Abstract
Aim: To examine the impact of tumor sidedness on clinical outcomes in Chinese patients with metastatic colorectal cancer treated with folinic acid/fluorouracil/oxaliplatin (FOLFOX-4) ± cetuximab in the TAILOR trial. Patients & methods: Clinical data from 391 patients were evaluated for tumor sidedness. Results: Patients with left-sided tumors who received cetuximab plus FOLFOX-4 had a significantly longer overall survival (medians: 22.0 vs 18.3 months; p = 0.007) and progression-free survival (medians: 9.3 vs 7.9 months; p = 0.006) compared with FOLFOX-4 alone. Overall survival (medians: 11.5 vs 9.4 months; p = 0.664) and progression-free survival (medians: 7.4 vs 4.5 months; p = 0.068) also improved in patients with right-sided tumors. Conclusion: Adding cetuximab to first-line FOLFOX-4 in patients with metastatic colorectal cancer improved clinical outcomes irrespective of primary tumor side.
Plain language summary
The effectiveness of treatment with cetuximab plus chemotherapy in people with RAS wild-type metastatic colorectal cancer based on the primary tumor location: Cetuximab is a drug used to treat people with advanced metastatic colorectal cancer (mCRC) with a gene called RAS wild-type (wt) and is given along with standard chemotherapy. The TAILOR study showed that in people with RAS wt mCRC cetuximab together with chemotherapy worked better than chemotherapy alone and had similar side effects. This analysis of the TAILOR study looked at whether Chinese people with RAS wt mCRC responded differently to treatment with cetuximab plus chemotherapy depending on the primary tumor location (whether left or right side of the colon). This analysis found that people with left- or right-sided primary tumors who received cetuximab plus chemotherapy lived longer and their cancer progressed more slowly compared with those who received chemotherapy alone.
Tweetable abstract
In the #TAILORstudy subanalysis #cetuximab plus chemotherapy in first-line treatment of RAS wild-type #metastaticcolorectalcancer improved #clinicaloutcomes irrespective of #primarytumorside.
Colorectal cancer (CRC) is the fourth most commonly diagnosed cancer globally (6.1% of total cancer cases) and the leading cause of cancer related mortality (9.2% of total cancer deaths) [Citation1]. Approximately 20% of patients with CRC are metastatic at the time of diagnosis (mCRC), and another 20% will go on to develop metastatic disease [Citation2,Citation3]. Despite continuous improvements in the treatment of CRC, the prognosis is still poor, with an estimated 5-year overall survival (OS) rate of 65% for all stages and 14% for mCRC [Citation2,Citation3].
CRC is a heterogeneous disease, with primary tumor site and RAS/BRAF mutation status exerting a large effect on prognosis and treatment response [Citation3]. Cetuximab is a monoclonal antibody that binds to the epidermal growth factor receptor (EGFR) and is indicated for the treatment of RAS wild-type (wt) mCRC and squamous cell carcinoma of the head and neck [Citation4,Citation5]. In patients with RAS wt mCRC, the addition of cetuximab to first-line (1L) FOLFOX (leucovorin calcium [folinic acid (FA)], fluorouracil [5-FU] and oxaliplatin) or FOLFIRI (leucovorin calcium [FA], 5-FU and irinotecan hydrochloride) has been shown in randomized trials to improve OS, progression-free survival (PFS) and response rates compared with chemotherapy alone [Citation6–8]. However, the impact of tumor side on clinical outcomes of patients with RAS wt mCRC treated with cetuximab plus FOLFOX is not fully understood.
The TAILOR study was the first prospective, phase III clinical trial to randomize a population of patients with RAS wt mCRC to FOLFOX-4 with or without the addition of cetuximab [Citation8]. Previous analyses of the TAILOR trial have shown that the addition of cetuximab to FOLFOX-4 significantly improved PFS, OS and overall response rate (ORR) compared with FOLFOX-4 alone, confirming that this combination is an effective 1L treatment option for patients with RAS wt mCRC [Citation8]. The safety profile of the combination was also comparable to those reported for prior randomized clinical trials, with no new or unexpected safety findings observed [Citation6,Citation8–10].
This subanalysis of the TAILOR trial, which included an additional 1 year of follow-up after the primary analysis, examined the impact of primary tumor side on efficacy and safety outcomes in patients with mCRC treated with cetuximab plus FOLFOX-4 or FOLFOX-4 alone. Here, we report the updated findings for clinical outcomes in subgroups by tumor side.
Patients & methods
Study design & patients
The TAILOR study (EMR62202-057; NCT01228734) has previously been reported [Citation8]. This was an open-label, randomized, multicenter, phase III trial of Chinese patients with RAS wt mCRC. Patients were included if they had their first occurrence of histologically confirmed RAS wt mCRC (not curatively resectable), ≥1 measurable lesion by computerized tomography or MRI using Response Evaluation Criteria in Solid Tumors (RECIST) v1.0, an Eastern Cooperative Oncology Group Performance Score (ECOG PS) of 0 or 1, and adequate hematologic and end-organ function.
Eligible patients were randomized to receive the FOLFOX-4 chemotherapy regimen alone or in combination with cetuximab. The FOLFOX-4 regimen consisted of a combination of oxaliplatin with a de Gramont schedule of 5-FU/FA. Oxaliplatin was administered intravenously at a dose of 85 mg/m2 over 120 min on day 1 of each cycle. FA was administered intravenously at a dose of 200 mg/m2 over 120 min on day 1 and day 2 of each cycle, immediately prior to the administration of 5-FU. 5-FU was administered as a bolus of 400 mg/m2 over 2–4 min followed by a 600 mg/m2 continuous infusion over 22 h on day 1 and day 2 of each cycle. The cycle was repeated every 2 weeks. In the group treated with the combination of cetuximab and FOLFOX-4, cetuximab 400 mg/m2 was administered on day 1, followed by 250 mg/m2 once weekly. Treatment was continued until disease progression or unacceptable toxicity.
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the ethics committees of all participating centers. All patients provided written informed consent prior to trial entry.
Outcomes & statistical analyses
The primary end point in the TAILOR study was PFS, while key secondary end points included OS, ORR and safety/tolerability [Citation8]. The cut-off dates for the primary and the final analyses in the TAILOR study were 25 January 2016 and 26 January 2017, respectively. This TAILOR subanalysis report presents the updated efficacy (including OS rates not previously reported) and safety data in subgroups by tumor side for the clinical data cut-off date of 26 January 2017.
At trial initiation, patients with KRAS exon 2 wt tumors were enrolled; however, the trial population was later restricted to patients with extended RAS (KRAS/NRAS exons 2 to 4) wt tumors [Citation8]. Primary efficacy analyses were performed on the RAS wt modified intention-to-treat population, while safety analyses were performed on the RAS wt modified safety population. All statistical tests comparing the treatment arms were performed 2-sided using a significance level of α = 0.05. The Kaplan–Meier method was used to evaluate PFS and OS. Log-rank tests were used to compare differences between treatment arms. A 2-sided Fisher exact test was used to compare ORRs between treatment groups.
Results
Baseline characteristics & primary tumor localization
The modified intention-to-treat population included 393 patients with RAS wt mCRC. A total of 391 patients were evaluable for tumor sidedness (clinical data cut-off: 26 January 2017). Tumors were categorized in evaluable patients as L-sided (splenic flexure, descending colon, sigmoid colon or rectum) or R-sided (appendix, cecum, ascending colon, hepatic flexure or transverse colon). Baseline characteristics were balanced in the left (L)- and right (R)-sided tumor location subgroups, with a greater proportion of patients with R-sided disease having BRAF mutant tumors and slightly more patients with L-sided disease undergoing prior chemotherapy ().
Table 1. Baseline characteristics.
Among the patients with L-sided tumors receiving cetuximab plus FOLFOX-4 or FOLFOX-4 alone, 48/146 (32.9%) and 67/162 (41.4%) patients, respectively, had primary tumors localized to the colon only; 98/146 (67.1%) and 95/162 (58.6%) patients had primary tumors localized to the rectum only. All patients with R-sided tumors receiving cetuximab plus FOLFOX-4 (45/83; 54.2%) or FOLFOX-4 alone (38/83; 45.8%) had primary tumors localized to the colon ().
Table 2. Primary tumor location by tumor side.
Treatment exposure/compliance by tumor side
The median (range) dose intensities (mg/m2) of cetuximab, oxaliplatin and 5-FU in patients with L-sided tumors were 225.46 (101.5–251.3), 70.99 (39.5–85.2) and 1581.68 (654.4–2333.3), respectively; in patients with R-sided tumors, they were 233.97 (162.4–251.1), 72.84 (28.5–85.5) and 1641.38 (969.0–2333.3), respectively. In patients with L-sided tumors receiving FOLFOX-4 alone, the median (range) dose intensities (mg/m2) of oxaliplatin and 5-FU were 72.60 (39.6–85.2) and 1642.27 (895.4–2338.9), respectively; in patients with R-sided tumors, they were 73.60 (35.5–85.9) and 1707.73 (933.3–2153.8), respectively.
Overall, >99% of enrolled participants discontinued treatment either prior to or at the clinical data cut-off date of 26 January 2017 (97.9% of patients with L-sided tumors receiving cetuximab plus FOLFOX-4 and 100% of patients in all other cohorts). Disease progression was the primary cause of treatment discontinuation for all groups, with 118/146 (80.8%) and 105/162 (64.8%) of patients with L-sided tumors receiving cetuximab plus FOLFOX-4 versus FOLFOX-4, respectively and 35/45 (77.8%) and 33/38 (86.8%) of patients with R-sided tumors in these same treatment groups. Other causes of treatment discontinuation included adverse events (AE), loss to follow-up, protocol non compliance, lack of efficacy, death and unspecified causes.
Efficacy outcomes
The 3-year OS rate following treatment with cetuximab plus FOLFOX-4 was double that achieved following treatment with FOLFOX-4 alone (32% [95% CI: 25–40%] vs 16% [95% CI: 11–22%]) in patients with L-sided tumors and >1.5-times higher in patients with R-sided tumors (23% [95% CI: 11–38%] vs 14% [95% CI: 5–28%]; ). Overall, in patients with L-sided tumors, the OS event rate was 79.5% (116/146) in those treated with cetuximab plus FOLFOX-4 versus 86.4% (140/162) in patients treated with FOLFOX-4 (A), while in patients with R-sided tumors the OS event rates were 84.4% (38/45) versus 89.5% (34/38), respectively (B). In patients with L-sided tumors, OS (months) was significantly longer in those who received cetuximab plus FOLFOX-4 compared with patients who were treated with FOLFOX-4 alone (medians: 22.0 [95% CI:18.7–25.5] vs 18.3 [95% CI: 15.2–20.2]; p = 0.007; A). Cetuximab plus FOLFOX-4 also resulted in longer median OS (months) in patients with R-sided tumors compared with FOLFOX-4 alone (11.5 [95% CI: 7.5–20.4] vs 9.4 [95% CI: 7.5–18.8]; p = 0.664; B).
Table 3. Compiled efficacy results by tumor side.
CET: Cetuximab; FOLFOX-4: Leucovorin calcium (folinic acid), fluorouracil and oxaliplatin; HR: Hazard ratio; OS: Overall survival; PFS: Progression-free survival.
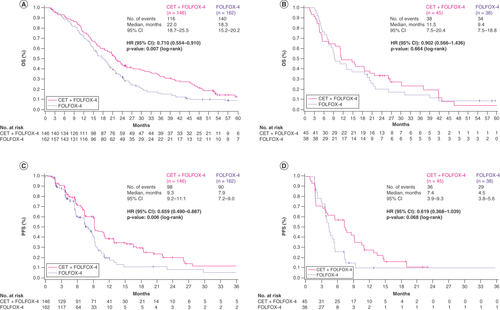
The 3-year PFS rates were improved regardless of tumor side in patients who received cetuximab plus FOLFOX-4 (L-sided tumors: 12% [95% CI: 5–21%]; R-sided tumors: not estimable) versus FOLFOX-4 alone (L-sided tumors: 5% [95% CI:1–15%]; R-sided tumors: 10% [95% CI: 2–25%]; ). PFS (months) was significantly longer in patients with L-sided tumors treated with cetuximab plus FOLFOX-4 compared with FOLFOX-4 (medians: 9.3 [95% CI: 9.2–11.1] vs 7.9 [95% CI: 7.2–9.0]; p = 0.006). Median PFS (months) was also longer in patients with R-sided tumors who received cetuximab plus FOLFOX-4 compared with patients who were treated with FOLFOX-4 (7.4 [95% CI: 3.9–9.3] vs 4.5 [95% CI: 3.8–5.6]; p = 0.068; C & D).
Patients treated with cetuximab plus FOLFOX-4 also showed higher ORRs versus those treated with FOLFOX-4 alone (). In the cetuximab plus FOLFOX-4 arm, 3.4% (L-sided tumors) and 2.2% (R-sided tumors) of patients achieved a complete response and 63.0% (L-sided tumors) and 42.2% (R-sided tumors) of patients achieved a partial response. In the FOLFOX-4 arm, 1.2% (L-sided tumors) and 0% (R-sided tumors) of patients achieved a complete response and 42.0% (L-sided tumors) and 23.7% (R-sided tumors) of patients achieved a partial response.
Safety outcomes
Rates of AEs were balanced among groups and consistent with those reported for the TAILOR study [Citation6]. Among the patients with L-sided tumors, 32/147 (21.8%) and 21/161 (13.0%) patients experienced ≥1 serious AE (SAE) in the cetuximab plus FOLFOX-4 and FOLFOX-4 treatment arms, respectively, including 8/147 (5.4%) and 9/161 (5.6%) that were considered related to study treatment, respectively. In the group with R-sided tumors, 9/45 (20.0%) patients in the cetuximab plus FOLFOX-4 treatment arm experienced ≥1 SAE compared with 6/38 patients (15.8%) in the FOLFOX-4 group; 3/45 (6.7%) and 2/38 (5.3%) had SAEs considered related to study treatment, respectively. AEs leading to death (i.e., the proportion of patients having ≥1 AE with fatal outcome) occurred in 2.0% and 1.9% of patients with L-sided disease in the cetuximab plus FOLFOX-4 and FOLFOX-4 treatment arms, respectively (0.7 and 0% considered related to study treatment), and in 11.1 and 2.6% of patients with R-sided disease in these treatment groups (4.4 and 0% considered related to study treatment). The most common treatment emergent AEs (TEAE) of grade ≥3 affecting ≥10% of patients in the cetuximab-treated groups included neutropenia, leukopenia, rash, fatigue, thrombocytopenia and hypokalemia, with no major differences between L- and R-sided disease ().
Table 4. Most common grade ≥3 treatment-emergent adverse events.
Anticancer therapy after study
Only about half of all patients across the groups received anticancer treatment after study discontinuation, including 177/308 patients (57.5%) with L-sided tumors and 49/83 patients (59.0%) with R-sided tumors (). Second-line (2L) and later-line treatments were most common in patients with R-sided disease who received FOLFOX-4 alone. Fewer than 25% of patients received targeted agents in later lines of treatment. The most common anticancer treatment received after discontinuation among all groups was chemotherapy, followed by radiotherapy and surgery. The rates of anticancer surgery after study discontinuation were 7.1% (22/308) for L-sided and 7.2% (6/83) for R-sided tumors.
Table 5. Anticancer therapy after study.
Discussion
TAILOR was the first prospective, phase III clinical trial to randomize a population of patients with RAS wt mCRC to FOLFOX-4 with or without the addition of cetuximab [Citation8]. Results from this analysis of patients from the TAILOR trial in subgroups by tumor location remained consistent with previous observations showing that the addition of cetuximab to 1L FOLFOX-4 or FOLFIRI improves OS, PFS and ORR in this patient population [Citation6–8,Citation11]. Notably, patients with tumors localized to the transverse colon were included in this analysis. Tumors originating from the transverse colon are rare and are classified as R-sided in most subgroup analyses of randomized trials, despite mutation clustering analyses indicating that their molecular landscape appears to be closer to that of L-sided tumors [Citation12,Citation13]. The median age was similar between the L- and R-sided groups, but differences were observed in terms of sex (more female patients in R-sided subgroups), prior chemotherapy (fewer patients in R-sided subgroups), tumor localization (colon only in R-sided subgroups) and BRAF status (more BRAF mutant tumors in R-sided subgroups). The differences between treatments within the subgroups in our study were small or similar. The OS and PFS data from this TAILOR study subanalysis showed statistically significant improvements in patients with L-sided tumors treated with the combination of cetuximab and FOLFOX-4 compared with patients treated with FOLFOX-4 alone. Although not statistically significant, numerical improvements in OS and PFS in the subgroup of patients with R-sided tumors who were treated with cetuximab plus FOLFOX-4 were also observed. The lack of statistically significant differences in these efficacy outcomes reported for R-sided tumors may be due to the small sample size and/or unstratified randomization and/or higher rates of BRAF mutant tumors in this group. The improvements in ORR, OS and PFS reported here for both L-sided and R-sided disease reinforce that the combination of cetuximab and FOLFOX-4 is an effective 1L, standard-of-care treatment regimen for patients with RAS wt mCRC, irrespective of primary tumor location.
No new or unexpected safety findings were observed in this analysis, and the safety profile of cetuximab plus FOLFOX-4 in Chinese patients with RAS wt mCRC was similar to that observed in other groups [Citation11]. Furthermore, the results by tumor side for patients from TAILOR who received additional therapy after progressing on the 1L regimen were consistent with the results for the overall population from the previously published final OS analysis [Citation8].
Our study has several limitations that should be noted, including the lack of comparator arm, small sample size in the R-sided group, as well as the unstratified randomization and higher rates of BRAF mutant tumors in the R-sided group. In addition, the proportion of patients treated with prior chemotherapy was lower in both R-sided subgroups compared with L-sided subgroups. The study was conducted in a Chinese population only and was limited to the Chinese healthcare system; therefore, the findings cannot be extrapolated to a broader population.
Despite these limitations, the findings from our initial analysis [Citation8] and our current subanalysis of the TAILOR study with updated clinical outcomes data, encompassing the additional 1-year of follow-up, provide evidence for the role of cetuximab in patients with R-sided disease in China. This conclusion is supported by prior studies, such as the nonrandomized, phase II, APEC trial, which showed improvements in median PFS, OS and ORR in patients with RAS wt R-sided mCRC [Citation11]. A pooled analysis of data from two phase II trials for mCRC, JACCRO CC-05 and CC-06, also demonstrated that, although patients with L-sided disease derived greater clinical benefit from 1L cetuximab combination therapy, some patients with R-sided disease were also able to achieve rapid and deep responses [Citation14].
Conclusion
After >4 years of follow-up, results of the TAILOR study remained consistent with previously published results showing that the addition of cetuximab to 1L FOLFOX-4 improved OS, PFS and ORR irrespective of primary tumor side in the TAILOR patient population, with no new or unexpected safety findings. The results of this subanalysis of patients from the TAILOR trial in subgroups by tumor side provide further evidence for the efficacy of the 1L combination of cetuximab and FOLFOX-4 in improving the outcomes of patients with mCRC, regardless of primary tumor side.
Evidence shows that in patients with RAS wild-type metastatic colorectal cancer (mCRC), the addition of cetuximab to chemotherapy improves overall survival (OS), progression-free survival (PFS) and response rates, compared with chemotherapy alone; however, the impact of tumor side on clinical outcomes is not fully understood.
In the TAILOR study, an open-label, randomized, multicenter, phase III trial in Chinese patients with RAS wild-type mCRC, the addition of cetuximab to FOLFOX-4 (folinic acid, fluorouracil and oxaliplatin) significantly improved PFS, OS and overall response rates compared with FOLFOX-4 alone.
The TAILOR study subanalysis examined the impact of primary tumor side on clinical outcomes in 391 patients treated with cetuximab plus FOLFOX-4 or FOLFOX-4 alone.
This report presents the updated data for PFS and OS in subgroups by tumor side.
Patients with left-sided tumors treated with cetuximab plus FOLFOX-4 had significantly longer OS and PFS compared with patients treated with FOLFOX-4 alone (OS medians: 22.0 vs 18.3 months; p = 0.007; PFS medians: 9.3 vs 7.9 months; p = 0.006).
Median OS and PFS improved numerically in patients with right-sided tumors (OS: 11.5 vs 9.4 months; p = 0.664; PFS: 7.4 vs 4.5 months; p = 0.068).
The 3-year OS rate following treatment with cetuximab plus FOLFOX-4 was double that achieved after treatment with FOLFOX-4 alone in patients with left-sided tumors (32% [95% CI: 25–40%] vs 16% [95% CI: 11–22%]) and >1.5 higher in the right-sided subgroup (23% [95% CI: 11–38%] vs 14% [95% CI: 5–28%]).
These updated results of the TAILOR study subanalysis provide further supportive evidence for the combination of cetuximab and FOLFOX-4 in first-line treatment of patients with mCRC, regardless of primary tumor side.
Author contributions
Conception and design: S Qin, T Liu, J Xu, Qi Li, Y Cheng, A Zhang, R Esser, H Chang, J Li. Provision of study materials or patients: S Qin. Collection and assembly of data: A Zhang, H Chang. Data analysis and interpretation: S Qin, T Liu, J Xu, Q Li, Y Cheng, A Zhang, R Esser, H Chang, J Li. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Ethical conduct of research
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the ethics committees of all participating centers. All patients provided written informed consent prior to trial entry.
Acknowledgments
The authors would like to thank the patients, investigators, co-investigators and the study teams at each of the participating centers and at Merck (CrossRef Funder ID: 10.13039/100009945) and Merck Serono Co., Ltd, Beijing, China, an affiliate of Merck KGaA.
Financial& competing interests disclosure
This study was funded by Merck (CrossRef Funder ID: 10.13039/100009945). A Zhang and H Chang disclose employment with Merck Serono Co., Ltd, Beijing, China, an affiliate of Merck KGaA. R Esser discloses employment at the time of the study and during its analysis with Merck Healthcare KGaA, Darmstadt, Germany and stock ownership in Merck KGaA, Darmstadt, Germany. J Li discloses consulting fees from Novartis, honoraria from Henrui and Roche, participation on an advisory board for Henrui and is president of the CSCO Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support was provided by Aleksandra Erac-Zganec, ClinicalThinking, which was also funded by Merck in accordance with Good Publication Practice (GPP) guidelines (https://www.ismpp.org/gpp-2022).
Additional information
Funding
References
- Bray F , FerlayJ, SoerjomataramI, SiegelRL, TorreLA, JemalA. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.68(6), 394–424 (2018).
- Riihimaki M , HemminkiA, SundquistJ, HemminkiK. Patterns of metastasis in colon and rectal cancer. Sci. Rep.6, 29765 (2016).
- Lakatos G , KöhneCH, BodokyG. Current therapy of advanced colorectal cancer according to RAS/RAF mutational status. Cancer Metastasis Rev.39(4), 1143–1157 (2020).
- European Medicines Agency. Merck . Summary of product characteristics, Erbitux. (2022). www.ema.europa.eu/en/medicines/human/EPAR/erbitux
- US Food and Drug Administration . Erbitux. Prescribing Information. EMD Serono, Inc., USA (2021). www.accessdata.fda.gov/drugsatfda_docs/label/2021/125084s277s280lbl.pdf
- Van Cutsem E , LenzHJ, KöhneCHet al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J. Clin. Oncol.33(7), 692–700 (2015).
- Bokemeyer C , KöhneCH, CiardielloFet al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur. J. Cancer51(10), 1243–1252 (2015).
- Qin S , LiJ, WangLet al. Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal cancer: the open-label, randomized, phase III TAILOR trial. J. Clin. Oncol.36(30), 3031–3039 (2018).
- Cheng A-L , CornelioG, ShenLet al. Efficacy, tolerability, and biomarker analyses of once-every-2-weeks cetuximab plus first-line FOLFOX or FOLFIRI in patients with KRAS or all RAS wild-type metastatic colorectal cancer: the phase II APEC study. Clin. Colorectal Cancer16(2), e73–e88 (2017).
- Bokemeyer C , BondarenkoI, HartmannJTet al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann. Oncol.22(7), 1535–1546 (2011).
- Price T , ShenL, MaBet al. Phase II APEC trial: the impact of primary tumor side on outcomes of first-line cetuximab plus FOLFOX or FOLFIRI in patients with RAS wild-type metastatic colorectal cancer. Asia Pac. J. Clin. Oncol.15(4), 225–230 (2019).
- Cremolini C , BenelliM, FontanaEet al. Benefit from anti-EGFRs in RAS and BRAF wild-type metastatic transverse colon cancer: a clinical and molecular proof of concept study. ESMO Open4(2), e000489 (2019).
- Loree JM , PereiraAAL, LamMet al. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin. Cancer Res.24(5), 1062–1072 (2018).
- Sunakawa Y , TsujiA, FujiiM, IchikawaW. No benefit from the addition of anti-EGFR antibody in all right-sided metastatic colorectal cancer?Ann. Oncol.28(8), 2030–2031 (2017).
