Abstract
Aim: To explore the incorporation of novel agents in the first-line setting for acute myeloid leukemia patients. Materials & methods: Observational study based on data from a multi-country cross-sectional retrospective web-based survey sent to 518 physicians in Europe between 2020 and 2021. Information from 2040 patients was analyzed. Results: 604 patients (29.6%) received novel agents in both intensive and non-intensive setting. Comorbidities were not a barrier for the use of novel agents. The presence of tumor mutations was observed to be an important element for treatment decision. Conclusion: There is a progressive incorporation of novel agents for newly diagnosed acute myeloid leukemia patients.
Plain Language Summary
Use of new treatments as first choice of therapy in patients with leukemia
What is this article about?
We now have new treatments for patients suffering from a type of blood cancer called acute myeloid leukemia (acronym AML). They are available as the first choice of therapy. In this study we explored how these new treatments are included in daily patient care.
What were the results?
We reviewed the data of 2040 patients in Europe, obtained from an online survey sent to physicians in two waves (between 2020 and 2021). The use of these new AML treatments was more frequent in patients who presented some specific gene alterations (changes in their DNA sequence) and were in worse health due to other diseases and old age. Most of the new treatments were administered together with other milder chemotherapies.
What do the results of the study mean?
The results of this study help us understand how new AML treatments are being used.
Tweetable abstract
Observational study with 2040 AML patients showed incorporation of novel therapies into 1st line in combination with hypomethylating agents. Presence of tumor mutations observed to be an important element for treatment decision.
Acute myeloid leukemia (AML) is a clonal disorder affecting pluripotent stem cells in bone marrow and is characterized by ineffective hematopoiesis, which can be fatal without treatment. It is an entity with high prevalence in elderly population and incidence rate is rising every year because of the aging population and successful use of chemotherapy in other cancers, with a subset of these survivors developing therapy-related myeloid neoplasms.
Treatment of AML experienced a significant improvement in the late ’80s when the combination of anthracyclines and cytarabine (classical chemotherapeutic agents) was developed and long-term survival patients increased from less than 5 to 20–30% [Citation1]. Apart from the development of novel antineoplastic agents, improvements in supportive care, such as new antibiotics, antifungals, antiemetics, critical care protocols and transfusion support as well as parallel advances in the hematopoietic stem-cell transplantation field, were also crucial to achieve this success.
Since then, decades of research in molecular biology and genomics have brought up new personalized treatments for patients. It is well known that AML is a highly heterogeneous disorder that arises from multiple acquired genetic lesions accumulating in hematopoietic progenitors. Next generation sequencing methods have confirmed that early mutations may be present many years before the disease develops [Citation2] and cooperating mutations would arise later driving the disease’s clinical manifestation.
Cytogenetic and molecular analyses at the time of diagnosis are critical to define prognostic risk, and predict overall survival, relapse and remission rate [Citation3]. Chromosomal abnormalities such as monosomies, trisomy 8 and deletions in chromosomes 5 or 7 are common in AML. Other frequent chromosomal abnormalities are balanced translocations between chromosomes 8 and 21 [t(8;21)(q22;q22)]; chromosomes 17 and 16 [t(7;16)(q31;q22)], chromosomes 15 and 17 [t(15;17)]; and chromosomes 9 and 11 t(9;11) and inv(16). Defects in the long arm of chromosome 11 (11q) are also common in AML [Citation4].
Patients with normal cytogenetics at diagnosis may also present heterogeneity in terms of treatment response. This is probably due to a considerable variability in gene expression and gene mutation. These alterations fall into two groups: class I and class II. Class I includes mutations that trigger signal transduction pathways, hence increasing the proliferation and/or survival of hematopoietic progenitor cells. Class II consists of mutations that affect cell cycle components and transcription factors leading to impaired differentiation. One example of class I mutations are the changes observed in the FMS-like tyrosine kinase 3 (FLT3) receptor. This receptor is expressed by immature hematopoietic stem cells. It plays a key role in normal development of the stem cells and is frequently overexpressed in AML [Citation5]. The FLT 3 gene is located in the long arm of chromosome 13. The two major types of FLT3 mutations include internal tandem duplications (ITD) that affect the juxtamembrane region in the receptor and point mutations in the tyrosine kinase domain (TKD) such as mutation at the aspartic acid 835. Both mutations cause a constitutive activation of the receptor´s tyrosine kinase activity in the absence of a ligand. Some class II mutations involve CCAAT/enhancer-binding protein alpha (CEBPA) [Citation6] and nucleoplasmin 1 (NPM1) [Citation7].
Other mutations identified as therapeutic targets are Isocitrate dehydrogenase -1 and -2 (IDH1 and IDH2) mutations that are found in 20% of adult patients with AML [Citation8]. The enzymes encoded by IDH1 and IDH2 are involved in epigenetic processes such as histone demethylation and DNA modification. The alteration in such pathways may alter cell differentiation and promote oncogenesis.
Results from conventional cytogenetics analysis and from NPM1, FLT3, CEBPA, ASXL 1, IDH1, IDH2 and TP53 mutational screening among others, are currently being used in routine diagnosis of AML following European Leukemia Net (ELN) recommendations [Citation9] and in line with National Comprehensive Cancer Network (NCCN) guidelines [Citation10]. The main objective is to identify patients who may benefit from currently available targeted therapies. On the other hand, molecular markers may provide information regarding the temporal evolution and growth of minor clones that may be responsible for resistant or relapsed disease and are also useful for detecting minimal residual disease (MRD) to monitor treatment response. Molecular testing should also be performed at the time of relapse to check for newly emerged targetable mutations that were absent at diagnosis.
With respect to treatment in patients with AML, it is remarkable that majority of patients diagnosed with AML are around 60–70 years old and this biological age together with other patient´s condition (comorbidities and psychosocial factors) may have a prognostic impact on disease outcome. Intensive treatment regimens that include conventional chemotherapy (anthracycline and cytarabine based combinations) present an increased toxicity in this patient population, as there is a decreased drug clearance and prolonged exposure to chemotherapeutics due to pharmacokinetic and pharmacodynamic changes. In addition, the decreased immune competence of elderly patients results in less tolerability of infections that arise as consequence of chemotherapy induced cytopenia. Less intensive modality of treatments (considered as non-intensive regimens) has been developed based on the administration of hypomethylating agents (decitabine, azacitidine) [Citation11] and low dose cytarabine (LDAC) with acceptable response rates and good quality of life observed during treatment. When deciding first-line treatment for AML patients, it is now mandatory to pre-assess cognitive and physical functions using different algorithms to better select patients suitable for intensive regimens. This approach led to a decreased rate of treatment-related mortality in patients that received conventional chemotherapy [Citation12]. Nevertheless, treatment outcomes are still poor in AML and new therapeutic options are needed.
The increased knowledge about genomic and molecular markers for AML led to the development of new targeted therapies in 2017, which have substantially changed the prognosis for patients in the last 5 years.
New targeted drugs recently approved for newly diagnosed (ND) AML patients include venetoclax [Citation13,Citation14] a B-cell lymphoma 2 (BCL2) inhibitor, midostaurin [Citation15] for leukemic cells that present FLT3 mutations, ivosidenib [Citation16] to target IDH1 and enasidenib [Citation17] for IDH2 mutations. Other additions include reapproval of gemtuzumab ozogamicin (GO) [Citation18] a monoclonal antibody binding to CD33 positive leukemic cells, glasdegib [Citation19] a hedgehog pathway inhibitor, and a liposomal formulation of daunorubicin and cytarabine (CPX-351) [Citation20]. The spectrum for approved drugs in the relapse or refractory (R/R) setting is broader. Gilteritinib is a second-generation FLT3 inhibitor approved for adults with relapsed or refractory AML and FLT3 mutation [Citation21]. Finally, the benefits of post-remission maintenance treatment with oral azacitidine (CC-486) [Citation22] a hypomethylating agent has also been reported in a randomized trial that led to its FDA approval in September 2020.
The new landscape in leukemia treatment is bringing personalized medicine to patients but some uncertainty is reported with respect to the incorporation of these novel agents in routine clinical practice for the first line setting, demonstrating the importance of exploring real-world data (RWD) in ND leukemia patients. The aim of this research is to describe the use of novel agents in first-line treatment of AML patients using retrospective data.
Materials & methods
This was a two-wave multi-country cross-sectional retrospective online web-based survey sent to healthcare professionals (HCP) treaters of AML patients in Europe (France, Germany, Italy, Spain [EU4] and UK). First wave from January to March 2020 and second wave from December 2020 to February 2021. The study included both deceased and living patients with AML who initiated cytotoxic and/or targeted therapies following AML diagnosis within the last 24 months prior to the survey.
518 physicians (255 physicians in the first wave and 263 physicians in the second wave) were asked to complete a survey in which they provided information on three to four AML patients treated by them, adding up to 2040 AML patients in total.
The survey was conducted through an online computer assisted web-based structured questionnaire in a case report form format lasting on average 60 minutes. Informed consent was obtained from the physicians who participated in the survey.
Questionnaire had two main sections: the first aimed to assess respondent physician’s caseload of cancer patients aged 18 and above, whom they personally treated within the last 6 months prior to the survey; the second section aimed at collecting details on AML patients personally treated by the respondent physician. The following variables have been collected during this survey.
General cancer patient caseload
Number of patients with any hematological malignancy, number of AML patients, percentage of FLT3 mutated AML patients, AML treatments received (standard AML treatment, hypomethylating agents, supportive care, or palliative care, etc.), percentage in clinical trials, details on eligibility and receipt of stem cell transplant (SCT).
Patient case report form
Patient’s clinical characteristics, status (living/deceased), ECOG performance status at diagnosis and at start of each therapy, date (mm/yyyy) and cause of death, date (mm/yyyy) of AML diagnosis, time from AML diagnosis to initiation of AML treatment, CNS involvement, percentage bone marrow blasts at AML diagnosis, white blood cells count at AML diagnosis, molecular profile at time of treatment decision, molecular and genetic risk stratification at diagnosis, cytogenetic abnormalities at diagnosis, comorbidities at diagnosis, healthcare utilization resource usage, AML treatments received and duration of treatments, details on ineligibility for highly intensive induction chemotherapy, percentage patients in clinical trials, physician’s reason for treatment choice and satisfaction with treatment, details on treatment discontinuation, number of relapses experienced by the patient, time from historic treatment to relapse, details on eligibility and receipt of SCT.
The questionnaire was firstly developed in English and later translated into the local languages. Translations were validated at a local level before the language overlay took place. The survey was then tested, and quality checked before going live. Physicians received honoraria payments for their participation in the online survey, which was in line with fair market value and local regulations. Information for all AML patients was gathered during a single survey with each HCP.
AML-treating healthcare professionals participating in the survey were oncologists, hematologists, or hemato-oncologists. In addition, respondents were required to meet the following criteria: have spent at least 3 years practicing/specialising; personally initiate or recommend/prescribe treatment for AML; recall the treatment initiated for their last four adult patients with AML; and devote at least 40% of their time to seeing and treating patients. Only those respondents who met the screening criteria and who agreed with the data privacy disclaimer provided were able to proceed to the main survey. AML non-treating physicians or those who may have possible conflicts of interest (i.e., employed by regulatory bodies or pharmaceutical companies) were excluded.
Included patients were: aged 18 years or older; with primary AML (i.e., not related to prior chemotherapy, not secondary to other blood cancer, e.g., MDS, MPN or other blood cancers); at any stage of AML at the time of the survey, including living or deceased patients; whose AML current and/or previous treatment(s) are known to the physician participating in the survey; and who initiated cytotoxic and/or targeted therapies following AML diagnosis within the last 24 months prior to the survey. Patients with acute promyelocytic leukaemia (APL) were not eligible for inclusion. In the first wave (January–March 2020) the inclusion criteria required that FLT3 mutation status of the patient should be known at the time of the survey, but this inclusion criterion was removed during the second wave (December 2020–February 2021).
New targeted agents approved since 2017 by FDA or EMA for AML treatment were considered as Novel Agents in this study, including midostaurin, gilteritinib, enasidenib, ivosidenib, CPX-351, gemtuzumab ozogamicin, venetoclax and glasdegib.
The definition for intensive versus non-intensive treatment was made based on the nature of the compounds administered and the objective of treatment. Intensive treatment consisted of high dose chemotherapy, and non-intensive treatment included low dose chemotherapy and hypomethylating agents. Candidates for non-intensive regimens were frail older adults or those with patient-specific factors that (according to the treating physician) made them ineligible for high dose chemotherapy.
Molecular and genetic risk stratification was performed using ELN 2017 definitions [Citation9].
Treatment response was assessed based on HCPs criteria with definition of four categories in the questionnaire:
Complete response: as meeting all the following criteria: <5% blasts in the bone marrow, no extramedullary disease (e.g., CNS, soft tissue disease), neutrophils ≥1000/μl, platelets ≥100,000/μl, transfusion independent.
Partial response: defined as a ≥50% decrease in bone marrow blasts with normalization of peripheral blood counts, or some other measure of hematologic improvement.
Progressive disease: defined as the appearance of disease after any type of response was met. It includes an increase in the % blasts in the marrow or peripheral blood, presence of extramedullary disease or disease persistence determined by a physician upon clinical assessment.
Stable disease: if none of the above was met.
Descriptive statistics analysis was performed using numbers and percent within category with 95% CI for categorical variables.
The use of novel agents was compared against the whole treatment spectrum due to the study design (retrospective study) and limited sample size.
Time-to-event variables such as duration of therapy were analyzed using the Kaplan–Meier (KM) method. KM survival analyses was conducted and summarized to include the number of patients (N), number of events (duration of therapy) observed, percentage of patients censored (on-going treatment at time of survey), median duration of therapy and probability of duration of therapy (95% CI). All the analyses were conducted using R software version 4.1.0. (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL www.R-project.org/).
Results
Patient characteristics
Data was collected for 2040 patients who received induction treatment. There were two waves of data collection, first from January to March 2020 (1012 pts) and second from December 2020 to February 2021 (1028 pts). compares the baseline demographic and clinical characteristics for the entire study population and for those patients who received novel agents (in monotherapy/combination) in first-line therapy.
Table 1. Patient characteristics.
Median age range was similar in both groups (in 60s) and there was a slight predominance of males. The majority of patients in both groups (80%) had an Eastern Cooperative Oncology Group (ECOG) status 0–2. For patients with worse condition and ECOG ≥2 there were no differences between patients who received novel agents and all patients.
The presence of main comorbidities was studied in these patient populations (), and we found similarity between both groups. We did not identify any specific trend of treating patients with novel agents based on the presence of any previous medical condition. There were fewer patients with cardiac disease (arrhythmias, heart failure and ischemia) and severe renal failure (patient on dialysis, underwent renal transplant) who received novel agents (among patients who received novel agents, 16.4 and 0.8% treated patients presented cardiac disease and severe renal failure, respectively vs 17.7 and 1.2% in the overall population). This small difference may be explained due to a pre-selection performed for patients that received novel agents within a clinical trial´s context.
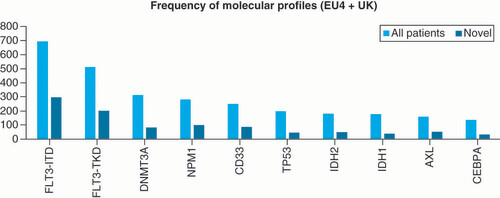
Different gene mutations that activate signal transduction pathways and transcription factors were analyzed in this patient population (). As novel agents used in the first-line setting mainly consist of selective therapy against molecular targets, there were a higher number of patients harboring a specific mutation (FLT3-ITD, FLT3-TKD, NPM1 or expression of antigen CD33) who were treated with novel agents. Those patients presented a higher incidence of FLT3 mutation (either ITD or TKD mutations) compared with overall population. The incidence of FLT3-ITD mutation was 50% among patients treated with novel versus 34.2% in the overall population. For FLT3-TKD mutated patients, incidence in the population treated with novel agents was 34.3 vs 25.2% in the entire patient sample. With respect to NPM1, the difference was less marked with 17.4% mutation incidence in patients treated with novel agents versus 14.1% in overall population. The expression of CD33 was also investigated in this sample and it was observed that 15.1% of patients treated with novel agents harbored CD33 expression, versus 12.4% in the overall population.
A wide spectrum of cytogenetic abnormalities was also analyzed in this set of patients: different translocations as t(8;21), t(15;17), t(9;11), trisomy 8 and deletion for chromosome 5 and 7 among others. Differently from gene mutations, we did not observe any trend of treating patients presenting cytogenetic abnormalities that received new agents compared with the overall population. There was a significant exception for patients with inv(16) or t(16;16) as higher proportion (6.3%) of patients receiving conventional therapy were harbouring these cytogenetic conditions versus 3.0% that received targeted therapy (novel agents). This selection of treatment is in line with the high rate of complete remission (CR) and favorable overall survival (OS) observed when those patients are treated with high dose cytarabine [Citation23].
Although cytogenetic was not considered for using new agents as preferred treatment in first-line AML patients different from mutational status, when leukemia prognostic factors based in molecular and genetic risk were assessed [Citation9] there was a trend toward selecting patients classified as having adverse risk prognosis to be treated with novel agents compared with the overall population (36.6% of novel agent receiving patients presented adverse risk factors vs 28.3% patients classified as adverse risk in the overall population). Nevertheless, for patients with favorable risk, novel agents as therapeutic option was less frequently selected: only 17.4% of novel agents receiving patients belonged to favorable risk group compared with 27.3% in the overall population.
As approval status for some of the new agents was varied across the five European countries selected for this retrospective review, the type of treatment program for first-line therapy was analyzed in our patient population. In general, higher proportion of patients treated with novel agents were included in clinical trials or received therapy as part of a compassionate use program compared with the overall population (7.6 and 6.3% enrolled in a clinical trial and under a compassionate use program respectively in the novel agent´s setting vs 5.3 and 4.0% in the overall population).
Use of novel agents in first-line AML patients
Out of 2040 patients that received induction (first-line) treatment, 604 patients (29.6%) were treated with novel agents (). New agents were administered in combination for most of the patients (598 subjects, 99% of total patients who received novel agents as first-line treatment) and less than 1% received novel agents as monotherapy.
Table 2. Use of novel agents in first-line acute myeloid leukemia patients by type of regimen.
Novel agents were used in both intensive and non-intensive settings.
The different anti-leukemic agents administered with novel agents in combination are presented in . These consist of conventional chemotherapy (mainly based in anthracycline and high dose cytarabine combo regimen), hypomethylating agents (HMA) such as azacitidine and decitabine, and low dose cytarabine (LDAC).
Table 3. Novel agents in combination administered in first-line treatment acute myeloid leukemia patients.
In the intensive setting, majority of novel agents treated patients were receiving them in combination with conventional chemotherapy. For patients receiving novel agent in combination with HMA agents, it was noted that only 8.3% of patients received this combination in an intensive setting while 91.7% were treated in a non-intensive setting. As HMA agents offer a valuable alternative for older and medically non-fit patients, we believe that the use of HMA + novel agent combinations classified as intensive regimens by treating physician, was based exclusively on patients´ age (younger population) and not based on other characteristics considered for fitness evaluation.
The use of novel agents in induction for newly diagnosed AML patients was evaluated over time during the two waves of data collection and is reflected in . Around 30% of patients diagnosed with AML received a novel agent for first-line treatment. No relevant changes in treatment patterns were observed from January–March 2020 and December 2020–February 2021 except for a decreasing trend in the administration of novel agents as monotherapy.
Table 4. Use of novel agents across time.
When the different novel agents in combination with other anti-leukemic treatments were analyzed across time (), chemotherapy and a novel agent combination was the selected schema used more frequently. There was a significant decrease, of 17%, in the use of this combination in December 2020–February 2021 compared with the previous year and a remarkable increase of 14% toward the use of the combination of HMA with novel agents, reflecting the FDA´s approval for some of the new agents (i.e., venetoclax) that year.
Table 5. Different treatments combination with novel agents across time.
Treatment response
Leukemia response data were available for 1024 subjects (50.2%) out of the total sample of 2040 patients with newly diagnosed AML who received Induction treatment. Within the 1024 subjects with tumor response data available in questionnaires, 752 patients (73.4%) received an intensive regimen. Among patients on intensive regimen, 196 (26.1%) received a novel agent. 272 out of 1024 patients with leukemia response data (26.6%) received a non-intensive regimen and 75 (27.6%) of those were on a novel agent (). The proportion of patients receiving a novel agent was similar in both types of treatment regimens, intensive (26.1%) and non-intensive setting (27.6%).
Table 6. Treatment response.
Considering these 1024 leukemia patients with data available for efficacy assessment, the overall response rate (ORR) for patients receiving an intensive regimen based on chemotherapy was higher (622 patients out of 752 with ORR 82.7%) compared with the ORR observed in patients receiving a non-intensive regimen (272 ORR 37.1%).
When the response was assessed based on the administration of a novel agent, there was a slight improvement in the ORR in the non-intensive regimen setting for patients who received a novel agent (ORR 41.3%) compared with patients treated with LDAC or HMA alone (ORR 35.5%).
For patients treated under an intensive regimen, there was no difference in the ORR based on the use of a novel agent but a trend toward better CR rates (72.3% without novel agent vs 66.9% when a novel agent was incorporated) was noted. In addition, lower rates for disease progression were observed in patients receiving new agents (8.7%) versus patients treated with chemotherapy alone (12.0%), in the intensive setting.
Duration of treatment
Intensive regimen
Duration of treatment for all administered regimens (intensive and non-intensive) was evaluated using KM analysis and is the time frame from start of treatment (mid of month for given month and year) to end of treatment (mid of month for given month and year), or survey inclusion date (censored). When duration of treatment is evaluated, it is important to consider the intrinsic characteristics of each regimen as non-intensive treatments are administered continuously in an ambulatory environment, while intensive treatments are administered over few days, intravenously, with patient being hospitalized for a long period to permit the bone marrow recovery.
In the intensive setting () the median duration considering all patients for Induction therapy was 3.02 months (IQR 25–75 = 1.02–6.05 months) similar to the duration observed for patients receiving novel agents, 2.99–months (IQR 25–75 = 1.02–5.03 months).
LOT: Line of treatment.
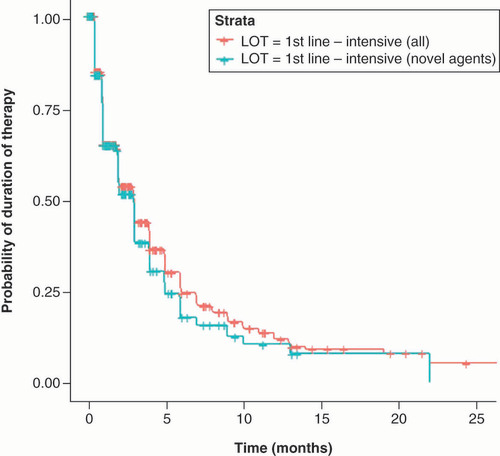
showed the probability of still being treated with first-line intensive regimen across time considering overall population and patients that received a novel agent. A similar percentage of permanence was observed for both groups at 3 months with a slightly higher discontinuation trend in patients receiving a novel agent during the following months.
Table 7. Probability of still being treated across time in newly diagnosed acute myeloid leukemia patients receiving intensive regimens.
Non-intensive regimen
For patients who received non-intensive regimen () a median duration of 7 months (IQR 25–75 = 3.02–24.0 months) was observed for the overall patient population and 9.04 months for patients who received novel agents (IQR 25–75 = 4.01 months not reached).
LOT: Line of treatment.
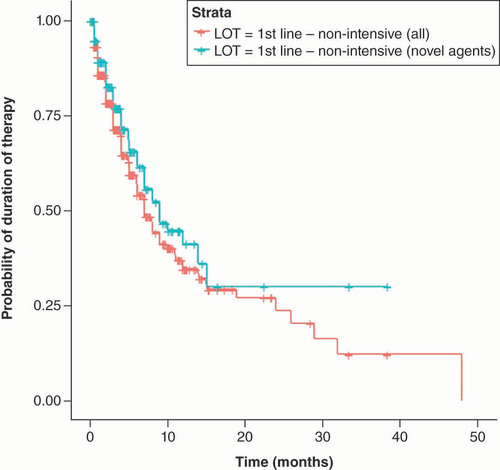
Although patients who received novel agents did not reach 75 interquartile and comparison between the curves is not possible, there is a trend toward a longer duration of treatments when novel agents were incorporated into the non-intensive setting.
The probability of still being treated across time in first-line patients who received a non-intensive regimen was higher for those patients that incorporated a novel agent in the anti-leukemic treatment (). This advantage for patients receiving novel agents persisted and increased over time.
Table 8. Probability of still being treated across time in newly diagnosed acute myeloid leukemia patients receiving non-intensive regimens.
Discussion
We are entering into a new era in the treatment of AML patients due to recent approval for new agents, many of them targeting specific mutations (midostaurin, gilteritinib, ivosidenib, enasidenib) or pathways (venetoclax, glasdegib). Although some registries [Citation24] have shown valuable data for treatment patterns in first-line therapy, there is a lack of real-world evidence (RWE) data for the use of novel agents in AML patients. The aim of this retrospective analysis with available data for more than 2000 patients in five EU countries, is to describe the use of novel agents in first-line and identify possible areas of growth for those new therapies.
Novel agents were administered to 604 patients out of 2040 total subjects (29.6%) mainly in combination with other anti-leukemic treatment in both intensive and non-intensive settings.
Considering patients’ characteristics, it was remarkable to see no differences between the use of novel agents based on different comorbidities. Novel agents were perceived as possible treatment options for patients with different conditions such as cerebrovascular disease, diabetes, hypertension, chronic pulmonary disease among others. We believe this finding confirms that mechanism of action of novel drugs (considered as targeted therapy) is the main attribute evaluated when selecting those as part of anti-leukemia treatment. An acceptable safety profile may contribute to their use in patients with significant comorbidities.
The presence of some mutations in our patient´s population seemed to be an important element for treatment decision when adding novel agents to the different anti-leukemia treatments. There was a significant increase in patients harboring FLT3-ITD, FLT3-TKD and NPM1 mutations among those subjects who received novel agents. A similar increase was also shown for patients with CD33 positive leukemia. Patients with IDH1 and IDH2 mutations did not have a higher presence in the novel agents’ group probably due to the non-approval status in EU for both compounds.
Cytogenetics characteristics were observed to be less important in determining the incorporation of novel agents into first-line treatment in our patient population. There were no differences for both groups when several chromosomal abnormalities were tested. The only difference was found for Inv (16) or t(16;16), considered a cytogenetic abnormality that confers favorable prognosis, and it may explain the higher number of patients treated under conventional chemotherapy.
Although isolated cytogenetics did not seem to influence the selection of novel agents for first-line treatment, it was important to consider chromosomal abnormalities when combined with mutations to define genetic risk stratification. There was a trend toward selecting adverse risk patients to be treated with novel agents reflecting the huge unmet need for efficacious targeted therapies for those patients harboring molecular and cytogenetic abnormalities associated with poor prognosis. A higher number of patients with adverse risk prognosis received novel agents (36.6%) compared with overall population (28.3%). The opposite was observed, when a smaller number of patients classified as having a favorable prognosis risk received novel agents (17.4%) compared with overall population (27.3%).
The use of novel agents during the 2 years of study duration did not experience a significant increase over time. A trend to use HMA as preferred combination schema (14% increase) in the non-intensive setting was observed, probably due to the good results published around that time for the combination with venetoclax that granted FDA approval in 2020 [Citation25,Citation26].
The benefit of novel agents in terms of leukemia response improvement was more evident when combined with HMA and in the non-intensive setting. This beneficial effect was also observed when duration of treatment was evaluated although due to the nature of our data it is difficult to draw clear conclusions on that matter.
The lack of differences in terms of duration of treatment in the intensive setting when novel agents are incorporated may reflect no relevant safety concerns that led to early treatment discontinuation.
Our study, being retrospective research in design and using a web-based structure questionnaire, presents some limitations. The definition for intensive versus non-intensive treatment for any of the regimens was made by the treating physician enabling some bias, for example the identification of 8.26% of patients treated with HMA agent and novel therapy in an intensive setting. Leukemia response was also assessed by physician and the definition for stable disease in leukemia setting was difficult to interpret.
Based on the available data, it was not possible to capture different reasons for treatment decisions made upon the various non-disease factors and availability for the novel agents; hence results should be interpreted cautiously. However, we think this study explores the real-world use of novel agents and provides useful insight about how new treatments may be incorporated into the standard of care in a new landscape with available personalized therapies.
Conclusion
Due to an unprecedented advance in genomic research in the last 5 years, new targeted therapies for specific mutations and intracellular pathways related to leukemogenesis have been developed and are now getting approved. This research has explored how novel agents are being incorporated into the first-line in AML patients of five EU countries.
Some barriers are still present that may limit incorporation of novel agents into first-line setting: the availability of new agents in EU is being delayed compared with recent approvals in US; use of novel agents is linked to the need for identification of actionable mutations, not just at diagnosis but also at relapse, and this high requirement for diagnosis leads to increased costs. In addition, more information is needed to decide which drug to use when several therapeutic options may be available; in that sense to set up goals of care and evaluate the cost of new agents, may play a key role. Finally, it is important to understand how to anticipate, mitigate and manage common complications associated with these new agents that are used in a subset of ‘frail’ patients.
Our descriptive study based on RWE data from 2040 patients showed a progressive incorporation of novel agents for newly diagnosed AML patients, with a significant impact on the treatment of patients with specific mutations. There are overlapping new treatment options, especially in older and unfit population in this first-line setting and we believe novel agents are a game changer for these patients.
There are novel targeted drugs approved for acute myeloid leukemia (AML) in the first-line setting although there is uncertainty with respect to their incorporation in routine clinical practice.
Data from 2040 patients from a European multi-country cross-sectional retrospective online web-based survey have been analyzed and presented in this article.
In our study population, 29.6% of patients were treated with novel agents in the first-line setting mainly in combination and as part of non-intensive regimens.
There were a higher number of patients harboring a specific mutation (FLT3-ITD, FLT3-TKD, NPM1 or expression of CD33) who were treated with novel agents in the first-line setting.
We observed a trend toward selecting patients classified as having adverse risk prognosis to be treated with novel agents compared with the overall population.
A higher proportion of patients treated with novel agents were included in clinical trials or received therapy as part of a compassionate use program compared with the overall population.
The benefit of novel agents in terms of leukemia response improvement was more evident when combined with hypomethylating agents (HMA) and in the non-intensive setting.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Participating physicians provided written informed consent prior to taking part in the survey. As this was not an investigation of clinical outcomes with any particular intervention, neither Ethics Committee approval nor clinical trial registration was required. The patient data was fully anonymized, and no primary or identifying data was collected. The survey was conducted in accordance with the EphMRA guidelines.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- Freireich EJ, Wiernik PH, Steensma DP. The leukemias: a half-century of discovery. J. Clin. Oncol. 32(31), 3463–3469 (2014).
- Genovese G, Kähler AK, Handsaker RE et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371(26), 2477–2487 (2014).
- Papaemmanuil E, Gerstung M, Bullinger L et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 374(23), 2209–2221 (2016).
- Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2(2), 95–107 (2011).
- Small D. Targeting FLT3 for the treatment of leukemia. Semin. Hematol. 45(2 Suppl. 3), S17–S21 (2008).
- Nerlov C. C/EBPalpha mutations in acute myeloid leukaemias. Nat. Rev. Cancer. 4(5), 394–400 (2004).
- Grisendi S, Mecucci C, Falini B et al. Nucleophosmin and cancer. Nat. Rev. Cancer. 6(7), 493–505 (2006).
- Becker JS, Fathi AT. Targeting IDH Mutations in AML: Wielding the Double-edged Sword of Differentiation. Curr. Cancer Drug Targets. 20(7), 490–500 (2020).
- Döhner H, Estey E, Grimwade D et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4), 424–447 (2017).
- Pollyea DA, Bixby D, Perl A et al. NCCN Guidelines Insights: Acute Myeloid Leukemia, Version 2.2021. J. Natl Compr. Canc. Netw. 19(1), 16–27 (2021).
- Quintás-Cardama A, Ravandi F, Liu-Dumlao T et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 20(24), 4840–4845 (2012).
- Othus M, Kantarjian H, Petersdorf S et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given ‘intense’ induction regimens: a report from SWOG and MD Anderson. Leukemia. 28(2), 289–292 (2014).
- Wei AH, Strickland SA Jr , Hou JZ et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J. Clin. Oncol. 37(15), 1277–1284 (2019).
- DiNardo CD, Pratz K, Pullarkat V et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133(1), 7–17 (2019).
- Stone RM, Mandrekar SJ, Sanford BL et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 377(5), 454–464 (2017).
- Roboz GJ, DiNardo CD, Stein EM et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood 135(7), 463–471 (2020).
- Stein EM, DiNardo CD, Pollyea DA et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130(6), 722–731 (2017).
- Castaigne S, Pautas C, Terré C et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, Phase III study. [published correction appears in Lancet. 2018 Feb 8]. Lancet 379(9825), 1508–1516 (2012).
- Cortes JE, Heidel FH, Hellmann A et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 33(2), 379–389 (2019).
- Lancet JE, Uy GL, Cortes JE et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 36(26), 2684–2692 (2018).
- Perl AE, Martinelli G, Cortes JE et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 381(18), 1728–1740 (2019).
- Wei A, Dohner H, Pocock C et al. The QUAZAR AML-001 Maintenance Trial: Results of a Phase III International, Randomized, Double-Blind, Placebo-Controlled Study of CC-486 (Oral Formulation of Azacitidine) in Patients with Acute Myeloid Leukemia (AML) in First Remission. Blood 134(Suppl. 2), LBA3 (2019).
- Eghtedar A, Borthakur G, Ravandi F et al. Characteristics of translocation (16;16)(p13;q22) acute myeloid leukemia. Am. J. Hematol. 87(3), 317–318 (2012).
- Labrador J, Martínez-Cuadrón D, de la Fuente A et al. Azacitidine Vs. Decitabine in Unfit Newly Diagnosed Acute Myeloid Leukemia Patients: Results from the Pethema Registry. Blood 136(Suppl. 1), 25–27 (2020).
- DiNardo CD, Jonas BA, Pullarkat V et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 383(7), 617–629 (2020).
- Wei AH, Montesinos P, Ivanov V et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a Phase III randomized placebo-controlled trial. Blood 135(24), 2137–2145 (2020).