Abstract
Several anti-HER2 agents are approved for third-line treatment and beyond (after first-line and second-line); however, no specific treatment strategy is recommended for third-line and beyond. Although these agents improve disease outcomes, HER2-positive metastatic breast cancer remains incurable and there is an unmet need for effective therapies in the later line setting. This review focuses on the development of margetuximab-cmkb, a novel, Fc-engineered, anti-HER2 monoclonal antibody, and its role in the systemic treatment of adult patients with metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease.
Plain Language Summary
A new treatment option for patients with HER2-positive metastatic breast cancer
In about 20% of patients with breast cancer, their tumor cells make too many copies of a protein called HER2. We call them HER2-positive breast cancer cells. HER2 is a protein that signals to breast cancer cells to make them grow. Certain drugs, known as antibodies, are able to bind to the HER2 proteins on the surface of the tumor cells. This stops their signaling and slows down the growth of the tumor cells. These antibodies are called anti-HER2 antibodies. In addition to its ‘head’ region binding to HER2, the ‘tail’ region of the anti-HER2 antibody can bind to certain other proteins (receptors) found on the surface of immune cells. When the anti-HER2 antibodies bind to the receptors on immune cells, this starts an anticancer immune response against the HER2-positive breast cancer cells and kills them. This review explains how anti-HER2 antibodies may block and destroy HER2-positive breast cancer cells. In particular, we focus on the beneficial and adverse effects of margetuximab, an anti-HER2 antibody. The tail region of margetuximab has been changed to boost the immune responses against HER2-positive cancer cells. Margetuximab is approved in the USA for patients with HER2-positive metastatic breast cancer after they have already received two or more anti-HER2 therapies. The decision to approve this was based on the pivotal clinical trial SOPHIA.
HER2-positive breast cancer
The occurrence of newly diagnosed, invasive breast cancer in the USA in 2022 is estimated at 300,590 new cases, with 43,700 breast cancer deaths [Citation1]. Current guidelines recommend that testing for HER2 should be performed on all primary and newly metastatic breast cancers (MBC) [Citation2,Citation3]. In fact, approximately 20% of breast cancers overexpress HER2, based on assessments of HER2 immunohistochemistry and/or ERBB2 gene amplification [Citation4].
The 5-year survival rate for patients with HER2-positive distant MBC from 2011 to 2017 was 39–46%, depending on hormone receptor status [Citation5]. Newer treatments may improve that rate. The current standard of care for HER2-positive MBC is dual anti-HER2 antibody therapy with pertuzumab (Perjeta®) and trastuzumab (Herceptin®) plus a chemotherapy as first-line treatment, followed by fam-trastuzumab deruxtecan-nxki (Enhertu®) or ado-trastuzumab emtansine (Kadcyla®) or tucatinib (Tuksya®) plus trastuzumab plus capecitabine (Xeloda®) as second-line treatment and beyond [Citation3,Citation6,Citation7]. Resistance to anti-HER2 therapy remains a major therapeutic challenge.
Until December 2019, treatment options for third-line and beyond included chemotherapy with trastuzumab or lapatinib (Tykerb®, Tyverb®), and the combination of trastuzumab with lapatinib [Citation6,Citation7]. With these regimens, the median overall survival (OS) ranged from 11.8 to 18.8 months [Citation8–12]. More recently, novel anti-HER2 agents (trastuzumab deruxtecan, neratinib [Nerlynx®], tucatinib, and margetuximab-cmkb [Margenza®]) have become available in this setting [Citation3,Citation6,Citation13–16]. Although trastuzumab deruxtecan is used as monotherapy [Citation14], neratinib [Citation16] and margetuximab [Citation13] are both used in combination with chemotherapy, and tucatinib [Citation15] is used in combination with trastuzumab and capecitabine. With these new agents, the median OS ranges from 21.0 to 28.4 months [Citation16–19]. Despite these advances, the need for improved treatments of MBC in third-line treatment and beyond persists.
Preclinical development of margetuximab & its mechanism of action
Margetuximab is a novel HER2/neu receptor antagonist, approved by the US FDA in December 2020, in combination with chemotherapy, for the treatment of adult patients with metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease [Citation13].
Margetuximab is a fragment crystallizable (Fc)-engineered, HER2-targeted IgG1 monoclonal antibody (mAb). The fragment antigen-binding (Fab) domain of margetuximab is derived from the same parental murine antibody (4D5) as trastuzumab, and margetuximab and trastuzumab bind HER2 with similar high affinity [Citation20,Citation21]. Direct binding of margetuximab to HER2 on the surface of tumor cells disrupts HER2 signaling and causes antiproliferative effects that are comparable with those mediated by trastuzumab [Citation20–22] (, Left). The engineered Fc region of margetuximab features five amino acid substitutions (L235V, F243L, R292P, Y300L, and P396L) compared with the native Fc region of trastuzumab [Citation21,Citation23,Citation24]. This engineering enhances binding to FcγRIIIa (CD16A), an activating Fc gamma receptor (FcγR), and diminishes binding to FcγRIIb (CD32B), an inhibitory FcγR (). As a result, margetuximab stimulates Fc-dependent innate immune responses, such as antibody-dependent cellular cytolysis (ADCC) mediated by natural killer (NK) cells, more potently than trastuzumab in vitro [Citation20,Citation21,Citation24] and ex vivo [Citation25] (, Right).
Figure 1. Mechanism of action of anti-HER2 mAb margetuximab and differences between trastuzumab and margetuximab.
(A) Margetuximab mediates antiproliferative (Fc-independent) and immune activation (Fc-dependent) effects. (B) The Fc engineering of margetuximab alters Fcγ receptor affinities. The red X in panel A (on the left side) indicates inhibition.
ADCC: Antibody-dependent cellular cytotoxicity; ADCP: Antibody-dependent cellular phagocytosis; CD: Cluster of differentiation; Fab: Fragment antigen-binding; Fc: Fragment crystallizable; FcγR: Fragment crystallizable gamma receptor; IgG: Immunoglobin G; M: Margetuximab; mAb: Monoclonal antibody; MHC II: Major histocompatibility complex class II; NK: Natural killer; T: Trastuzumab; TAA: Tumor-associated antigen.
(A) reproduced from [Citation26], with permission from BMJ Publishing Group Ltd.
![Figure 1. Mechanism of action of anti-HER2 mAb margetuximab and differences between trastuzumab and margetuximab.(A) Margetuximab mediates antiproliferative (Fc-independent) and immune activation (Fc-dependent) effects. (B) The Fc engineering of margetuximab alters Fcγ receptor affinities. The red X in panel A (on the left side) indicates inhibition.ADCC: Antibody-dependent cellular cytotoxicity; ADCP: Antibody-dependent cellular phagocytosis; CD: Cluster of differentiation; Fab: Fragment antigen-binding; Fc: Fragment crystallizable; FcγR: Fragment crystallizable gamma receptor; IgG: Immunoglobin G; M: Margetuximab; mAb: Monoclonal antibody; MHC II: Major histocompatibility complex class II; NK: Natural killer; T: Trastuzumab; TAA: Tumor-associated antigen.(A) reproduced from [Citation26], with permission from BMJ Publishing Group Ltd.](/cms/asset/10049847-87f9-4ab4-902e-606c04f7a96c/ifon_a_12333771_f0001.jpg)
Importantly, the engineered Fc of margetuximab has increased binding to both polymorphic variants of CD16A, which have either valine (V) or phenylalanine (F) at position 158 (). These CD16A variants bind IgG1 Fc domains with differing affinities that correlate with relative ADCC potency [Citation26]. The CD16A-158V variant has a higher affinity for IgG1 Fc compared with the CD16A-158F variant, and NK cells from donors homozygous for the higher affinity variant (VV genotype) generally mediate more potent ADCC in vitro than NK cells from donors homozygous for the lower affinity variant (FF genotype) [Citation26]. The VV, FF, and VF genotypes are found in about 12, 43 and 45% of patients, respectively [Citation26]. These percentages are consistent with frequencies worldwide; in fact, F carriers (VF and FF genotypes) make up approximately 88% of the worldwide population. Margetuximab mediates ADCC in vitro more potently than trastuzumab with NK cells obtained from donors of all CD16A genotypes [Citation20,Citation21]. Margetuximab also exhibits improved in vivo antitumor activity against breast cancer tumor xenografts in mice transgenic for human CD16A-158F, the lower affinity Fc receptor variant, compared with a wild-type (nonengineered) Fc version of margetuximab [Citation21]. In addition to enhancing innate immune responses, margetuximab has also been shown to potentiate adaptive immune responses in treated patients, as shown by increased clonality of the T-cell repertoire, enhanced HER2-specific T-cell responses, and increased levels of endogenous HER2-specific antibodies in blood samples compared with pretreatment samples [Citation27].
Clinical studies of HER2-positive breast cancer, including MBC, found different outcomes related to the expression of the CD16A-158 F or V variants [Citation28–31]. These data suggest that patients with MBC carrying the F allele (FF homozygous or FV heterozygous genotype) may have lower objective response rates than VV homozygous and shorter median progression-free survival (PFS) after trastuzumab therapy [Citation29]. In the adjuvant setting, patients receiving trastuzumab who had the FF genotype appeared to have a worse prognosis than those with VV or VF genotypes and shorter disease-free survival [Citation28]. However, contradictory findings from studies of adjuvant and advanced patients treated with trastuzumab showed no correlation between CD16A genotype and disease-free survival outcomes [Citation32,Citation33].
Preclinical toxicology of margetuximab has been examined in enhanced prenatal and postnatal developmental studies [Citation13,Citation34]. Maternofetal transfer of margetuximab was confirmed. Based on findings in pregnant cynomolgus monkeys and known class effects of anti-HER2 antibodies, margetuximab may cause embryo-fetal harm [Citation13,Citation34]. In an animal reproduction study, intravenous (IV) administration of margetuximab to pregnant cynomolgus monkeys once every 3 weeks (Q3W) starting at gestational day 20 until delivery resulted in oligohydramnios and delayed infant kidney development [Citation34]. In postmarketing studies, use of other HER2-directed antibodies during pregnancy resulted in cases of oligohydramnios and oligohydramnios sequence manifesting as pulmonary hypoplasia, skeletal abnormalities and neonatal death [Citation13]. Hence, before margetuximab treatment, patients should be advised of the risk and the need for effective contraception during and 4 months after treatment.
Clinical development of margetuximab
Phase I
In a phase I, multicenter, open-label, dose-escalation and expansion study (CP-MGAH22-01/NCT01148849), margetuximab monotherapy was evaluated in 66 patients with refractory HER2-overexpressing carcinomas for whom no standard therapy was available, including 24 patients with breast cancer [Citation25]. Two regimens of margetuximab via IV administration were tested as follows: 34 patients received margetuximab weekly at doses ranging from 0.1 to 6.0 mg/kg and 32 patients received margetuximab Q3W at doses ranging from 10.0 to 18.0 mg/kg. Margetuximab was well tolerated at all doses, with common toxicities primarily of grades 1 and 2. Among the 66 patients evaluable for safety, the most common treatment-emergent adverse events (TEAE; ≥20%) were fatigue, pyrexia, anemia, and nausea. Treatment-related infusion-related reactions (IRR) were observed in 18% of patients. No patients experienced abnormalities in the left ventricular ejection fraction (LVEF). Overall, seven of 60 patients (12%) evaluable for response had confirmed partial response (PR) according to the Response Evaluation Criteria in Solid Tumours guidelines (version 1.1). Among 24 patients with breast cancer evaluable for response, 11 (46%) experienced tumor reduction, with confirmed partial responses in four (17%) [Citation35]. Durable responses, lasting for at least 5.25 years, were seen in three patients with HER2-positive MBC who had received 3–4 prior lines of therapy, with no cardiac toxicities, nor treatment-related adverse events (TRAE) of grade ≥3 [Citation35]. Pharmacokinetics (PK) of margetuximab monotherapy followed a two-compartment model with parallel linear and Michaelis–Menten elimination, and the estimated PK parameters aligned with those expected for a mAb of the human IgG1 isotype [Citation25]. Terminal half-life was estimated at 15.5 days [Citation25].
Phase II
To test whether margetuximab may have utility in patients with low HER2-expressing breast tumors, a single-arm, open-label, phase II study (CP-MGAH22-02/NCT01828021) of margetuximab monotherapy in patients with relapsed or refractory breast cancer was conducted. Patients with low HER2 expression, whose tumors have a HER2 immunohistochemistry (IHC) score of 2+ and lack evidence of HER2 gene amplification by FISH or have a HER2 IHC score of 1+, were included in the study [Citation36]. Overall, 25 patients with low HER2 expression were enrolled as follows: 14 patients treated at 6.0 mg/kg via IV weekly infusions and 11 patients treated at 15 mg/kg via IV Q3W infusions. Futility criteria were met; no PR or complete response (CR) among 21 response-evaluable patients was observed, indicating that margetuximab monotherapy does not provide clinical benefit in low HER2 expressors with breast cancer (NCT01828021).
Phase III SOPHIA
Margetuximab safety and efficacy were described in phase III data from the SOPHIA study (CP-MGAH22-04/NCT02492711) in patients with HER2-positive MBC treated with margetuximab 15 mg/kg IV Q3W in combination with physician’s choice chemotherapy compared with trastuzumab 6 mg/kg (8 mg/kg for loading dose) IV Q3W plus chemotherapy after at least two previous anti-HER2 therapies including pertuzumab [Citation37]. Dual primary end points, tested sequentially, were used to independently assess PFS and OS.
Efficacy
A total of 536 patients were randomized to receive margetuximab plus chemotherapy (n = 266) or trastuzumab plus chemotherapy (n = 270). Margetuximab improved median PFS, the primary end point, by central review over trastuzumab with 24% relative risk reduction (hazard ratio [HR], 0.76; 95% CI, 0.59–0.98; p = 0.03; median, 5.8 [95% CI, 5.5–7.0] months vs 4.9 [95% CI, 4.2–5.6] months; ) [Citation37]. Margetuximab recipients had higher investigator-assessed objective response rate than trastuzumab recipients (26% vs 14) [Citation19]. Importantly, Fc domain engineering to enhance immune effector function was shown to be clinically beneficial in SOPHIA. Efficacy analysis by CD16A-158 allele expression in 506 genotyped patients from the SOPHIA study showed that PFS benefit of margetuximab over trastuzumab was increased in F carriers, representing 86% of the population examined (median PFS, 6.9 vs 5.1 months; HR, 0.68; 95% CI, 0.52–0.90; p = 0.005) [Citation37]. Conversely, no margetuximab PFS benefit over trastuzumab was seen in VV homozygotes, representing 14% of the population examined (median PFS, 4.8 vs 5.6 months; HR, 1.78; 95% CI, 0.87–3.62; p = 0.110) [Citation37].
Figure 2. SOPHIA progression-free survival (A) and overall survival (B) in the intent-to-treat population.
HR: Hazard ratio; ITT: Intent-to-treat; mo: Month; OS: Overall survival; PFS: Progression-free survival.
(A) Reproduced from [Citation37], with permission from Rugo HS. (B) Reproduced from [Citation19], https://ascopubs.org/journal/jco/.
![Figure 2. SOPHIA progression-free survival (A) and overall survival (B) in the intent-to-treat population.HR: Hazard ratio; ITT: Intent-to-treat; mo: Month; OS: Overall survival; PFS: Progression-free survival.(A) Reproduced from [Citation37], with permission from Rugo HS. (B) Reproduced from [Citation19], https://ascopubs.org/journal/jco/.](/cms/asset/838ec4b2-1481-4475-8420-d4822c65d689/ifon_a_12333771_f0002.jpg)
Physician’s choice of chemotherapy was allowed in SOPHIA, and patients received either capecitabine (Cap; n = 143), eribulin (Halaven®, Eri; n = 136), gemcitabine (Gemzar®, Gem; n = 66), or vinorelbine (Navelbine®, Vin; n = 191). A subgroup analysis of PFS by chemotherapy backbone (data cut-off October 2018) in SOPHIA revealed that margetuximab improved PFS over trastuzumab across all chemotherapy subgroups () [Citation37,Citation38].
Table 1. PrespecifiedTable Footnote† exploratory progression-free survival and overall survival subgroup analyses by chemotherapy backbone.
The primary analysis of PFS by central review of SOPHIA led to the FDA approval of margetuximab with chemotherapy in patients with HER2-positive MBC who have received ≥2 prior anti-HER2 regimens, at least one of which was for metastatic disease [Citation13]. Based on a recent publication [Citation19], the SOPHIA final OS analysis for the intent-to-treat population did not demonstrate a statistically significant advantage in the margetuximab group compared with the trastuzumab group (median OS: 21.6 vs 21.9 months; HR: 0.95; 95% CI: 0.77–1.17; p = 0.620; ), whereas a numerical OS advantage was observed in the subgroup of patients homozygous for the CD16A-158 F allele. Specifically, a preplanned, exploratory analysis of CD16A genotyping demonstrated a numerical OS advantage for margetuximab in FF homozygous patients versus trastuzumab (median OS: 23.6 vs 19.2 months; HR: 0.72; 95% CI: 0.52–1.00; nominal p = 0.052) and a numerical OS advantage for trastuzumab in VV homozygous patients versus margetuximab (median OS: 31.1 vs 22.0 months; HR: 1.77; 95% CI: 1.01–3.12; nominal p = 0.044; ). There was an imbalance for the VV homozygotes in poor prognostic features between the margetuximab and trastuzumab arms, which may potentially serve as a biological basis for the observation that margetuximab provided less clinical benefit compared with trastuzumab in VV homozygotes [Citation19]. A subgroup analysis of OS by chemotherapy backbone (data cut-off June 2021) in SOPHIA confirmed not statistically significant differences among chemotherapy subgroups () [Citation19]. The HR differences among chemotherapy subgroups may have been driven by small number of patients for some subgroup, along with selection bias and/or tumor sensitivity to individual chemotherapies.
Figure 3. SOPHIA overall survival analysis, per CD16A genotype by treatment group.
(A) Kaplan–Meier estimates of OS by treatment group in CD16A-158F carriers (FF or FV), (B) CD16A-158FF homozygotes, (C) CD16A-158FV heterozygotes and (D) CD16A-158VV homozygotes.
HR: Hazard ratio; OS: Overall survival.
Reproduced from [Citation19], https://ascopubs.org/journal/jco/.
![Figure 3. SOPHIA overall survival analysis, per CD16A genotype by treatment group.(A) Kaplan–Meier estimates of OS by treatment group in CD16A-158F carriers (FF or FV), (B) CD16A-158FF homozygotes, (C) CD16A-158FV heterozygotes and (D) CD16A-158VV homozygotes.HR: Hazard ratio; OS: Overall survival.Reproduced from [Citation19], https://ascopubs.org/journal/jco/.](/cms/asset/cd8252f7-ffdb-4dbf-97df-51028864c76f/ifon_a_12333771_f0003.jpg)
Safety
In SOPHIA, margetuximab safety was comparable with trastuzumab [Citation19,Citation37]. Common TEAEs occurring in ≥20% of patients were fatigue (42 vs 36%), nausea (33% each), diarrhea (26 vs 25%), and neutropenia (29 vs 21%), respectively, in both groups, as well as vomiting (21%) and pyrexia (20%) in the margetuximab group and anemia (24%) in the trastuzumab group. Grade ≥3 TEAEs in ≥5% of patients were neutrophil count decreased (9 vs 11%) and anemia (5 vs 6%), respectively in both groups, as well as fatigue (5%) in the margetuximab group and febrile neutropenia (5%) in the trastuzumab group.
To provide a comprehensive overview of margetuximab safety, a pooled integrated safety analysis [Citation39] of margetuximab from the phase I margetuximab monotherapy study (NCT01148849) [Citation25], the phase II margetuximab monotherapy study (NCT01828021) [Citation36], and the phase III SOPHIA study of margetuximab plus chemotherapy (NCT02492711) [Citation37] was undertaken. Among a total of 355 patients, 98% had ≥1 TEAE (60% TRAEs) and 49% had ≥1 TEAE of grade ≥3 [Citation39]. Serious TEAE incidence across studies was 16% (2% serious TRAEs), and 6% of patients discontinued margetuximab because of TEAEs. The most frequently reported TEAEs (≥20%) were fatigue (35%), nausea (29%), diarrhea (21%) and neutropenia (21%). Across the three studies, margetuximab demonstrated an acceptable safety profile and has been administered without long-term safety issues. Adverse events of special interest included IRRs and left ventricular (LV) dysfunction.
In SOPHIA, all-grade IRRs were more common with margetuximab than with trastuzumab (14 vs 3%, respectively) [Citation19]. In patients receiving margetuximab, most IRRs were grade 1 or 2 severity (86%). Among margetuximab recipients, grade ≥3 IRRs were reported in 2% of patients and IRRs leading to discontinuation occurred in 1% of patients. No trastuzumab recipients had grade ≥3 IRRs or IRRs leading to discontinuation. Most IRRs in both groups occurred on cycle 1/day 1 and resolved within 24 h [Citation37]. All IRRs were medically manageable with supportive care or interruption of margetuximab [Citation40]. Premedication for margetuximab within 30 minutes of administration is recommended, if not already given with chemotherapy, and includes standard doses of acetaminophen or ibuprofen, diphenhydramine, ranitidine and dexamethasone or equivalents [Citation37]. IRRs on first infusion of margetuximab appear to resemble those observed after first infusion of trastuzumab, based on the trastuzumab USA prescribing information and a retrospective review of IRRs in patients receiving trastuzumab [Citation41,Citation42].
In parallel with SOPHIA, a single-arm, nonrandomized infusion substudy was conducted on 88 patients with HER2-positive MBC to determine whether infusion duration of margetuximab, from cycle 2 onward, could be safely reduced from 120 to 30 minutes [Citation43]. Among 88 patients enrolled, 69 received margetuximab plus chemotherapy and 19 received margetuximab alone. Margetuximab was administered at 15 mg/kg Q3W IV over 120 minutes for the initial dose, then over a minimum of 30 minutes for all subsequent doses. The overall rate of IRRs was 21%, all IRRs were grade ≤2 and most events occurred during the first 120 minutes of administration of margetuximab. This infusion substudy shows that acceptable safety and tolerability of margetuximab were maintained after infusion time was reduced to 30 minutes from cycle 2 onward.
All-grade LV dysfunction was reported in 3% in each treatment group [Citation19,Citation37]. Rates of grade ≥3 LV dysfunction adverse events were 1% in margetuximab recipients and 0.4% in trastuzumab recipients. Monitoring for LVEF led to dose delays or discontinuations in 2% of margetuximab-treated patients and in 3% of trastuzumab-treated patients [Citation19]. All LVEF reductions detected by monitoring were asymptomatic and reversible with complete follow-up [Citation37]. LVEF is a known class effect of HER2-directed therapies and was included in the Warnings and Precautions section in FDA labeling of anti-HER2 agents [Citation13,Citation14,Citation41,Citation44–46]. Margetuximab has not been studied in patients with a pretreatment LVEF value of <50%, a prior history of myocardial infarction or unstable angina within 6 months, or congestive heart failure New York Heart Association class II–IV.
Quality of life
In SOPHIA, patient-reported health-related quality of life (HRQOL) mean total scores, including symptoms and functioning domains found in the Network-Functional Assessment of Cancer Therapy-Breast Cancer Symptom Index-16 and the EuroQol 5-dimension 5-level questionnaires, were maintained at similar levels to baseline after treatment with either margetuximab or trastuzumab [Citation47]. Overall, no significant differences between margetuximab and trastuzumab treatment groups were observed for HRQOL outcomes.
Bridging phase II study in China
In January 2020, Zai Lab initiated the registrational bridging, randomized phase II trial (NCT04262804) of margetuximab plus chemotherapy (capecitabine, gemcitabine, or vinorelbine) versus trastuzumab plus chemotherapy in Chinese patients with HER2+ MBC who had received at least two prior lines of anti-HER2–directed therapy in the metastatic setting. This study met its primary end point (median PFS by central review) with acceptable safety and tolerability, showing that the efficacy of this combination in Chinese patients was consistent with that seen in the global population of the SOPHIA trial, with an HR for PFS of 0.69 favoring the margetuximab arm [Citation48]. Based on these data, Zai Lab filed a new drug application in China for margetuximab in this indication in January 2022 [Citation49].
Other HER2-directed agents for MBC
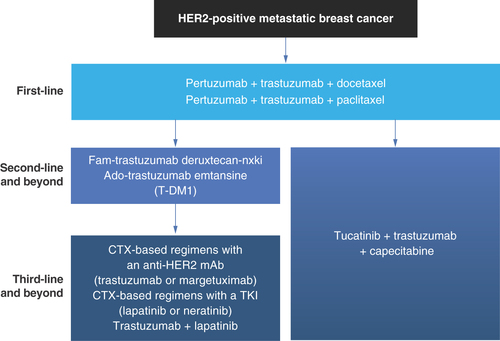
The overall landscape of treatments for HER2-positive MBC in the first-, second-, and third-line settings continues to evolve () [Citation3,Citation9–11,Citation13–16,Citation18,Citation22,Citation37,Citation41,Citation44–46,Citation50–72]. First-line therapy for HER2-positive MBC is pertuzumab plus trastuzumab plus docetaxel (Taxotere®) or paclitaxel (Taxol®) [Citation41,Citation44,Citation52,Citation53,Citation59,Citation64]. Since 2013, the antibody–drug conjugate ado-trastuzumab emtansine has been indicated for use as a single agent in patients with HER2-positive MBC who have progressed after treatment with trastuzumab and a taxane and it has been used in the second-line setting [Citation45,Citation71]. Since December 2019, multiple targeted therapies including antibody–drug conjugates (trastuzumab deruxtecan) [Citation14,Citation66,Citation73], tyrosine kinase inhibitors (TKI; tucatinib and neratinib) [Citation15,Citation16,Citation18,Citation69], and margetuximab [Citation13,Citation19,Citation37] have been approved by the FDA for second-line therapy and beyond (trastuzumab deruxtecan and tucatinib) or third-line therapy and beyond (neratinib, and margetuximab) in patients with HER2-positive MBC. Chemotherapy-based regimens in combination with either anti-HER2 mAb (trastuzumab or margetuximab) or TKIs (lapatinib or neratinib) and the combination of anti-HER2 mAb (trastuzumab) with TKI (tucatinib) and chemotherapy are generally used in third-line settings and beyond, but tucatinib plus trastuzumab plus capecitabine is also an option for the second-line setting [Citation3]. Tucatinib is a choice for second- and third-line treatment, but might be preferred in the second line in some patients with difficult-to-treat brain metastases. Trastuzumab deruxtecan has also demonstrated clinical benefit in patients with low HER2-expressing MBC who had received previous lines of chemotherapy [Citation74]. This success is attributed to the bystander effect of the cleaved toxin, which leads to the killing of adjacent breast cancer cells expressing low levels of HER2.
Third line & beyond: sequencing of agents
Margetuximab represents an effective treatment strategy that can be combined safely with chemotherapy of choice for patients who have undergone multiple lines of treatment for HER2-positive MBC. Margetuximab demonstrated an acceptable safety profile and has been administered without long-term safety issues. Margetuximab will probably be used in the later-line setting in combination with chemotherapy, as trastuzumab deruxtecan and the triplet of tucatinib and trastuzumab plus chemotherapy move to earlier lines.
Fc domain engineering to enhance immune effector function was shown to be clinically beneficial in SOPHIA. Although the indication for margetuximab plus chemotherapy is broad and includes patients with all CD16A genotypes, the exploratory data on genotypic differences suggest that patients with the F alleles may receive more benefit from margetuximab plus chemotherapy than from trastuzumab plus chemotherapy. Quest Diagnostics has developed a CD16A (FCGR3A) F158V polymorphism analysis test to identify FF, FV or VV genotypes [Citation75], although there is no current FDA requirement for a companion diagnostic for margetuximab [Citation13].
Future perspective
Margetuximab appeared to be more efficacious in CD16A-158 FV heterozygous patients and FF homozygous patients compared with VV homozygous patients in an exploratory analysis [Citation19,Citation29,Citation37]. A future direction for the use of margetuximab, given the unique mechanism of action due to Fc engineering of the molecule that may lead to increased immune engagement, may be in replacement use for trastuzumab in FV heterozygous patients or in FF homozygous patients. Using a test for CD16A-158 FF, FV and VV genotypes, the ongoing investigator-initiated phase II neoadjuvant MARGOT study (NCT04425018) is comparing the rate of pathologic CR in patients with early-stage HER2-positive breast cancer preoperatively treated with margetuximab plus pertuzumab plus paclitaxel versus trastuzumab plus pertuzumab plus paclitaxel.
Conclusion
Margetuximab is approved in the USA for patients with HER2-positive MBC, after receipt of two or more prior anti-HER2 therapies, based on data from the pivotal clinical trial SOPHIA. Margetuximab combined with chemotherapy of choice is an effective treatment option, with an acceptable safety profile, for patients with HER2-positive MBC who have undergone multiple lines of therapy.
HER2-positive breast cancer
A clinical unmet need exists for patients with HER2-positive distant metastatic breast cancer (MBC), as the 5-year survival rate is 39–46%.
Preclinical development of margetuximab & its mechanism of action
Margetuximab is a novel, Fc-engineered, HER2-targeted monoclonal antibody approved in the USA for use in HER2-positive metastatic breast cancer (MBC) in combination with chemotherapy in the third-line setting and beyond.
The improved binding of the engineered Fc component of margetuximab to the Fcγ receptor CD16A enhances innate immunity, via induction of antibody-dependent cellular cytotoxicity; margetuximab may also potentiate adaptive immunity.
Clinical development of margetuximab
The median progression-free survival was 5.8 months for patients receiving margetuximab plus chemotherapy and 4.9 months for patients in the trastuzumab group.
Margetuximab safety was comparable with trastuzumab. Common adverse events were fatigue, nausea, diarrhea, and neutropenia in both groups, as well as vomiting and pyrexia in the margetuximab group and anemia in the trastuzumab group.
Bridging phase II study in China
The registrational bridging, randomized phase II trial (NCT04262804) of margetuximab plus chemotherapy versus trastuzumab plus chemotherapy in Chinese patients with HER2+ MBC who had received at least two prior lines of anti-HER2–directed therapy in the metastatic setting met its primary end point of progression-free survival, with acceptable safety and tolerability.
The efficacy of margetuximab plus chemotherapy in Chinese patients was consistent with that seen in the global population of the SOPHIA trial.
Other HER2-directed agents for MBC
Multiple targeted therapies including antibody–drug conjugates (trastuzumab deruxtecan), tyrosine kinase inhibitors (tucatinib and neratinib), and margetuximab have been approved by the FDA for second-line therapy and beyond (trastuzumab deruxtecan and tucatinib) or third-line therapy and beyond (neratinib, and margetuximab) in patients with HER2-positive MBC.
Third line & beyond: sequencing of agents
Margetuximab represents an effective new treatment option that can be combined safely with chemotherapy for patients with HER2-positive MBC who have undergone two or more lines of prior anti-HER2 treatments, at least one of which was for metastatic disease.
Author contributions
The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
Financial & competing interests disclosure
This literature review and manuscript were sponsored by MacroGenics. WJ Gradishar has received consulting fees from AstraZeneca, Daiichi Sankyo, Merck, and Seagen. R O’Regan has received grants from Novartis; consulting fees and honoraria from Pfizer, Genomic Health, Biotheranostics, Novartis, Lilly, Puma Biotechnology, Genentech, Immunomedics, and MacroGenics; and financial support for attending ASCO, AACR, and ESHOS. MF Rimawi has received a grant from Pfizer, consulting fees from Novartis, Genentech, AstraZeneca, SeaGen; and honoraria from MacroGenics for an advisory board. JL Nordstrom is an employee and stockholder of MacroGenics. MK Rosales was an employee and stockholder of MacroGenics at the time of study conduct. HS Rugo has received research support for clinical trials through the University of California from Pfizer, Merck, Novartis, Lilly, Roche, Daiichi Sankyo, Seattle Genetics, MacroGenics, Sermonix, Boehringer Ingelheim, Polyphor, AstraZeneca, Ayala, Astellas, and Gilead; and honoraria from Puma, Samsung, Mylan, Chugai Pharma, Blueprint Medicines, and Napo Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing and/or editorial assistance was provided by E Cullinan, E Marcantoni and F Balordi of The Lockwood Group (Stamford, CT, USA), funded by MacroGenics.
References
- American Cancer Society . Cancer facts & figures 2023.American Cancer Society, 1–84 (2023). www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html
- Wolff AC , HammondMEH, AllisonKHet al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline focused update. J. Clin. Oncol.36(20), 2105–2122 (2018).
- NCCN . Clinical practice guidelines in oncology, breast cancer (v1.2023). www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Owens MA , HortenBC, DaSilva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin. Breast Cancer5(1), 63–69 (2004).
- NCI . Cancer stat facts: female breast cancer subtypes.Surveillance, Epidemiology, and End Results (SEER) program. https://seer.cancer.gov/statfacts/html/breast-subtypes.html
- Gennari A , AndreF, BarriosCHet al. on behalf of the ESMO Guidelines Committee . ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol.32(12), 1475–1495 (2021).
- Giordano SH , FranzoiMAB, TeminSet al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J. Clin. Oncol.40(23), 2612–2635 (2022).
- Cameron D , CaseyM, OlivaC, NewstatB, ImwalleB, GeyerCE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist15(9), 924–934 (2010).
- Blackwell KL , BursteinHJ, StornioloAMet al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J. Clin. Oncol.28(7), 1124–1130 (2010).
- Krop IE , KimSB, González-MartinAet al. on behalf of the TH3RESA study collaborators. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol.15(7), 689–699 (2014).
- Krop IE , KimSB, GonzalezMartin Aet al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol.18(6), 743–754 (2017).
- von Minckwitz G , SchwedlerK, SchmidtMet al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur. J. Cancer47(15), 2273–2281 (2011).
- MARGENZA® (margetuximab-cmkb): US prescribing information. MacroGenics, Inc., MD, USA (2020). www.margenza.com/pdf/prescribing-information.pdf
- ENHERTU® (fam-trastuzumab deruxtecan-nxki). US prescribing information. Daiichi Sankyo, Inc., NJ, USA (2022). https://daiichisankyo.us/prescribing-information-portlet/getPIContent?productName=Enhertu&inline=true
- TUKYSA® (tucatinib) tablets: US prescribing information. Seagen Inc., WA, USA (2023). https://seagendocs.com/TUKYSA_Full_Ltr_Master.pdf
- NERLYNX® (neratinib) tablets: US prescribing information. Puma Biotechnology, Inc., Los Angeles, CA (2022). https://nerlynxhcp.com/pdf/full-prescribing-information.pdf
- Saura Manich C , ModiS, KropIet al. Trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): updated survival results from a phase II trial (DESTINY-Breast01). Ann. Oncol.32(Suppl. 5), Abstract 279P (2021).
- Murthy RK , LoiS, OkinesAet al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med.382(7), 597–609 (2020).
- Rugo HS , ImS-A, CardosoFet al. Margetuximab versus trastuzumab in patients with previously treated HER2-positive advanced breast cancer (SOPHIA): final overall survival results from a randomized phase 3 trial. J. Clin. Oncol.41(2), 198–205 (2023).
- Liu L , YangY, BurnsRet al. Margetuximab mediates greater Fc-dependent anti-tumor activities than trastuzumab or pertuzumab in vitro. Cancer Res.79(Suppl. 13), Abstract 1538 (2019).
- Nordstrom JL , GorlatovS, ZhangWet al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res.13(6), R123 (2011).
- Slamon DJ , Leyland-JonesB, ShakSet al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med.344(11), 783–792 (2001).
- Stavenhagen JB , GorlatovS, TuaillonNet al. Enhancing the potency of therapeutic monoclonal antibodies via Fc optimization. Adv. Enzyme Regul.48, 152–164 (2008).
- Stavenhagen JB , GorlatovS, TuaillonNet al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res.67(18), 8882–8890 (2007).
- Bang YJ , GiacconeG, ImSAet al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol.28(4), 855–861 (2017).
- Musolino A , GradisharWJ, RugoHSet al. Role of Fcγ receptors in HER2-targeted breast cancer therapy. J. Immunother. Cancer10(1), e003171 (2022).
- Nordstrom JL , MuthJ, ErskineCLet al. High frequency of HER2-specific immunity observed in patients (pts) with HER2+ cancers treated with margetuximab (M), an Fc-enhanced anti-HER2 monoclonal antibody (mAb). J. Clin. Oncol.37(Suppl. 15), Abstract 1030 (2019).
- Gavin PG , SongN, KimSRet al. Association of polymorphisms in FCGR2A and FCGR3A with degree of trastuzumab benefit in the adjuvant treatment of ERBB2/HER2-positive breast cancer: analysis of the NSABP B-31 trial. JAMA Oncol.3(3), 335–341 (2017).
- Musolino A , NaldiN, BortesiBet al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol.26(11), 1789–1796 (2008).
- Musolino A , NaldiN, DieciMVet al. Immunoglobulin G fragment C receptor polymorphisms and efficacy of preoperative chemotherapy plus trastuzumab and lapatinib in HER2-positive breast cancer. Pharmacogenomics J.16(5), 472–477 (2016).
- Tamura K , ShimizuC, HojoTet al. FcγR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann. Oncol.22(6), 1302–1307 (2011).
- Hurvitz SA , BettingDJ, SternHMet al. Analysis of Fcγ receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin. Cancer Res.18(12), 3478–3486 (2012).
- Norton N , OlsonRM, PegramMet al. Association studies of Fcγ receptor polymorphisms with outcome in HER2+ breast cancer patients treated with trastuzumab in NCCTG (Alliance) Trial N9831. Cancer Immunol. Res.2(10), 962–969 (2014).
- Royce M , OsgoodCL, AmatyaAKet al. FDA approval summary: margetuximab plus chemotherapy for advanced or metastatic HER2-positive breast cancer. Clin. Cancer Res.28(8), 1487–1492 (2022).
- Im SA , BangYJ, OhDYet al. Long-term responders to single-agent margetuximab, an Fc-modified anti-HER2 monoclonal antibody, in metastatic HER2+ breast cancer patients with prior anti-HER2 therapy. Cancer Res.79(Suppl. 4), Abstract P6-18-11 (2019).
- Pegram MD , Tan-ChiuE, MillerKet al. A single-arm, open-label, phase 2 study of MGAH22 (margetuximab) [fc-optimized chimeric anti-HER2 monoclonal antibody (mAb)] in patients with relapsed or refractory advanced breast cancer whose tumors express HER2 at the 2+ level by immunohistochemistry and lack evidence of HER2 gene amplification by FISH. J. Clin. Oncol.32(Suppl. 15), Abstract TPS671 (2014).
- Rugo HS , ImSA, CardosoFet al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol.7(4), 573–584 (2021).
- Escrivá S , ImSA, CardosoFet al. SOPHIA analysis by chemotherapy (CTX) choice: a phase III (P3) study of margetuximab (M) + CTX versus trastuzumab (T) + CTX in patients (pts) with pretreated HER2+ metastatic (met) breast cancer (MBC). J. Clin. Oncol.38(Suppl. 15), Abstract 1040 (2020).
- Im SA , CardosoF, CortesJet al. Integrated safety summary of single agent and combination margetuximab in phase 1, 2, and 3 studies of HER2-positive advanced cancers and metastatic breast cancer (MBC). Cancer Res.81(Suppl. 4), Abstract PS10-12 (2021).
- Cortes J , CardosoF, CuriglianoGet al. Infusion related reactions in the phase 3 SOPHIA trial of margetuximab + chemotherapy vs trastuzumab + chemotherapy in patients with pretreated HER2+ metastatic breast cancer. Cancer Res.81(Suppl. 4), Abstract PS10-24 (2021).
- HERCEPTIN® (trastuzumab): US prescribing information. Genentech, Inc., CA, USA (2021). www.gene.com/download/pdf/herceptin_prescribing.pdf
- Thompson LM , EckmannK, BosterBLet al. Incidence, risk factors, and management of infusion-related reactions in breast cancer patients receiving trastuzumab. Oncologist19(3), 228–234 (2014).
- Gradishar WJ , ImS-A, CardosoFet al. Phase 3 SOPHIA study of margetuximab + chemotherapy vs trastuzumab + chemotherapy in patients with HER2+ metastatic breast cancer after prior anti-HER2 therapies: Infusion time substudy results. Cancer Res.80(Suppl. 4), Abstract P1-18-04 (2020).
- PERJETA® (pertuzumab): US prescribing information. Genentech, Inc., CA, USA (2021). www.gene.com/download/pdf/perjeta_prescribing.pdf
- KADCYLA® (ado-trastuzumab emtansine): US prescribing information. Genentech, Inc.; CA, USA (2022). www.gene.com/download/pdf/kadcyla_prescribing.pdf
- TYKERB® (lapatinib) tablets: US prescribing information. Novartis Pharmaceuticals Corp., NJ, USA (2022). www.novartis.us/sites/www.novartis.us/files/tykerb.pdf
- Cardoso F , CortesJ, GradisharWet al. Health-related quality of life for margetuximab + chemotherapy vs. trastuzumab + chemotherapy in the phase 3 SOPHIA trial of patients with pretreated HER2+ metastatic breast cancer. Cancer Res.81(Suppl. 4), Abstract PS9-11 (2021).
- Zai Lab . Zai Lab announces margetuximab achieved primary objective in bridging study in advanced HER2+ breast cancer in greater China. https://ir.zailaboratory.com/node/9636/pdf
- Zai Lab . Zai Lab announces NDA acceptance of margetuximab for patients with pretreated metastatic HER2-positive breast cancer in China by the NMPA. https://ir.zailaboratory.com/node/10036/pdf
- Andersson M , LidbrinkE, BjerreKet al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J. Clin. Oncol.29(3), 264–271 (2011).
- Esfahani K , FerrarioC, LeP, PanasciL. The trastuzumab and vinorelbine combination: an alternative to taxane-based chemotherapy for early-stage and locally advanced HER2-positive breast cancer. Curr. Oncol.21(5), e723–727 (2014).
- Dang C , IyengarN, DatkoFet al. Phase II study of paclitaxel given once per week along with trastuzumab and pertuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol.33(5), 442–447 (2015).
- Smyth LM , IyengarNM, ChenMFet al. Weekly paclitaxel with trastuzumab and pertuzumab in patients with HER2-overexpressing metastatic breast cancer: overall survival and updated progression-free survival results from a phase II study. Breast Cancer Res. Treat.158(1), 91–97 (2016).
- Blackwell KL , BursteinHJ, StornioloAMet al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J. Clin. Oncol.30(21), 2585–2592 (2012).
- Swain SM , BaselgaJ, KimSBet al. for the CLEOPATRA Study Group . Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med.372(8), 724–734 (2015).
- Swain SM , KimSB, CortesJet al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol.14(6), 461–471 (2013).
- Swain SM , MilesD, KimSBet al. on behalf of the CLEOPATRA study group . Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol.21(4), 519–530 (2020).
- Bartsch R , WenzelC, AltorjaiGet al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J. Clin. Oncol.25(25), 3853–3858 (2007).
- Baselga J , CortésJ, KimSBet al. for the CLEOPATRA Study Group . Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med.366(2), 109–119 (2012).
- Burstein HJ , KeshaviahA, BaronADet al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer110(5), 965–972 (2007).
- Cobleigh MA , VogelCL, TripathyDet al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol.17(9), 2639–2648 (1999).
- Esteva FJ , ValeroV, BooserDet al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J. Clin. Oncol.20(7), 1800–1808 (2002).
- Geyer CE , ForsterJ, LindquistDet al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med.355(26), 2733–2743 (2006).
- Leyland-Jones B , GelmonK, AyoubJPet al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J. Clin. Oncol.21(21), 3965–3971 (2003).
- Marty M , CognettiF, MaraninchiDet al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J. Clin. Oncol.23(19), 4265–4274 (2005).
- Modi S , SauraC, YamashitaTet al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med.382(7), 610–621 (2020).
- Perez EA , SumanVJ, RowlandKMet al. Two concurrent phase II trials of paclitaxel/carboplatin/trastuzumab (weekly or every-3-week schedule) as first-line therapy in women with HER2-overexpressing metastatic breast cancer: NCCTG study 983252. Clin. Breast Cancer6(5), 425–432 (2005).
- Robert N , Leyland-JonesB, AsmarLet al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J. Clin. Oncol.24(18), 2786–2792 (2006).
- Saura C , OliveiraM, FengYHet al. for the NALA Investigators . Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥2 HER2-directed regimens: phase III NALA trial. J. Clin. Oncol.38(27), 3138–3149 (2020).
- Seidman AD , BerryD, CirrincioneCet al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J. Clin. Oncol.26(10), 1642–1649 (2008).
- Verma S , MilesD, GianniLet al. for the EMILIA Study Group . Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med.367(19), 1783–1791 (2012).
- von Minckwitz G , du BoisA, SchmidtMet al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03–05 study. J. Clin. Oncol.27(12), 1999–2006 (2009).
- Cortés J , KimS-B, ChungW-Pet al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med.386(12), 1143–1154 (2022).
- Modi S , JacotW, YamashitaTet al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med.387(1), 9–20 (2022).
- Quest Diagnostics . CD16A (FCGR3A) F158V polymorphism analysis. https://testdirectory.questdiagnostics.com/test/test-detail/34493/cd16a-fcgr3a-f158v-polymorphism-analysis?cc=MASTER