD-L1 agents. Patients who were treated outside of the University of Arizona Cancer Center, who received chemoradiotherapy, or who received pegfilgrastim the day after chemotherapy were excluded. The study was approved by the Institutional Review Board of The University of Arizona. Prophylactic pegfilgrastim was administered approximately 15 min after completion of chemotherapy. In patients who received chemotherapy at ≤14-day intervals (e.g., every week [QW]; every 2 weeks [Q2W]; days 1, 8 and 15 of a 28-day cycle), pegfilgrastim was administered on day 1 and on day 15 (i.e., 14 days after the previous dose of pegfilgrastim). In patients who received chemotherapy on consecutive days (e.g., days 1–3 of a 21-day cycle), pegfilgrastim was administered on day 3. In patients who received chemotherapy on days 1 and 8 of a 21- or 28-day cycle, pegfilgrastim was administered on day 8 (i.e., ≥14 days between pegfilgrastim administration and the next chemotherapy administration).
Data collection
FN was defined as an ANC of ≤500 cells/mm3 and a temperature of ≥38°C or a chart documentation of FN. Grade 3 and grade 4 neutropenia were defined as an ANC of 500–<1000 cells/mm3 and <500 cells/mm3 (without fever), respectively.
Baseline demographics collected included age, sex and race (). Disease characteristics collected were type of lung cancer, Eastern Cooperative Oncology Group (ECOG) performance status (PS), chemotherapy received prior to study period, prior cancer-related surgeries and baseline ANC. Treatment-related data collected were the chemotherapy regimen administered during the study period; risk of neutropenia based on chemotherapy regimen (low, <10%; intermediate, 10–20%; and high, ≥20%); number of chemotherapy regimens received during the study period; administration of pegfilgrastim as primary prophylaxis; completion of four cycles of chemotherapy with same-day pegfilgrastim administration; ANC following chemotherapy for up to four chemotherapy cycles; incidences of FN, grade 3 neutropenia and grade 4 neutropenia; and associated chemotherapy dose delays, hospitalizations and their causes. Per institutional protocol, same-day pegfilgrastim was administered within 15 min after the completion of chemotherapy.
Table 1. Baseline characteristics of the patient population.
Outcomes
The primary outcome was the incidence of FN after the first chemotherapy cycle and across all cycles of chemotherapy. Secondary outcomes included the incidence of grade 3/4 neutropenia, chemotherapy dose delays due to FN or grade 3/4 neutropenia and hospitalizations due to FN or grade 3/4 neutropenia. Each of the secondary outcomes was assessed after the first chemotherapy cycle and across all cycles. All outcomes were analyzed for the entire study cohort, and subgroups were defined by the type of lung cancer (NSCLC and SCLC).
Statistical analysis
The proportions of each outcome of interest (FN, grade 3/4 neutropenia, chemotherapy delays and hospitalizations) for the entire cohort and for the lung cancer type subgroups across all cycles were calculated by dividing the number of chemotherapy cycles with same-day pegfilgrastim during which patients experienced any of the outcomes by the number of total cycles in which same-day pegfilgrastim was administered. Similarly, the proportions of outcomes of interest in the first cycle were determined by dividing the number of patients experiencing a given outcome by the number of patients who received pegfilgrastim in the first chemotherapy cycle. All data were analyzed descriptively as counts and percentages for discrete variables, and as medians (with ranges) and means (with standard deviations) for continuous variables.
Results
Patient population
Of the 1181 patients whose electronic health records were reviewed, 114 met the inclusion criteria and were included in the study. The demographics and baseline characteristics of patients are shown in . The median age of patients was 68 years (range, 22–84), more than half of the study population was male (55.3%) and had an ECOG PS of 0 or 1 (63.2%) and most patients were white (86.8%). NSCLC was the most common diagnosis (76.3%), and 73% of patients received a chemotherapy regimen associated with low risk of FN but were in the intermediate- or high-risk category after inclusion of patient-specific risk factors (e.g., age, previous FN).
The most commonly administered chemotherapy regimen was carboplatin plus pemetrexed every 3 weeks (Q3W) in patients with NSCLC and carboplatin plus etoposide Q3W in patients with SCLC (). Same-day prophylactic pegfilgrastim was administered in 384 cycles of chemotherapy, including 44 first cycles. A total of 31 patients (27.2%) received pegfilgrastim in four cycles of chemotherapy.
Table 2. Baseline characteristics of the patient population.
Primary outcomes
Among patients who received same-day pegfilgrastim prophylaxis, FN was reported in one (2.3%) patient after the first chemotherapy cycle and in six (1.6%) patients across all chemotherapy cycles ( & ). Of the six patients who developed FN across all cycles, most were male (n = 5), were aged 56–75 years and had an ECOG PS of 0 (n = 4; not available for two patients). Furthermore, half of these patients had NSCLC (n = 3) and half had SCLC (n = 3); four patients received chemotherapy regimens with low risk of FN (>10%); and two received regimens with intermediate risk (10–20%). Two patients developed FN after receiving carboplatin + etoposide Q3W, one patient after receiving cisplatin + etoposide Q4W, one patient after receiving pemetrexed Q3W, one patient after receiving irinotecan QW (cycle 1) and one patient after receiving vinorelbine on days 1, 8 and 15 of a 28-day cycle. The baseline ANC count ranged from 2.86 × 109 to 8.52 × 109/l. Previous chemotherapy was received by two patients, and only one patient received prophylactic pegfilgrastim after the first chemotherapy cycle.
NSCLC: Non-small-cell lung cancer; SCLC: Small-cell lung cancer.
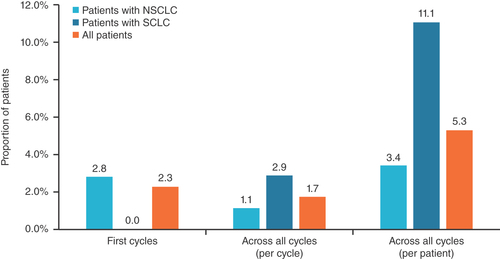
Table 3. Incidences of febrile neutropenia, grade 3/4 neutropenia and associated chemotherapy dose delays and hospitalizations.
Secondary outcomes
Incidences of grade 3/4 neutropenia, chemotherapy dose delays and hospitalizations are presented in . Grade 3/4 neutropenia was reported in 40 cycles (10.4%), including 11 first cycles (25.0%). Although 46 (12.0%) chemotherapy dose delays were noted across all cycles, including seven (15.9%) in the first cycle, only 12 (3.1%) of these were directly attributable to FN or grade 3/4 neutropenia. Similarly, of the 34 (8.9%) cycles in which patients required hospitalization, including 12 (27.3%) first cycles, only seven (1.8%) were for FN or grade 3/4 neutropenia. The incidences of FN, grade 3/4 neutropenia, chemotherapy dose delays and hospitalizations in the subgroups of patients with NSCLC or SCLC followed similar patterns. A trend of lower incidences was observed in the setting of SCLC, but should be noted with caution due to the small sample size.
Most chemotherapy dose delays and hospitalizations observed during this study occurred for reasons not directly related to FN or grade 3/4 neutropenia but showed some differences by type of lung cancer. In the setting of NSCLC, chemotherapy delays were attributed to thrombocytopenia (n = 8), fatigue (n = 5), hospitalization (n = 4), anemia (n = 2), nausea and vomiting (n = 2), renal impairment (n = 2), altered mental status (n = 1), neuropathy (n = 1) and pain (n = 1); and hospitalizations to sepsis (n = 8), pneumonia (n = 4), altered mental status (n = 1), cellulitis (n = 1), dehiscence of intrathecal pump (n = 1), fatigue (n = 1), intractable nausea and vomiting (n = 1), non-ST segment elevation myocardial infarction (n = 1), pleural effusion (n = 1), pneumonitis (n = 1), pneumothorax (n = 1) and port-a-cath line infection (n = 1). In the setting of SCLC, chemotherapy delays were due to thrombocytopenia (n = 4), hospitalization (n = 2), anemia (n = 1), fatigue (n = 1), nausea and vomiting (n = 1) and pain (n = 1); and hospitalizations to gastrointestinal bleeding (n = 2), Clostridium difficile infection (n = 1), acute kidney injury (n = 1) and chest pain (n = 1).
Discussion
Prophylactic administration of pegfilgrastim, initially approved by the US FDA in 2002 [Citation12], has been instrumental in reducing the incidence of neutropenia and FN in patients with cancer who receive myelosuppressive chemotherapy regimens. Since then, OBI delivery of originator pegfilgrastim and several pegfilgrastim biosimilars in prefilled syringes have become additional product options for patients, providers and payers [Citation20–23]. Although not stated in the pegfilgrastim prescribing information, administration of pegfilgrastim on the same day as chemotherapy is commonly used in clinical practice and has the potential to minimize the burden on patients and healthcare providers by avoiding a second clinic visit 24 h after chemotherapy administration [Citation24]. Same-day administration has been recommended during the COVID-19 pandemic as an alternative to OBI use [Citation25], and has been acknowledged as an option that can be considered in the NCCN Guidelines for hematopoietic growth factors [Citation6]. Because the extent of prophylactic effect achieved with same-day pegfilgrastim may depend on the chemotherapy regimen administered [Citation26], several studies have investigated the incidence in tumor type-defined patient populations in real-world settings [Citation10,Citation27–32].
Contributing to a growing body of evidence, this retrospective, single-institution study assessed the incidence of FN, grade 3/4 neutropenia and associated chemotherapy dose delays and hospitalizations in patients with lung cancer who received pegfilgrastim as FN prophylaxis on the same day as their myelosuppressive chemotherapy regimen. In contrast to prospective clinical trials [Citation33], this real-world study included patients with an ECOG PS of 2 or 3 and patients with metastases. Consistent with the standard-of-care during the study period, the most commonly administered chemotherapy regimens were platinum-based chemotherapy doublet regimens: carboplatin in combination with pemetrexed or nab-paclitaxel in patients with NSCLC, and carboplatin or cisplatin in combination with etoposide in patients with SCLC. Across the 384 chemotherapy cycles evaluated in this study, the incidence of FN was low overall (1.6%; first cycles, 2.3%). Most patients experiencing FN were male, had an ECOG PS of 0, had lung cancer stage ≥3 and did not receive pegfilgrastim in their first chemotherapy cycle. The incidence of FN across all cycles was numerically higher in patients with SCLC (2.9%) compared with patients with NSCLC (1.1%). A similar trend was observed for grade 3/4 neutropenia across all cycles, with an incidence of 14.4% in patients with SCLC and 8.9% in patients with NSCLC. In all patients, chemotherapy dose delays occurred in 0.5% of cycles due to FN and in 2.6% of cycles due to grade 3/4 neutropenia. Similarly, in all patients, the incidence of hospitalizations related to FN or grade 3/4 neutropenia was low (1.0 vs 0.8%). Similarly, most hospitalizations observed in this study were not related to neutropenia. Of note, although 73% of patients received low-risk chemotherapy regimens, all patients were considered to be at intermediate or high risk for FN after assessment of patient-specific risk factors (based on NCCN guidelines and physician assessment).
Consistent with the low incidence of FN reported here, a retrospective cohort study based on healthcare claims data reported FN incidences of 3.3% (using a broad definition of FN) and 1.3% (using a narrow definition of FN) across all chemotherapy cycles administered to 21,994 patients with lung cancer who received G-CSF prophylaxis [Citation34]. The FN incidence in first cycles was 2.8% [Citation34]. The most commonly administered chemotherapy regimens were carboplatin plus paclitaxel and carboplatin plus etoposide [Citation34].
The low FN incidence rates observed in our study are also consistent with the results of two studies that included a cohort of patients with NSCLC [Citation10,Citation35]. In a cohort study by Lokich, no cases of FN were reported among 24 patients with NSCLC who received pegfilgrastim Q2W on the same day as their weekly chemotherapy regimens [Citation35]. Of note, most patients included in this study received a weekly regimen composed of alternating doublet chemotherapy (cisplatin plus docetaxel, alternating with vinorelbine plus gemcitabine) or triplet chemotherapy (cisplatin plus docetaxel plus irinotecan, alternating with cisplatin plus gemcitabine plus vinorelbine) for 6 or 12 weeks [Citation35], suggesting compatibility of weekly administration of chemotherapy and same-day administration of pegfilgrastim every other week. Similarly, no cases of FN were observed among 44 patients with NSCLC who received carboplatin plus docetaxel and same-day pegfilgrastim in a prospective, randomized phase II study [Citation10].
To our knowledge, this is the first article about same-day pegfilgrastim administration in patients with SCLC [Citation36]. Among the patients in our study, no cases of FN occurred in the first chemotherapy cycle and only three cases occurred across all cycles. Although these data in SCLC should be interpreted with caution because of the limited number of patients with SCLC in this study, our results warrant future studies of same-day pegfilgrastim in this patient population.
Overall, data from this study in combination with data from the aforementioned studies in patients with lung cancer show that the incidence of FN and related outcomes are low when same-day or next-day pegfilgrastim is administered as prophylaxis against the myelosuppressive effects of common NSCLC or SCLC chemotherapy regimens (i.e,. platinum-based doublets). These data support that same-day administration provides reliable FN prophylaxis for patients with lung cancer. In addition, same-day administration of pegfilgrastim offers the benefit of minimizing the burden associated with a next-day clinic visit for patients and healthcare providers. Minimizing clinic visits has become a crucial component of healthcare during the current COVID-19 pandemic, because doing so reduces the exposure risk in this sensitive patient population with lung cancer [Citation37].
The present study has some limitations, while also identifying areas for future research. This was a retrospective, noncontrolled, electronic heath records-based analysis from a single center, but it confirmed observations from previous studies with newly gained real-world insights. Because a randomized, prospective clinical trial of same-day versus next-day pegfilgrastim administration is unlikely to be considered, additional multicenter studies would be useful to further investigate the broad applicability of same-day pegfilgrastim to the most recent standard-of-care chemotherapy combination treatments, including chemo-immunotherapy regimens in patients with lung cancer. As a real-world study, our patient population had a higher degree of heterogeneity than is typically seen in prospective clinical trial populations. Although arguably heterogeneity may introduce bias, data from such populations are reflective of real-world clinical practice and are, therefore, more directly applicable than those obtained in tightly controlled clinical trials. Our data support same-day prophylactic pegfilgrastim in patients with lung cancer; however, they may not be directly transferable to other tumor types and other chemotherapy regimens. As such, additional real-world studies are needed to investigate the timing of pegfilgrastim administration that ensures effective FN prophylaxis in patient populations across the broad spectrum of solid and hematological malignancies and the various treatment options for each.
Conclusion
In this retrospective study, the incidence of FN or grade 3/4 neutropenia was low in patients with NSCLC or SCLC who received pegfilgrastim on the same day as platinum-based myelosuppressive doublet chemotherapy. The observed incidences were similar to those found by other studies that evaluated FN and associated outcomes in patients with lung cancer. Consistent with these findings, neutropenia- and FN-related chemotherapy dose delays or hospitalizations were rare in this patient population. Overall, these findings suggest that same-day administration of pegfilgrastim is a safe and effective option for patients with lung cancer that allows reduction of healthcare burden and clinic visits in the real-world setting.
In patients receiving myelosuppressive immunotherapy, febrile neutropenia prophylaxis is achieved by administration of pegfilgrastim 24–72 h after chemotherapy (per US FDA-approved package insert).
To minimize outpatient visits, same-day administration of pegfilgrastim is common in clinical practice, but remains controversial, despite its mention as an option by National Comprehensive Cancer Network guidelines.
This retrospective, real-world, single-institution study evaluated the incidence of febrile neutropenia, grade 3/4 chemotherapy-induced neutropenia, hospitalizations and chemotherapy dose delays in a heterogeneous population of patients with non-small-cell lung cancer or small-cell lung cancer.
Consistent with previous studies, the incidence of febrile neutropenia and related outcomes after same-day pegfilgrastim was low in both patient populations.
The study findings suggest that same-day pegfilgrastim may be a safe administration option in patients with lung cancer; thereby, reducing outpatient hospital visits and healthcare burden.
Author contributions
Conception and design: N Alrawashdh and J Vraney; collection and assembly of data: J Vraney; data analysis and interpretation: N Alrawashdh and BM Choi; manuscript writing: N Alrawashdh, J Vraney and BM Choi; final approval of manuscript: all authors. Accountable for all aspects of the work: all authors.
Disclaimer
The contents of this manuscript are solely the opinions of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the Saudi Food and Drug Authority.
Previous publication
This is an original work and is not under consideration by any other journal. Data included in this work have been presented as an abstract at ASCO 2021 (https://meetings.asco.org/abstracts-presentations/199279) and as a poster at ASCO Quality Care Symposium 2021 (https://meetings.asco.org/abstracts-presentations/201759).
Financial & competing interests disclosure
This study was funded by The University of Arizona Cancer Center. I Abraham owns equity in Matrix45, which provides research and consulting services to, among others, pharmaceutical companies on a non exclusivity basis. Matrix45 has been contracted by Coherus Biosciences for such services, but not for the work reported here. By Matrix45 company policy, employees and equity owners are prohibited from owning equity in client organizations (except through mutual funds or other independently administered collective investment instruments) or contracting independently with client organizations. A McBride owns stock in Bristol Myers Squibb. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing and editorial assistance were provided by N Parkar and S Biechele (ApotheCom, CA, USA) and funded by Coherus BioSciences (CA, USA).
Data sharing statement
All data relevant to the study are included in the article. Data are available upon reasonable request.
Acknowledgments
The authors would like to thank A Henglefeldt for assisting in the development of this project.
Additional information
Funding
References
- World Health Organization . Cancer Fact Sheet (2022). www.who.int/news-room/fact-sheets/detail/cancer
- Centers for Disease Control and Prevention . Cancer statistics at a glance. https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/
- American Cancer Society . Key statistics for lung cancer (2022). www.cancer.org/cancer/lung-cancer/about/key-statistics.html
- Onoi K , ChiharaY, UchinoJet al. Immune checkpoint inhibitors for lung cancer treatment: a review. J. Clin. Med.9(5),1362 (2020).
- Wang C , KulkarniP, SalgiaR. Combined checkpoint inhibition and chemotherapy: new era of 1(st)-line treatment for non-small-cell lung cancer. Mol. Ther. Oncolytics13, 1–6 (2019).
- National Comprehensive Cancer Network . NCCN practice guidelines in Oncology. Hematopoietic growth factors. Version 1.2022. Plymouth Meeting, PA, USA (2021). www.nccn.org/guidelines/guidelines-detail?category=3&id=1493
- Tai E , GuyGP, DunbarA, RichardsonLC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J. Oncol. Pract.13(6), e552–e561 (2017).
- Aapro MS , BohliusJ, CameronDAet al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur. J. Cancer47(1), 8–32 (2011).
- Eckstrom J , BartelsT, AbrahamIet al. A single-arm, retrospective analysis of the incidence of febrile neutropenia using same-day versus next-day pegfilgrastim in patients with gastrointestinal cancers treated with FOLFOX or FOLFIRI. Support. Care Cancer27(3), 873–878 (2019).
- Burris HA , BelaniCP, KaufmanPAet al. Pegfilgrastim on the same day versus next day of chemotherapy in patients with breast cancer, non-small-cell lung cancer, ovarian cancer, and non-Hodgkin's lymphoma: results of four multicenter, double-blind, randomized phase II studies. J. Oncol. Pract.6(3), 133–140 (2010).
- Weycker D , BensinkM, LonshteynA, DoroffR, ChandlerD. Risk of chemotherapy-induced febrile neutropenia by day of pegfilgrastim prophylaxis in US clinical practice from 2010 to 2015. Curr. Med. Res. Opin.33(12), 2107–2113 (2017).
- Neulasta©(pegfilgrastim) injection, for subcutaneous use, package insert. Amgen Inc, CA, USA (2021).
- Stuessy P , SanchezFA, SchoberM. Retrospective review of pegfilgrastim on-body injector delivery rates in a large health system. 35(Suppl. 15), e18273–e18273 (2017).
- Mahler LJ , DiblasiR, PerezA, GaspardJ, MccauleyD. On-body injector: an administration device for pegfilgrastim. Clin. J. Oncol. Nurs.21(1), 121–122 (2017).
- Townley C , PorterC, McmullenN. Comparing grade 4 neutropenia associated with pegfilgrastim administered via the Onpro device versus manual injection with a prefilled syringe. J. Hematol. Oncol. Pharm.8(3), 119–125 (2018).
- Smith TJ , BohlkeK, LymanGHet al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol.33(28), 3199–3212 (2015).
- Lyman GH , AllcottK, GarciaJet al. The effectiveness and safety of same-day versus next-day administration of long-acting granulocyte colony-stimulating factors for the prophylaxis of chemotherapy-induced neutropenia: a systematic review. Support. Care Cancer25(8), 2619–2629 (2017).
- Li Y , KlippelZ, ShihX, WangH, ReinerM, PageJH. Trajectory of absolute neutrophil counts in patients treated with pegfilgrastim on the day of chemotherapy versus the day after chemotherapy. Cancer Chemother. Pharmacol.77(4), 703–712 (2016).
- U.S. Centers for Medicare & Medicaid Services . Drug coverage under different parts of Medicare. (2021). www.cms.gov/outreach-and-education/outreach/partnerships/downloads/11315-p.pdf
- Fulphila® (pegfilgrastim-jmdb) injection, for subcutaneous use, package insert. Mylan Institutional LLC, IL, USA (2021).
- Udenyca® (pegfilgrastim-cbqv) injection, for subcutaneous use, package insert. Coherus Biosciences, CA, USA (2021).
- Ziextenzo® (pegfilgrastim-bmez) injection, for subcutaneous use, package insert. Sandoz Inc, NJ, USA (2021).
- Nyvepria™ (pegfilgrastim-apgf) injection, for subcutaneous use, package insert. Hospira, Inc., a Pfizer Company, IL, USA2021).
- Ludwig H , GascónP, BokemeyerCet al. Outcomes of chemotherapy-induced (febrile) neutropenia prophylaxis with biosimilar filgrastim (Zarzio®) initiated “same-day” (<24 h), “per-guidelines” (24–72 h), and “late” (>72 h): findings from the MONITOR-GCSF study. Support. Care Cancer27(6), 2301–2312 (2019).
- National Comprehensive Cancer Network . NCCN hematopoietic growth factors. short-term recommendations specific to issues with COVID-19 (SARS-CoV-2). Plymouth Meeting, PA, USA (2020). www.iononline.com/-/media/assets/ion/pdf/covid19-resources/nccn_hgf_covid-19_19may20.pdf?la=en&hash=9FB98741E9E83D354F8FEFF66AC5F5188E139471
- Gerberich AJ , AttilioMR, SvobodaA. Revisiting same day administration of pegfilgrastim in the age of biosimilars: a review of literature. J. Oncol. Pharm. Pract.26(8), 1970–1976 (2020).
- McBride A , AlrawashdhN, BartelsT, MooreL, PerskyD, AbrahamI. Same-day versus next-day pegfilgrastim or pegfilgrastim-cbqv in patients with lymphoma receiving CHOP-like chemotherapy. Future Oncol.17(26), 3485–3497 (2021).
- Moore L , BartelsT, PerskyDO, AbrahamI, KumarA, McBrideA. Outcomes of primary and secondary prophylaxis of chemotherapy-induced and febrile neutropenia in bendamustine plus rituximab regimens in patients with lymphoma and chronic lymphocytic leukemia: real-world, single-center experience. Support. Care Cancer29(8), 4867–4874 (2021).
- Bilen MA , CauleyDH, AtkinsonBJet al. Safety of same-day pegfilgrastim administration in metastatic castration-resistant prostate cancer treated with cabazitaxel with or without carboplatin. Clin. Genitourin. Cancer15(3), e429–e435 (2017).
- Cheng C , GallagherEM, YehJY, EarlMA. Rates of febrile neutropenia with pegfilgrastim on same day versus next day of CHOP with or without rituximab. Anticancer Drugs25(8), 964–969 (2014).
- Athar U , RajanA, GajraA, LynchTP. Incidence of febrile neutropenia (FN) is not altered by the day of administration of pegfilgrastim (PEG). J. Clin. Oncol.25(Suppl. 18), 19636–19636 (2007).
- Billingsley CC , JacobsonSN, CraftonSMet al. Evaluation of the hematologic safety of same day versus standard administration (24- to 72-hour delay) of pegfilgrastim in gynecology oncology patients undergoing cytotoxic chemotherapy. Int. J. Gynecol. Cancer25(7), 1331–1336 (2015).
- Cobb PW , MoonYW, MezeiKet al. A comparison of eflapegrastim to pegfilgrastim in the management of chemotherapy-induced neutropenia in patients with early-stage breast cancer undergoing cytotoxic chemotherapy (RECOVER): a phase III study. Cancer Med.9(17), 6234–6243 (2020).
- Weycker D , LiX, FigueredoJ, BarronR, TzivelekisS, HagiwaraM. Risk of chemotherapy-induced febrile neutropenia in cancer patients receiving pegfilgrastim prophylaxis: does timing of administration matter?Support. Care Cancer24(5), 2309–2316 (2016).
- Lokich J . Same-day pegfilgrastim and chemotherapy. Cancer Invest.23(7), 573–576 (2005).
- Choi B . Institutional chart review on same-day pegfilgrastim administrayion in small cell lung cancer (SCLC) patients receiving myelotoxic chemotherapy. J. Clin. Oncol.39(Suppl. 28), 71 (2021).
- Al-Shamsi HO , AlhazzaniW, AlhuraijiAet al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist25(6), e936–e945 (2020).
