Abstract
Aim: To evaluate the safety and effectiveness of carfilzomib in a real-world setting. Methods: A post-marketing surveillance of Japanese patients with relapsed or refractory multiple myeloma who received carfilzomib treatment was performed. Results: Overall incidences of adverse events of any grade, ≥grade 3 treatment-related adverse events and serious adverse events were 63.5, 44.6 and 37.7% of patients, respectively. No new safety findings were observed. Treatment-related adverse events of special interest (≥5%) were hematological toxicities, infectious disease, cardiac disorders (including cardiac failure, myocardial infarction and QT prolongation), renal disorders, liver failure or liver dysfunction, and hypertension or hypertensive crisis. The overall response rate was 46.5%. Conclusion: Carfilzomib was found to be a safe and effective treatment for relapsed or refractory multiple myeloma in Japanese patients.
Plain language summary
Carfilzomib is a medicine that was recently approved for the treatment of cancer of bone marrow (multiple myeloma) that comes back or does not respond to previous treatment (relapsed or refractory). Data gathered from the hospitals, where the medicine is commonly used, was used to generate evidence. We looked at how well carfilzomib works in Japanese participants and if it is safe. Overall, 63.5% of participants treated with carfilzomib had side effects and 37.7% had serious side effects. Death occurred in 3.1% of participants during the study. Decrease in bone marrow and blood cells, infections, heart and kidney disorder, liver failure or dysfunction, and high blood pressure occurred in 5% or more participants. In 46.5% of participants the tumors had disappeared or shrank. In Japanese participants, carfilzomib was found to be safe and effective treatment for cancer of bone marrow that comes back or does not respond to previous treatment.
Multiple myeloma is a disease with recurring relapses and a poor prognosis. Proteasome inhibitors, immunomodulators and monoclonal antibodies are considered essential parts of standard-of-care regimens. Bortezomib was developed as a first-generation proteasome inhibitor and has contributed to prolonging the survival of the disease [Citation1].
Carfilzomib is an irreversible and selective inhibitor of chymotrypsin-like activity in the proteasomes and has been developed as a second-generation proteasome inhibitor [Citation2,Citation3]. In vitro experimental systems using tumor cell lines showed the accumulation of ubiquitinated proteins and proapoptotic markers with carfilzomib, suggesting its role in cancer cell death [Citation3]. In July 2012 the US FDA granted accelerated approval of carfilzomib for the treatment of multiple myeloma in patients who had received at least two prior therapies, including bortezomib and immune-modulating drugs, and who had disease progression during therapy or within 60 days of completion of the last therapy [Citation4]. Carfilzomib in combination with lenalidomide and dexamethasone (KRd therapy) for treating adult patients with multiple myeloma who have received at least one prior therapy was approved by the EMA in November 2015 [Citation5]. In July 2016 KRd therapy was approved in Japan for the treatment of relapsed or refractory multiple myeloma. In May 2017 carfilzomib in combination with dexamethasone (Kd therapy) was approved for treating relapsed or refractory multiple myeloma as a twice-weekly dosing regimen.
There is limited experience with carfilzomib therapy in Japanese patients. The phase III ASPIRE study comparing KRd therapy with lenalidomide and dexamethasone alone for patients with relapsed multiple myeloma did not enroll any patients from Japan [Citation6]. A Japanese phase I, open-label study (ONO-7057-05) enrolled 26 patients with relapsed or refractory multiple myeloma who had received at least one prior therapy [Citation7]. In the randomized, phase III, open-label multicenter ENDEAVOR study (ONO-7057-03), patients with relapsed or refractory multiple myeloma who had one to three previous treatments received either carfilzomib (n = 464) or bortezomib (n = 465) in combination with dexamethasone. Of these, 102 patients who received carfilzomib were from the Asia–Pacific region [Citation8]. In Japan, data from the ASPIRE study (not including Japanese patients) and the ONO-7057-05 study supported KRd approval, and the ENDEAVOR study supported Kd approval. The small number of Japanese patients enrolled in these studies means that further evaluation is required of the safety and effectiveness of carfilzomib therapy in Japanese patients with relapsed or refractory multiple myeloma.
Carfilzomib therapy is associated with clinically significant cardiovascular adverse events (AEs) [Citation9,Citation10]. In a meta-analysis of 24 prospective studies of carfilzomib in 2594 patients with multiple myeloma, 18.1 and 8.2% patients, respectively, experienced AEs of any grade and grade 3 or higher cardiovascular AEs [Citation9]. The risk of cardiac AEs with carfilzomib is reported to be high in patients of Asian ethnicity [Citation11]; however, there is limited experience of the safety and effectiveness of carfilzomib, including risks for cardiovascular safety, in clinical settings in Japan. This post-marketing surveillance study describes the safety and effectiveness of carfilzomib in a real-world setting for Japanese patients with relapsed or refractory multiple myeloma. This study also identifies factors influencing the safety of carfilzomib. The primary objectives were to understand the background information of patients, collect data for safety and effectiveness and confirm the occurrence of key AEs of special interest (identified in the risk management plan for carfilzomib) for Japanese patients who received carfilzomib therapy.
Patients & methods
Study design & treatment
This multicenter, noninterventional, post-marketing surveillance study was conducted in Japan from August 2016 to May 2021. The surveillance used anonymous data collected in 591 clinical centers. Japanese patients who received KRd therapy or Kd therapy were included. Treatment was administered at the discretion of the prescribing physicians in accordance with the regimens in the approved labels of carfilzomib. In the 28-day treatment cycle of KRd therapy, carfilzomib was administered as a 10-min infusion on days 1, 2, 8, 9, 15 and 16 (starting dose: 20 mg/m2 on days 1 and 2 of cycle 1; target dose of 27 mg/m2 thereafter) during cycles 1–12. From cycle 13 onwards, carfilzomib was administered on days 1, 2, 15 and 16. Lenalidomide (25 mg oral) was given on days 1–21, and dexamethasone (40 mg oral or intravenous) on days 1, 8, 15 and 22 of the 28-day cycle. Patients received a maximum of 18 cycles unless treatment was discontinued for disease progression or unacceptable toxicity. In the 28-day cycle of Kd therapy, patients received carfilzomib (20 mg/m2 on days 1 and 2 of cycle 1; 56 mg/m2 thereafter; 30-min intravenous infusion) on days 1, 2, 8, 9, 15 and 16, and dexamethasone (20 mg oral or intravenous infusion) on days 1, 2, 8, 9, 15, 16, 22 and 23.
Patients were observed from the start of carfilzomib therapy until before the start of cycle 7. Patients who completed or discontinued the use of carfilzomib before the start of cycle 7 were observed until the end or discontinuation of carfilzomib.
The study was conducted in accordance with the Japanese Ministry Directive on Good Post-Marketing Study Practice. The Ministry of Health and Labor and Welfare of Japan approved the surveillance protocol. This study was registered with the Japan Pharmaceutical Information Center clinical trials registry (JapicCTI-No.184068). The approval of the ethics committees of each institution and informed consent from patients were not obtained as the Japanese regulations for post-marketing surveillance do not have a specific mandate.
Study population
Japanese patients with relapsed or refractory multiple myeloma who received treatment with KRd or Kd therapy were included in this study. Patients who received carfilzomib for any other indication or in another dosing regimen were excluded from the effectiveness analysis (effectiveness analysis set), and safety analysis was done for all patients (safety analysis set). Cases with incomplete documentation or records with poor data reliability were excluded from the study.
Study end points
This survey recorded patient background information, safety and effectiveness of carfilzomib therapy in Japanese patients with relapsed and refractory multiple myeloma.
Safety evaluation
AEs were monitored throughout the study. The primary safety outcomes included the incidence of serious AEs and treatment-related AEs (TRAEs). Survey items of special interest for TRAEs were cardiac disorders (heart failure, myocardial infarction, QT prolongation, pericarditis, pericardial effusion), interstitial lung disease, pulmonary hypertension, hypertension or a hypertensive crisis, renal disorder, tumor lysis syndrome, infusion reaction, bleeding, hematological toxicity, venous thromboembolism, hepatic failure or dysfunction, thrombotic microangiopathy, infection, reversible posterior leukoencephalopathy syndrome, gastrointestinal perforation and encephalopathy. All AEs were coded using terminology from the Medical Dictionary for Regulatory Activities (v.22.0) and graded using the Common Terminology Criteria for Adverse Events (v.4). Patients with AEs were followed until resolution. Among the priority survey items including cardiac TRAEs, cardiac TRAEs were selected and the instances were analyzed by grade, time of onset, treatment and outcome, and risk factors for onset. The status of prophylactic administration of antiviral drugs was confirmed.
Effectiveness evaluation
The treating physician determined the tumor shrinkage effects after administration of carfilzomib in accordance with the criteria described by the International Myeloma Working Group [Citation12,Citation13]. The primary effectiveness outcomes included the overall response rate, stringent complete response, complete response, very good partial response, partial response, stable disease, progressive disease and ‘not evaluable’.
Statistical methods
The target sample size was based on the inclusion of all on-label carfilzomib users following consultation with the Japanese health authorities and the Pharmaceuticals and Medical Devices Agency. In the ASPIRE study [Citation6] and the ONO-7057-05 study [Citation7], the lowest incidence of the AEs of interest with carfilzomib was observed for tumor lysis syndrome (0.72%). A sample of size n = 300 will ensure 88.6% power to detect at least one event. The same number of patients who received carfilzomib in combination with dexamethasone was included. Accordingly, it was decided to include ∼600 patients in this study. Safety and effectiveness outcomes were assessed using descriptive statistics. Factors excluded in the safety analysis set were patients with duplicate registration, results with unknown status of AEs, patients with non-use of carfilzomib, those whose questionnaire was completed by personnel other than the registered investigator, those who lost the original materials, those with responses outside the survey window or with responses from facilities not contracted in the survey, and those with unregistered responses.
The effectiveness analysis set excluded patients with: undetermined amount and duration of the use of carfilzomib; disease other than multiple myeloma leading to use of carfilzomib; undetermined reason for the use of treatment; no prior chemotherapy; no dexamethasone or lenalidomide in the first cycle of KRd therapy; administration of lenalidomide in the first cycle after enrollment in the Kd group; and no treatment information about the first cycle.
The incidences of AEs and cardiac TRAEs were assessed for demographic subgroups. Fisher’s exact test (two groups with unpaired nominal data), Wilcoxon rank-sum test, or χ-square test was used to compare between subgroup categories. The level of significance was set to p < 0.05 in two-tailed hypothesis tests. Study results with a response of ‘unknown’ or ‘not reported’ were excluded from the analysis.
Results
Study population
A total of 3280 patients were registered across 591 centers in Japan. Survey forms were collected for 1012 patients. Among the respondents, 583 patients received KRd therapy and 397 patients received Kd therapy. One patient was excluded from the analysis due to no drug administration after registration; 20 patients due to no agreement with some institutions on the disclosure of information; and 37 patients due to meeting exclusion criteria for the effectiveness analysis set (). The safety and effectiveness analyses sets included 991 and 954 patients, respectively.
*Data cutoff date for enrolled patients: 19 November 2019.
**Patients who could not be allocated to the KRd or Kd treatment groups; for instance, those who did not receive dexamethasone. The KRd group comprised patients whose fax registration forms were received by 30 November 2016. The Kd group comprised patients who were scheduled to initiate the study drugs by 4 February 2018.
CRF: Case report form; KD: Carfilzomib + dexamethasone; KRd: Carfilzomib + lenalidomide + dexamethasone.
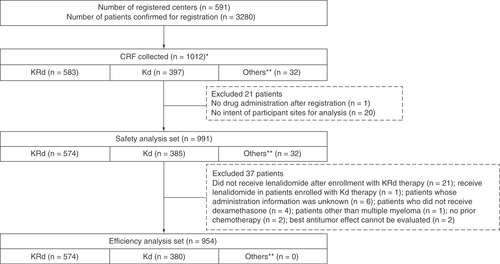
Baseline characteristics of patients in the safety analysis set are shown in . Approximately 80–90% of patients had received prior treatment with bortezomib and/or lenalidomide. Patients had received a median of four treatment regimens before the start of carfilzomib therapy. The median time from diagnosis to start of treatment was 1174.5 days (range: 10–9572; ). A higher percentage of patients aged ≥75 years received Kd therapy (33.8%) compared with KRd therapy (19.2%). Patients who received Kd therapy were older (median age: 71 years) than those who received KRd therapy (median age: 67 years). Patients with any stage for Eastern Cooperative Oncology Group performance status (ECOG PS) and New York Heart Association classification were included. ECOG PS ≥3 was observed in 22.3% of the total patient cohort and in 24.7 and 18.4% of patients treated with KRd and Kd therapy, respectively. Similarly, New York Heart Association Class III or higher was observed in 7.9% of patients overall and in 13.0 and 2.2% of patients treated with KRd and Kd therapy, respectively. The median number of cycles was four (range: one–six), and the median treatment duration was 112 days (range 13–474). The KRd and Kd regimens were given at the approved dose to 83 and 59% of patients, with a relative dose intensity of 98 and 84%, respectively. The Kd regimen was associated with a higher rate of dose reduction.
Table 1. Patient demographic and clinical characteristics.
Safety outcomes
Incidence of treatment-related AEs
Overall, any-grade AEs and grade ≥3 treatment-related AEs (TRAEs) were seen in 63.5 and 44.6% of patients, respectively. The types and frequencies of TRAEs (any grade and ≥ grade 3) were similar for KRd and Kd therapy (). Predominant TRAEs included a decreased platelet count (19.7%), decreased neutrophil count (9.1%), anemia (7.1%), decreased white blood cell count (5.8%) and heart failure (5.1%). KRd therapy was associated with a higher incidence of decreased white blood cell count than Kd therapy (7.5 vs 2.9%, respectively). The overall rate of recovery or improvement of AEs was 77.3%, while the mortality rate was 3.1%. The main causes of death were cardiac disorders (1.3%).
Table 2. Treatment-related adverse events with carfilzomib.
Prophylaxis with antiviral agents against herpes virus was used in 734 (74.1%) patients. Infectious diseases were seen in 0.1% of patients who received prophylactic antiviral treatment and 0.8% of patients who did not receive any prophylaxis. The incidence of TRAEs was significantly associated with International Staging System (ISS) stage, the number of prior chemotherapy regimens, the average number of doses of carfilzomib in each cycle and any prior treatment with bortezomib, lenalidomide or a bortezomib/lenalidomide combination ().
Table 3. Baseline factors associated with treatment-related adverse events.
Incidence of serious AEs
Serious AEs were observed in 37.7% of patients overall; the incidences were 36.9 and 40.5%, respectively, in patients treated with KRd therapy and Kd therapy ().
Table 4. Treatment-related serious adverse events with carfilzomib.
Treatment discontinuation
A total of 669 patients (67.5%) discontinued treatment. The most common reasons for treatment discontinuation were progressive disease (including deaths) in 241 patients (24.3%), AEs in 233 patients (23.5%) and no response to carfilzomib in 194 patients (19.6%). The treatment discontinuation rates were similar between KRd and Kd therapy (69.0 vs 65.5%, respectively).
TRAEs of special interest
shows the TRAEs of special interest in all patients and in those treated with KRd and Kd therapy. TRAEs of special interest with an overall frequency of ≥5% were hematological toxicity (31.6%), infection (15.1%), cardiac disorders (11.3%), renal disorders (6.1%), hepatic failure or liver dysfunction (5.9%) and hypertension or hypertensive crisis (5.4%). Cardiac disorders included cardiac failure (n = 70; 7.1%), myocardial infarction (n = 11; 1.1%) and QT prolongation (n = 14; 1.4%). There were no reports of pericarditis or pericardial effusion. Grade 3 or higher cardiac disorders were observed in 66 patients (6.7%). About two-thirds of the total cardiac disorders were seen within 28 days from the start of carfilzomib therapy (). Cardiac disorders occurred at a median of 15.5 days (range: 1–288) after initiation of carfilzomib therapy, cardiac failure at a median of 20 days (range: 2–288), myocardial infarction 11 days (range: 1–140) and QT extension 13.5 days (range: 2–71). There was no difference in the onset of cardiac disorders between KRd and Kd therapies. The overall rate of recovery or improvement of cardiac disorders was 75.0% (). The mortality rate for patients in whom a cardiac disorder developed was 11.6%. The median time to recovery in cardiac disorders was 19 days (range: 2–340). Discontinuation of carfilzomib was performed in 70.6% of patients with heart failure. There were significant differences in the incidence of cardiac disorders between patients of various age groups: 15–64 years (9.6%), 65–74 years (10.2%), 75–84 years (15.7%) and ≥85 years (13.0%; Wilcoxon rank-sum test: p = 0.0412). Cardiac disorders were significantly higher in patients who had heart-related comorbidities at baseline (24.8%) than in those with no comorbidities (9.8%; Fisher’s exact test: p < 0.0001). ECOG PS, ISS stage of the tumor, levels of β2 microglobulin and albumin, a history of treatment with bortezomib and/or lenalidomide, the number of treatment cycles and the average doses in each cycle were also significantly associated with the development of cardiac disorders as TRAEs ().
Table 5. Treatment-related adverse events of special interest with carfilzomib.
Kd: Carfilzomib + dexamethasone; KRd: Carfilzomib + lenalidomide + dexamethasone.
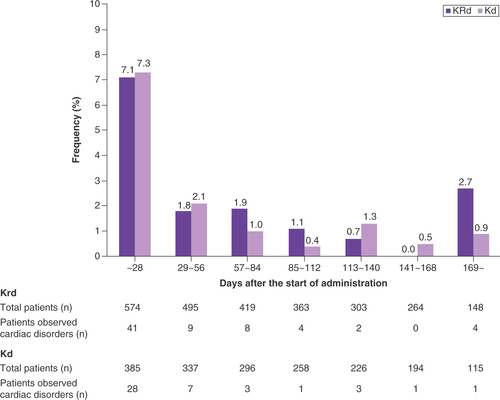
Table 6. Outcomes of cardiac disorders with carfilzomib.
Table 7. Baseline factors associated with cardiac disorders as treatment-related adverse events of special interest.
Effectiveness outcomes
The overall response rate was 46.5% (444/954 cases); response rates were 42.3% (243/574 cases) in the KRd group and 52.9% (201/380 cases) in the Kd group (). When ‘not evaluable’ cases were excluded from the sample, overall response rate was 62.6% (444/709 cases) in the total cohort, 57.3% (243/424 cases) in the KRd group and 70.5% (201/285 cases) in the Kd group.
Table 8. Tumor response with carfilzomib.
Discussion
Post-marketing surveillance is important in routine clinical practice to investigate the safety of a drug in a large nonselective patient population. In this study we evaluated the safety and effectiveness of carfilzomib in the Japanese population. This is the first study to report the background information, overall safety and cardiovascular safety in patients who received treatment with carfilzomib since its approval for use in Japan.
Patients’ characteristics in the study
The median number of pretreatment regimens in the current study was four, and a history of treatment with bortezomib or lenalidomide was reported in 93.6 and 85.9% of patients, respectively. The median number of pretreatment regimens reported in other studies for relapsed refractory multiple myeloma includes four (one–ten) in a phase I study in Japanese patients [Citation14], two (one–three) in the ASPIRE study [Citation6] and two (one–three) in the ENDEAVOR study [Citation8]. Bortezomib treatment history was reported as 65.9, 88.5 and 53.9%, and lenalidomide treatment history was 19.9, 61.5 and 38.1% of patients, respectively, in these three studies, indicating that patients prescribed with carfilzomib in real-world clinical practice were relatively in poorer health compared with patients in clinical studies. This study also included 22.3% of patients with ECOG PS of 3 or higher, who were not enrolled in the clinical studies. The median age of patients in our study was 68 years, which was higher than that of patients included in clinical trials (clinical studies in Japanese patients: 64 years; ENDEAVOR: 65 years; ASPIRE: 64 years) [Citation6,Citation8,Citation14]. The Kd regimen was used more frequently in the elderly in this study, but the reason for this is unknown. Similar median age was reported in a real-world study of carfilzomib-based treatment for relapsed/refractory multiple myeloma in Japan; median ages were 67 years in the KRd group and 70 years in the Kd group [Citation15]. The choice of regimen in this study may have been influenced by the fact that more than 80% of patients had previously received lenalidomide treatment, and elderly patients receiving lenalidomide are more likely to have decreased renal function, necessitating caution in dose selection.
Safety of carfilzomib
This surveillance study reported 63.5% TRAEs and 37.7% serious AEs in patients with relapsed or refractory multiple myeloma who were treated with carfilzomib. Overall, the rates of TRAEs were comparable for KRd therapy and Kd therapy. The predominant AEs included decreased platelet count, decreased neutrophil count, decreased white blood cell count, anemia and heart failure, consistent with previous clinical trials. The overall safety profile was similar to that reported for carfilzomib in other clinical studies in Japanese patients [Citation7,Citation14,Citation16] and real-world data (RWD) in Asian patients [Citation15,Citation17]. No new AEs were reported in this surveillance study. Advanced stage of disease and prior treatment are key factors associated with the development of TRAEs. In patients with ISS stage III, multiple myeloma progresses rapidly and the prognosis is poor. Given that the renal function is reduced at serum β2 microglobulin levels of 5.5 mg/l or higher, which is a criterion for ISS stage III, it is considered that side effects including renal dysfunction and cardiac disorders occur frequently. Patients who have received pretreatment with bortezomib and lenalidomide generally have more advanced disease present poorer health status than those without pretreatment because they were heavily pretreated.
Several reports of RWD with carfilzomib have been published, and cardiac disorders have been reported at a rate of 2–14% [Citation15,Citation17–21]. In our study, cardiac disorders were reported in 11.3% of patients (KRd: 11.8%; Kd: 11.4%). The incidences of individual cardiac disorders incidence were cardiac failure 7.1%, myocardial infarction 1.1% and QT prolongation 1.4%. Hypertension was seen in 5.4% of patients. The incidence of cardiac disorders tended to be slightly higher than that reported in some RWD, probably due to three reasons: cardiac disorders being listed as a safety item of special interest in this study; inclusion of patients with cardiac comorbidities; and enrollment of patients aged over 75 years. In our study the inclusion of 22.3% of patients with ECOG PS ≥3 may explain the higher frequency of cardiac events than that reported in the ASPIRE [Citation6] and ENDEAVOR studies [Citation8]. The ASPIRE and ENDEAVOR studies excluded patients with ECOG PS >2. The risk of cardiac AEs with carfilzomib is reported to be high in patients of Asian ethnicity [Citation22]. Upon performing subgroup analysis on the incidence of cardiac TRAEs using baseline characteristics, we identified the history of cardiac comorbidities as a risk factor. In the report by Kawaji-Kanayama et al., the incidence of cardiac disorders was low because carfilzomib was used after excluding patients with a history of heart disease [Citation15]. In addition, Bishnoi et al. reported that age is also a risk factor for cardiac disorder development and that the frequency is higher in patients aged 75 years or older [Citation23]. Approximately one-quarter of patients in our study were aged 75 years or older, suggesting a high incidence of cardiac disorders. As the incidence of cardiac TRAEs of carfilzomib increases with increasing age or with heart-related comorbidities, caution should be exercised when administering the drug to patients with these backgrounds in routine clinical practice. The other patients’ background and characteristics such as ISS stage, levels of β2 microglobulin and albumin, and pretreatment with bortezomib and lenalidomide, might have contributed to a higher incidence of cardiac events in our study.
About two-thirds of the total cardiac disorders in this study had an onset within 1 month of starting carfilzomib therapy, and the overall rates of recovery or improvement and mortality were 75.0 and 11.6%, respectively. The findings in our study support continued monitoring for cardiac comorbidities with electrocardiography in patients receiving carfilzomib. Plummer et al. reported adequate control of hypertension and consultation with a cardiologist, if necessary, as key measures to prevent cardiac AEs in patients with multiple myeloma who receive proteasome inhibitors [Citation24]. Cardiovascular AEs are common with the use of proteasome inhibitors for relapsed or refractory multiple myeloma. These are commonly encountered within the first 3 months of therapy and do not require discontinuation of therapy. For optimal treatment outcomes, it is important to identify patients who are at risk of cardiovascular AEs. Although serum B-type natriuretic peptide (BNP) was not tested in this study, it may be useful in predicting carfilzomib-induced cardiac injury in patients with multiple myeloma. Cornell et al. reported that the risk of cardiovascular AEs in patients receiving carfilzomib-based therapy was higher (odds ratio: 10.8; p < 0.001) in a subgroup of patients with elevated baseline BNP (>100 pg/ml) or N-terminal proBNP (>125 pg/ml); elevated BNP during the mid-first cycle of treatment also increased the risk of cardiovascular AEs (odds ratio: 36.0; p < 0.001) [Citation25]. Future research may validate the usefulness of BNP for predicting cardiovascular AEs during carfilzomib treatment. A position paper on assessing baseline cardiovascular risk in cancer patients receiving cardiotoxic cancer therapy has been published recently. Proteasome inhibitors are described, which may be helpful from the standpoint of cardio-oncology [Citation26].
In our study 74.1% of patients received prophylaxis with antiviral agents for herpes virus. All patients in the ASPIRE [Citation6] and ENDEAVOR [Citation8] studies received antiviral prophylaxis. In this study the number of people infected with the herpes virus was small, so it was impossible to evaluate the impact of prophylactic administration of antiviral drugs.
Effectiveness of carfilzomib
In our study the overall response rate was 46.5% among the total patient cohort, 42.3% for KRd-treated patients and 52.9% for those treated with Kd therapy. These data suggest that carfilzomib is an effective therapeutic agent for relapsed or refractory myeloma in clinical practice. In the other RWD studies, the overall response rates for KRd and Kd have been reported as 73–85 and 74%, respectively [Citation15,Citation17,Citation19–21]. The overall response rate observed in this study was lower than that in the ASPIRE [Citation6] and ENDEAVOR studies [Citation8] and the real-world studies. These differences could be attributed to numerous patients with more advanced disease and may be associated with a higher number of pretreatment regimens than in the other RWD studies, suggesting that these patients may have had a lower response to drugs. Indeed, in this study more than 85% of patients had received bortezomib or lenalidomide. Additionally, the tumor response could only be determined in 46% of the patients, and about one-quarter of cases in each of the two treatment groups were undetermined cases, which might have affected the overall effectiveness results. After excluding ‘not evaluable’ patients, 57.3 and 70.5% of patients had at least a partial response for KRd and Kd, respectively. Carfilzomib has been considered valuable for treating relapsed or refractory multiple myeloma because it demonstrated therapeutic effects even in patients with these risk factors.
Limitations
Limitations of our study include the short observation period of up to 6 months, the absence of a control group because this was a real-world post-marketing surveillance study, and lack of data on progression-free survival and overall survival as outcome indicators. As an indicator of effectiveness, this study only collected the best responses according to the International Myeloma Working Group response criteria [Citation13]. The follow-up period for this study was 6 months, which we thought was too short to assess overall survival. Furthermore, the study protocol did not specify the exact timing of response assessments. As a result, we did not collect progression-free survival or overall survival data.
Conclusion
Carfilzomib was demonstrated to be a safe and effective treatment option for patients with relapsed or refractory multiple myeloma in routine clinical practice in Japan. Patients receiving carfilzomib should be monitored for cardiac comorbidities during the course of the treatment. The findings of this study are expected to serve as a guide for the proper use of carfilzomib regimens in clinical settings. It is hoped that understanding the characteristics of carfilzomib-related AEs will aid in the early detection and management of AEs in a healthcare setting.
Carfilzomib (either carfilzomib + lenalidomide + dexamethasone or carfilzomib + dexamethasone) is approved for the treatment of relapsed or refractory multiple myeloma.
However, current experience with carfilzomib therapy in Japanese patients with relapsed or refractory multiple myeloma is limited.
This was a prospective surveillance study of Japanese patients with relapsed or refractory multiple myeloma who received treatment with carfilzomib. Tumor response to carfilzomib regimens, adverse events (AEs), serious AEs and treatment-related AEs (TRAEs), including those of special interest, were all documented.
Overall incidences of any-grade AEs, ≥grade 3 TRAEs and serious AEs were 63.5, 44.6 and 37.7%, respectively. Compared with previous clinical trials, no new safety findings were observed.
Hematological toxicities (31.6%), infectious disease (15.1%), cardiac disorders (11.3%), renal disorders (6.1%), liver failure or liver dysfunction (5.9%) and hypertension or hypertensive crisis (5.4%) were among the TRAEs of special interest.
Cardiac disorders included heart failure (7.1%), myocardial infarction (1.1%) and QT prolongation (1.4%).
The overall response rate was 46.5%.
The results suggest that carfilzomib is a safe and effective treatment option for patients with relapsed or refractory multiple myeloma in routine clinical practice in Japan.
Author contributions
A Kawasaki and M Matsushita contributed to the conception and design of the analysis, data collection, data/analysis tools and writing of the paper, and performed the analysis. H Murakami, T Chou and M Kizaki contributed to the conception and design of the analysis and wrote the paper. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work and have given final approval for the version to be published.
Financial & competing interests disclosure
A Kawasaki and M Matsushita are employees of Ono Pharmaceutical Co. Ltd. M Kizaki received the following support: consulting fees from Ono Pharmaceutical Co. Ltd; research grants from Ono Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, Kyowa Kirin Co. Ltd, Chugai Pharmaceutical Co. Ltd and Daiichi Sankyo Co. Ltd; lecture fees from Bristol Myers Squibb, Novartis Pharma, Janssen Pharmaceutical K. K., MSD, Sumitomo Dainippon Pharma Co. Ltd, Nippon Shinyaku Co. Ltd, Ono Pharmaceutical Co. Ltd and Takeda Pharmaceutical Co. Ltd. H Murakami received the following support: consulting fees from Ono Pharmaceutical Co. Ltd and Sanofi K. K.; research grants from Celgene Corp., Takeda Pharmaceutical Co. Ltd and Daiichi Sankyo Co. Ltd. T Chou received consulting fees from Ono Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd and BMS Japan. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
The authors thank J Tarveen and B Sreeharsha, Enago Life Sciences, India for providing medical writing support, which was funded by Ono Pharmaceuticals Co. Ltd, Japan.
Ethical conduct of research
The approval of the ethics committees of each institution and informed consent from patients were not obtained as the Japanese regulations for post-marketing surveillance do not have a specific mandate.
Data sharing statement
Qualified researchers may request Ono Pharma to disclose individual patient-level data from clinical studies through the following website: https://www.clinicalstudydatarequest.com/. For more information on Ono Pharma’s Policy for the Disclosure of Clinical Study Data, please see the following website: https://www.ono.co.jp/eng/rd/policy.html.
Additional information
Funding
References
- Kumar SK , RajkumarSV, DispenzieriAet al. Improved survival in multiple myeloma and the impact of novel therapies. Blood111(5), 2516–2520 (2008).
- Demo SD , KirkCJ, AujayMAet al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res.67(13), 6383–6391 (2007).
- Parlati F , LeeSJ, AujayMet al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood114(16), 3439–3447 (2009).
- Herndon TM , DeisserothA, KaminskasEet al. US Food and Drug Administration approval: carfilzomib for the treatment of multiple myeloma. Clin. Cancer Res.19(17), 4559–4563 (2013).
- Tzogani K , CamareroJiménez J, GarciaIet al. The European Medicines Agency review of carfilzomib for the treatment of adult patients with multiple myeloma who have received at least one prior therapy. Oncologist22(11), 1339–1346 (2017).
- Stewart AK , RajkumarSV, DimopoulosMAet al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med.372, 142–152 (2015).
- Suzuki K , RiM, ChouTet al. Carfilzomib, lenalidomide and dexamethasone in patients with heavily pretreated multiple myeloma: a phase 1 study in Japan. Cancer Sci.108(3), 461–468 (2017).
- Dimopoulos MA , MoreauP, PalumboAet al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol.17(1), 27–38 (2016).
- Waxman AJ , ClasenS, HwangWTet al. Carfilzomib-associated cardiovascular adverse events: a systematic review and meta-analysis. JAMA Oncol.4(3), e174519-e (2018).
- Mushtaq A , KapoorV, LatifAet al. Efficacy and toxicity profile of carfilzomib based regimens for treatment of multiple myeloma: a systematic review. Crit. Rev. Oncol. Hematol.125, 1–11 (2018).
- Bringhen S , MilanA, FerriCet al. Cardiovascular adverse events in modern myeloma therapy – incidence and risks. A review from the European Myeloma Network (EMN) and Italian Society of Arterial Hypertension (SIIA). Haematologica103(9), 1422–1432 (2018).
- Durie BG , HarousseauJL, MiguelJSet al. International uniform response criteria for multiple myeloma. Leukemia20, 1467–1473 (2006).
- Kumar S , PaivaB, AndersonKCet al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol.17(8), e328–e346 (2016).
- Maruyama D , TobinaiK, ChouT, TaniwakiM, ShumiyaY, IidaS. Weekly carfilzomib and dexamethasone in Japanese patients with relapsed or refractory multiple myeloma: a phase 1 and PK/PD trial. Cancer Sci.109(10), 3245–3252 (2018).
- Kawaji-Kanayama Y , KobayashiT, MuramatsuAet al. Prognostic impact of resistance to bortezomib and/or lenalidomide in carfilzomib-based therapies for relapsed/refractory multiple myeloma: the Kyoto Clinical Hematology Study Group, multicenter, pilot, prospective, observational study in Asian patients. Cancer Rep. (Hoboken)5(2), e1476 (2022).
- Sugiura I , SuzukiK, RiMet al. Final results of a phase I study of carfilzomib, lenalidomide, and dexamethasone for heavily pretreated multiple myeloma. Int. J. Hematol.111, 57–64 (2019).
- Lee JH , ParkY, KangKWet al. Carfilzomib in addition to lenalidomide and dexamethasone in Asian patients with RRMM outside of a clinical trial. Ann. Hematol.100(8), 2051–2059 (2021).
- Zhai Y , YeX, HuFet al. Cardiovascular toxicity of carfilzomib: the real-world evidence based on the adverse event reporting system database of the FDA, the United States. Front. Cardiovasc. Med.8, 735466 (2021).
- Palmieri S , RoccoS, VitaglianoOet al. KRD (carfilzomib and lenalidomide plus dexamethasone) for the treatment of relapsed or refractory multiple myeloma in the real-life: a retrospective survey in 123 patients. Ann. Hematol.99(12), 2903–2909 (2020).
- Mele A , PreteE, DeRisi Cet al. Carfilzomib, lenalidomide, and dexamethasone in relapsed/refractory multiple myeloma patients: the real-life experience of Rete Ematologica Pugliese (REP). Ann. Hematol.100(2), 429–436 (2021).
- Rocchi S , TacchettiP, PantaniLet al. A real-world efficacy and safety analysis of combined carfilzomib, lenalidomide, and dexamethasone (KRd) in relapsed/refractory multiple myeloma. Hematol. Oncol.39(1), 41–50 (2021).
- Dimopoulos MA , MoreauP, IidaSet al. Outcomes for Asian patients with multiple myeloma receiving once- or twice-weekly carfilzomib-based therapy: a subgroup analysis of the randomized phase 3 ENDEAVOR and A.R.R.O.W.trials. Int. J. Hematol.110(4), 466–473 (2019).
- Bishnoi R , XieZ, ShahCet al. Real-world experience of carfilzomib-associated cardiovascular adverse events: SEER–Medicare data set analysis. Cancer Med.10(1), 70–78 (2021).
- Plummer C , DriessenC, SzaboZ, MateosMV. Management of cardiovascular risk in patients with multiple myeloma. Blood Cancer J.9, 26 (2019).
- Cornell RF , KyB, WeissBMet al. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J. Clin. Oncol.37(22), 1946–1955 (2019).
- Lyon AR , DentS, StanwaySet al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail.22(11), 1945–1960 (2020).
