Abstract
Aim: Treatment effects among anticoagulant-treated patients with venous thromboembolism (VTE) and cancer across tumor types were evaluated. Methods: Patients initiating an anticoagulant within 30 days after VTE were identified. After inverse probability treatment weighting, patients were stratified by tumor type. Interactions between treatment and tumor type on recurrent VTE, major bleeding and clinically relevant non-major bleeding were assessed using Cox proportional hazard models. Results: Treatment effects were generally not significantly different among patients with or without the following cancer types: prostate, breast, lung, pancreatic or multiple myeloma. Few significant interactions were observed for lung and pancreatic cancer. Conclusion: Anticoagulant treatment effects were generally consistent across tumor types. The significant interactions may indicate tumor-specific effects of anticoagulants, but further research is needed.
Tweetable abstract
This real-world study evaluated the effectiveness and safety of apixaban, LMWH, and warfarin among patients with VTE and with common or high risk tumor type. Further research is needed to better understand effects of anticoagulants in patients with VTE and different tumor types
Keywords: :
Patients with cancer are at high risk of developing venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), which can result in increased morbidity and mortality [Citation1–4]. The risk of VTE varies depending on the patient’s age, treatment, cancer stage and tumor type [Citation5–7]. Low molecular weight heparin (LMWH) used to be a first-line treatment for VTE in patients with cancer [Citation4,Citation6,Citation8,Citation9], but oral anticoagulants like warfarin have also been commonly used. In recent years, direct oral anticoagulants (DOACs) have been included as another recommended treatment option for VTE in patients with cancer [Citation6,Citation10–16].
VTE outcomes vary depending on the type of cancer diagnosis [Citation17–20]. Breast cancer consistently had lower events per 100 patient-years (recurrent VTE and major bleeding [MB]) compared with prostate, colorectal and lung cancer when evaluating patients with VTE and active cancer in an international VTE registry [Citation17]. It was also found that patients with gastrointestinal (GI) cancer tended to experience more MB and recurrent VTE compared with those with other cancers such as breast and lung when treated with edoxaban or dalteparin (LMWH) in addition to differing effects of treatment [Citation20–23]. For example, edoxaban and rivaroxaban patients were less likely to have a recurrent VTE but more likely to have MB than dalteparin (LMWH) patient [Citation22,Citation23]. However, no excess of MB including GI bleeding was found for patients treated with apixaban compared with LMWH [Citation15,Citation18,Citation24]. Patient-specific factors (age/sex) and treatment-specific factors (chemotherapy/surgery) might also interact with tumor type to influence the effects of different DOACs [Citation4,Citation13,Citation16,Citation25,Citation26].
Recently, a regularly updated, living systematic review found that DOACs were associated with a 41% reduction of recurrent VTE compared with LMWH for patients with cancer-associated thrombosis (odds ratio [OR]: 0.59; 95% CI: 0.41 to 0.86) [Citation16,Citation27]. These effects did not differ by presentation (symptomatic vs incidental) or patient characteristics (e.g., age, cancer stage). The review noted that it was unclear whether treatment effects differed as a function of tumor type. One reason for this evidence gap is that most studies comparing anticoagulant treatments have not been designed to examine such differences. Most cancers represented in clinical trials (>87%) are solid tumors such as breast, colorectal or lung cancer [Citation9,Citation12,Citation15,Citation16,Citation23,Citation28]. These cancers are prevalent in the general population but are not necessarily at higher risk of VTE [Citation29]. Other cancers with a higher risk of VTE are less commonly observed in clinical trials due to lower prevalence (i.e., multiple myeloma and pancreatic cancer) [Citation15,Citation25,Citation29,Citation30]. Thus, evaluations of anticoagulants for specific tumor types are not always feasible because of small sample sizes or low event rates for these tumors.
Given the risk of VTE in patients with cancer, there is a need to understand the effectiveness and safety of different anticoagulant treatments, including DOACs, among patients with VTE and with different types of tumors. This real-world study evaluated the effectiveness (recurrent VTE) and safety (MB and clinically relevant non-major bleeding [CRNMB] events) of apixaban, LMWH and warfarin among patients with VTE and with common (prostate, breast, lung) or high risk (multiple myeloma and pancreatic) tumor type. Other high risk tumor types such as GI and brain cancer have been assessed in previous publications [Citation24] or a separate analysis [Citation31].
Methods
Data sources
Medical and pharmacy claims data were pooled from four commercial databases (Optum, Humana, PharMetrics Plus and MarketScan) and the Medicare Fee-for-Service (FFS) database. The study period ranged from 1 March 2014, through 30 June 2017 (MarketScan), 31 December 2017 (Optum, Humana and Medicare FFS), or 31 March 2018 (PharMetrics). Data were de-identified and data collection complied with the requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Study design & patient selection
A retrospective longitudinal cohort design analysis was used to examine adults 18 years of age or older who were diagnosed with VTE (i.e., they had a medical claim with an ICD-9-CM or ICD-10-CM diagnosis code for a VTE in any position on the claim) with an identification period spanning 1 September 2014, through the end of available data. VTE was defined as either a DVT or PE. Patients were included if they had active cancer. Active cancer was defined as either: 1) having two or more claims at least 1 day apart for a cancer diagnosis during the 6 months prior and 30 days after the index date; or 2) one claim for a cancer diagnosis and an additional claim for cancer treatment in the 6 months prior to the index date through 30 days after. Tumor types were identified by ICD-9 and ICD-10 diagnosis codes. Patients with VTE and active cancer were also required to be newly treated with an anticoagulant (apixaban, LMWH or warfarin) within 30 days after index VTE event. The date of anticoagulant treatment initiation was defined as index date and patients were required to be 18+ (commercial datasets) or 65+ years of age (Medicare) on the index date. Patients also had continuous enrollment in medical and pharmacy benefits for the 6 months prior to the index VTE event until the index date. Warfarin patients with LMWH bridging that had evidence of MB or recurrent VTE between warfarin initiation and the start of LMWH were excluded. The final study population excluded patients with VTE in the 6 months prior to their index VTE and atrial fibrillation/flutter, mechanical heart valve, or outpatient claims of anticoagulant use in the 6 months prior to the index date (i.e., baseline period). In addition, patients with evidence of an inferior vena cava filter, pregnancy or antiphospholipid syndrome during the study period were excluded.
Patient characteristics
Patient characteristics were measured as of the index date and included demographics (age and gender) and VTE-related factors (setting of index VTE event [inpatient, ambulatory, emergency room] and type of VTE diagnosis [DVT only, PE only, or PE with DVT]). Comorbidities (National Cancer Institute adaptation of the CCI [NCI Comorbidity Index], anemia, central venous catheter, cerebrovascular disease, thrombocytopenia, ischemic heart disease, dyspepsia/stomach discomfort, hyperlipidemia, obesity, pneumonia, sleep apnea, congestive heart failure, diabetes, hypertension, renal disease, liver disease, chronic obstructive pulmonary disorder [COPD], peripheral vascular disease, prior bleed and fracture/trauma of lower extremities), selected surgeries and cancer-related characteristics (evidence of metastasis and cancer-related treatment) were measured in the 6 months prior to the index VTE event through the index date.
Outcomes
Study outcomes included recurrent VTE, MB and CRNMB events and were measured from the index date through the earliest of death, disenrollment, switch to another anticoagulant, discontinuation of treatment, 6 months after the index date, or end of available data (i.e., follow-up period). Discontinuation was defined as no evidence of anticoagulant use for at least 30 days after the end of the patient days of supply. A recurrent VTE event was considered an inpatient admission with a diagnosis (ICD-9 or ICD-10) for VTE in the primary or first-listed position on the claim. Any inpatient admission for VTE within 7 days of the index VTE event was not considered a recurrent VTE. An MB event was an inpatient admission with a diagnosis (ICD-9 or ICD-10) for bleeding in the primary or first-listed position. The MB included gastrointestinal (GI) bleeding, intracranial hemorrhage and MB from other sites. A CRNMB event was an inpatient admission for a bleeding event that did not qualify as an MB event (i.e., had a bleed diagnosis in the secondary position and the bleeding was for a non-critical site) or an ambulatory care visit for non-critical site bleeding. The CRNMB was from GI locations or other non-intracranial sites.
Statistical analysis
Results are presented by initial anticoagulant used (warfarin, LMWH and apixaban). Inverse probability of treatment weighting (IPTW) was used to balance patient characteristics between treatment cohorts in overall population. Weights were generated as the inverse of the propensity score which was generated by a multinomial logistic model for the probability of receiving treatment (LMWH, apixaban or warfarin) with LMWH as the reference. Stabilized weights were used to reduce variability in the treatment weights caused by outliers. Standardized differences were used to determine differences between cohorts with a value of 10% (0.1) or higher indicating a relevant difference. Variables included in IPTW were those with a standardized difference >10% and included age, sex and NCI score. After IPTW, results were further stratified by those with and without a tumor type of interest (prostate cancer, breast cancer, lung cancer, multiple myeloma or pancreatic cancer). Outcomes (recurrent VTE, MB and CRNMB) were reported as incident rates (IR), which were calculated as the number of first events over person-years at risk for first event and reported as per 100 person-years. Cox proportional hazards models were used to determine the interaction of the specific tumor (yes and no) with initial treatment (apixaban, LMWH and warfarin). Unbalanced patient characteristics after the stratification (yes and no for a specific tumor) were included in the Cox models for further adjustment. Interactions were considered to be significant if p-values for interaction were <0.1.
Results
Patient characteristics
A total of 30,586 patients with VTE and cancer met all eligibility criteria (). From this overall cancer population, patients with lung cancer (19.9%), breast cancer (17.2%), prostate cancer (13.1%), pancreatic cancer (6.1%) and multiple myeloma (4.7%) were selected for this analysis.
*Identification period across databases were as follows:
MarketScan: 1 September 2014 – 30 June 2017.
Optum and Humana: 1 September 2014 – 31 December 2017.
PharMetrics: 1 September 2014 – 31 March 2018.
Medicare: 1 September 2014 – 31 December 2017.
◊ Study period across databases were as follows:
MarketScan: 1 March 2014 – 30 June 2017.
Optum and Humana: 1 March 2014 – 31 December 2017.
PharMetrics: 1 March 2014 – 31 March 2018.
Medicare: 1 March 2014 – 31 December 2017.
LMWH: Low molecular weight heparin; VTE: Venous thromboembolism.
shows demographics and clinical characteristics for patients with VTE and with the selected cancer after IPTW for the overall population. The percentage of patients using apixaban ranged from 23% (multiple myeloma) to 30% (prostate and pancreatic), using LMWH ranged from 26% (prostate) to 39% (pancreatic) and using warfarin ranged from 31% (pancreatic) to 44% (prostate). Mean age in years ranged from 71.0 (pancreatic) to 75.1 (prostate) for apixaban patients, 68.4 (breast) to 74.4 (prostate) for LMWH patients, and 70.6 (pancreatic) to 75.6 (prostate) for warfarin patients. Patient gender varied by tumor type. Multiple myeloma tended to be more common for male patients, whereas lung, and pancreatic cancers were more common for female patients. The setting in which the index VTE event was diagnosed was mostly inpatient (43.9–58.0%) or emergency room (ER; 36.4–50.8%) across tumor types.
Table 1. Characteristics of patients with common or high-risk cancer treated with apixaban, warfarin and LMWH (post-IPTW).
The index VTE event was more likely to be DVT only across different tumor types except for lung cancer which had more PE with or without DVT. The mean NCI comorbidity index score ranged from 2.7 (breast) to 3.5 (lung) for apixaban patients, 2.7 (breast) to 3.4 (pancreatic) for LMWH patients, and 2.8 (breast) to 3.5 (lung and pancreatic) for warfarin patients. The percentage of patients with a documented bleed during baseline ranged from 26.4% (breast) to 38.5% (pancreatic) for apixaban patients, 25.8% (breast) to 40.5% (prostate) for LMWH patients, and 26.1% (breast) to 37.6% (pancreatic) for warfarin patients. Cancer metastasis was higher for pancreatic (72.5–87.5%) and lung (72.3–77.0%) cancer compared with breast (43.1–50.5%), multiple myeloma (20.2–37%) and prostate cancer (29.1–45.8%). Additional patient characteristics can be found in Supplementary Table 1.
show data for the effects of apixaban, LMWH, and warfarin on the risks of recurrent VTE, MB and CRNMB stratified by the presence and absence of a specific tumor type.
*Hazard ratios were adjusted for the following unbalanced patient characteristics between treatment cohorts after the stratification for a specific cancer:
Apixaban vs warfarin: (prostate cancer: age group, antiarrhythmic; breast cancer: pneumonia, chemotherapy; lung cancer: selected surgeries; pancreatic cancer: sex, region, index VTE setting, anemia, ischemic heart disease, pneumonia; multiple myeloma: age group, sex, inpatient VTE, dyspepsia/stomach discomfort, spinal cord injury, selected surgeries, gastroprotective agent, Khorana risk, immunotherapy, radiation therapy).
Apixaban versus LMWH: (prostate cancer: age group, anemia, obesity, prior bleed, history of fall, metastasis, chemotherapy, cancer related surgery; breast cancer: age group, non-cancer provoked factors, central venous catheter, hyperlipidemia, hypertension, rheumatologic disease, metastasis, chemotherapy; lung cancer: age group, anemia, central venous catheter, hematologic disorder, hyperlipidemia, hypertension, selected surgeries, antiarrhythmic, chemotherapy, cancer related surgery; pancreatic cancer: age group, region, index VTE setting, anemia, hematological disorder, ischemic heart disease, hyperlipidemia, hypertension, prior bleed, metastasis, hormone therapy, chemotherapy, radiation therapy; multiple myeloma: age group, region, PE with or without DVT, hematologic disorder, pneumonia, hyperlipidemia, hypertension, prior bleed, fracture/trauma of lower extremities, statins, metastasis, chemotherapy).
Warfarin versus LMWH: (prostate cancer: age group, obesity, varicose veins, hyperlipidemia, hypertension, prior bleed, history of fall, selected surgeries, anti-platelet, Khorana risk scale, metastasis, chemotherapy; breast cancer: age group, central venous catheter, hyperlipidemia, hypertension, rheumatologic disease, metastasis, chemotherapy; lung cancer: age group, central venous catheter, hyperlipidemia, hypertension, NSAIDs, selected surgeries, metastasis, Khorana risk scale, chemotherapy, cancer related surgery; pancreatic cancer: age group, sex, region, hyperlipidemia, hypertension, inflammatory bowel disease, prior bleed, aromatase inhibitor, metastasis, hormone therapy, chemotherapy, radiation therapy; multiple myeloma: age group, sex, inpatient VTE, PE with or without DVT, hematologic disorder, dyspepsia/stomach discomfort, pneumonia, spinal cord injury, hypertension, history of fall, selected surgeries, antiarrhythmic, metastasis, chemotherapy, immunotherapy, radiation therapy).
DVT: Deep vein thrombosis; IPTW: Inverse proportional treatment weighting; IR: Incidence rate (in 100 person-years); LMWH: Low molecular weight heparin; PE: Pulmonary embolism; VTE: Venous thromboembolism.
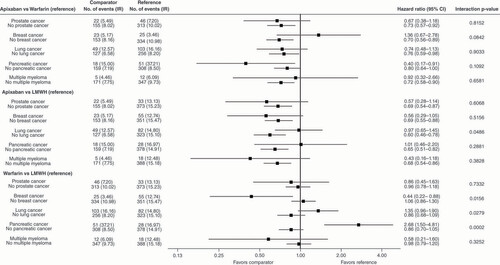
*Hazard ratios were adjusted for unbalanced patient characteristics between treatment cohorts after the stratification for a specific cancer. The list of unbalanced patient characteristics can be found in the footnote of .
IR: Incidence rate (in 100 person-years); IPTW: Inverse proportional treatment weighting; LMWH: Low molecular weight heparin.
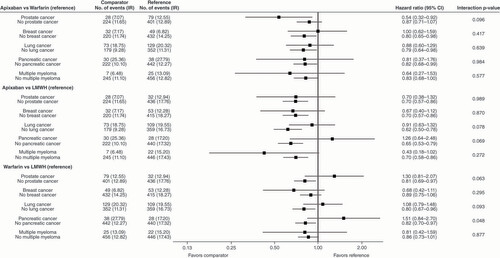
*Hazard ratios were adjusted for unbalanced patient characteristics between treatment cohorts after the stratification for a specific cancer. The list of unbalanced patient characteristics can be found in the footnote of .
CRNMB: Clinically-relevant non-major bleeding; IR: Incidence rate (in 100 person-years); IPTW: Inverse proportional treatment weighting; LMWH: Low molecular weight heparin.
Recurrent venous thromboembolism
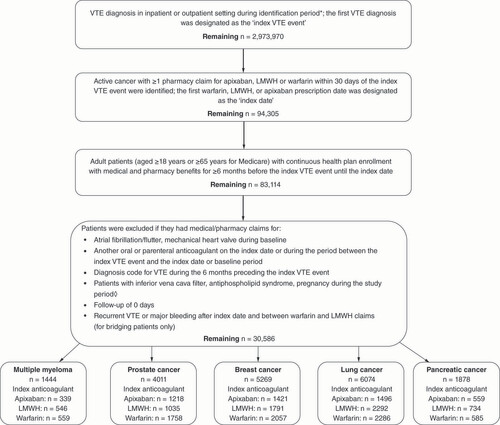
In general, effects of apixaban versus warfarin, apixaban versus LMWH and warfarin versus LMWH on recurrent VTE were not significantly different (p-values >0.1 for most interactions) between patients with a specific tumor versus patients without the specific tumor for each of the five tumor types (multiple myeloma, prostate cancer, breast cancer, lung cancer and pancreatic cancer; ). For most of the tumor types, apixaban tended to have lower incidence rates of recurrent VTE versus warfarin and versus LMWH regardless of tumor status.
There were few significant interactions between some treatments and some tumor types on recurrent VTE. For apixaban versus warfarin, there was a significant interaction between the treatment and breast cancer status (yes or no) on recurrent VTE (p < 0.1). Compared with warfarin, apixaban was associated with a lower incidence of recurrent VTE for patients without breast cancer, but higher incidence for patients with breast cancer. For apixaban versus LMWH, apixaban was associated with lower incidence of recurrent VTE for patients without lung cancer, but similar risk for those with lung cancer (p interaction = 0.049). For warfarin versus LMWH, the treatment effect on recurrent VTE was significantly different between patients with or without a specific tumor for three of the five tumor types (breast, lung and pancreatic; ). For each of the three tumor types, there was a significant interaction between treatment with warfarin versus LMWH and the specific tumor status (yes or no) on recurrent VTE (p for interactions <0.1) with different trends in treatment effects between patients with and without the tumor ().
Major bleeding
In general, most of the effects of apixaban versus warfarin, apixaban versus LMWH, and warfarin versus LMWH on MB were not significantly different (p > 0.1 for most interactions) between patients with a specific tumor and patients without the specific tumor for each of the five tumor types (). Regardless of tumor status, incidence rates of MB were in general, numerically lower for apixaban versus warfarin or versus LMWH (). However, for lung and pancreatic cancer, there were significant interactions between the treatment of apixaban versus LMWH and treatment of warfarin versus LMWH and tumor status (yes or no) on MB (p for interactions < 0.1). In general, the treatment effects trended differently for patients with versus those without lung cancer and for patients with versus those without pancreatic cancer (). There were also significant interactions between prostate cancer status (yes or no) and treatment of apixaban versus warfarin and treatment of warfarin versus LMWH on MB ().
Clinically-relevant non-major bleeding
In general, most of the effects of apixaban versus warfarin, apixaban versus LMWH, and warfarin versus LMWH on CRNMB were not significantly different (p > 0.1 for most interactions) between patients with a specific tumor and patients without the specific tumor (). Apixaban was generally associated with lower incidence of CRNMB versus warfarin and versus LMWH in patients with or without a specific tumor ().
Significant interactions were observed for some treatments and some tumor types on CRNMB. For apixaban versus LMWH, there was a significant interaction between the treatment and pancreatic cancer and breast cancer (). For apixaban versus warfarin, there was a significant interaction between the treatment and lung cancer (). For warfarin versus LMWH, there was a significant interaction between the treatment and lung cancer ().
Discussion
This real-world study evaluated the risk of recurrent VTE, MB and CRNMB among patients with VTE and active cancer who initiated apixaban, LMWH, or warfarin stratified by the presence or absence of one of the five tumor types. These tumors either have high prevalence (prostate, breast and lung cancer) or high VTE risk (multiple myeloma and pancreatic cancer) [Citation29,Citation32,Citation33]. In general, effects of apixaban versus warfarin, apixaban versus LMWH and warfarin versus LMWH on recurrent VTE, MB or CRNMB were not significantly different between patients with versus without a specific tumor type. Apixaban was generally associated with lower incidence rates of recurrent VTE, MB and CRNMB compared with LMWH or warfarin regardless of tumor status (yes or no). In a few cases, treatment effects significantly differed between patients with and without a specific cancer. The effects of apixaban versus LMWH on MB; and effects of warfarin versus LMWH on recurrent VTE, MB and CRNMB were significantly different between patients with lung cancer and patients without lung cancer. There were also significant differences in the effects of apixaban versus LMWH on MB and CRNMB and effects of warfarin versus LMWH on recurrent VTE and MB between patients with and without pancreatic cancer.
Limited studies have systematically examined the efficacy/effectiveness and safety of anticoagulant treatments as a function of tumor type. A post hoc analysis of the Hokusai VTE Cancer study that compared edoxaban with LMWH found a similar benefit-risk profile for lung, breast, hematological and gynecological cancers [Citation20]. But that post hoc analysis, as well as the SELECT-D trial, did report an increased risk of MB for edoxaban (Hokusai VTE Cancer) and rivaroxaban (SELECT-D), respectively, for gastrointestinal cancers, suggesting different treatment effect on MB for this tumor type [Citation23,Citation34]. A subgroup analysis of the CARVAGGIO trial compared apixaban with LMWH and found that the effects of apixaban were not different for gynecological, lung, genitourinary and gastrointestinal cancer [Citation18]. A subgroup analysis of a recent observational study which evaluated the effectiveness and safety of anticoagulants including apixaban, LMWH and warfarin in patients with VTE and active cancer found consistent treatment effects on recurrent VTE and MB for patients with and without gastrointestinal cancer [Citation24]. Consistent with the previous publications, our current study found that the treatment effects of apixaban, LMWH and warfarin were mostly not significantly different between patients with VTE who also have a specific tumor versus patients without a specific tumor for most tumor types including prostate, breast, lung, multiple myeloma and pancreatic cancer.
The findings from our study also suggest there may be tumor-specific treatment effects for some of the medications and some of the tumor types. One example is the comparison of apixaban or warfarin with LMWH for patients with or without lung cancer. Our study shows that the effects of apixaban versus LMWH and effects of warfarin versus LMWH on recurrent VTE and MB were significantly different between patients with versus without lung cancer. The treatment effects trended differently with lung cancer patients showing more favorable treatment effects of apixaban or warfarin (versus LMWH) than those without lung cancer. However, these findings are for hypothesis generation and require additional studies to confirm this observation.
Limitations
The present analysis includes limitations associated with retrospective claims analyses. Only associations and not causation can be inferred from this study and there may be confounding due to unmeasured variables (e.g., over-the-counter medications, laboratory values, etc.). Sample size for the high-risk tumor types such as multiple myeloma and pancreatic cancer is relatively small. When stratifying patients by presence and absence of a specific tumor, patients without the specific tumor are heterogeneous and include different types of tumors which may not have consistent treatment effects. In addition, the commercial databases for which the data were derived from do not have complete death information for the patients, and consequently, mortality and fatal recurrent VTE in patients were not assessed. The analysis assumes that medications were taken properly and that diagnosis codes were accurate. Nevertheless, coding errors and missing data may exist, which could contribute to underreporting of outcomes or misclassification of study subjects. For example, women with prostate cancer [ICD-9 185] maybe incorrectly coded. But 17 women had this potential coding error, which should not influence the overall conclusion for prostate cancer. Additionally, the algorithm used to determine CRNMB events (including ICD-9 and ICD-10 diagnosis codes) has not been validated; however, this algorithm does follow the suggested definition by the International Society on Thrombosis and Hematology (ISTH) as closely as possible [Citation35]. Finally, the findings from this analysis cannot be generalized to the entire US population of patients with VTE and active cancer as uninsured individuals, Medicaid recipients, and patients within the Veterans Affairs health system were not included. Additionally, the data were from US databases and may not generalize to other populations.
Conclusion
The findings from this study add to a growing body of research that evaluate effectiveness and safety of DOACs in patients with VTE and different tumor types. Though effectiveness and safety of apixaban, LMWH and warfarin were generally consistent across patients with or without a specific tumor for five tumor types evaluated in this study, there may be tumor specific treatment effects for some of the medications and some tumor types. Further research is needed to better understand effects of anticoagulants in patients with VTE and different tumor types.
Patients with cancer are at high risk of developing venous thromboembolism (VTE), including deep vein thrombosis and pulmonary embolism, which can result in increased morbidity and mortality.
In recent years, direct oral anticoagulants have been included as another treatment option for VTE in patients with cancer; however, clinical profiles of VTE outcomes could vary depending on the type of cancer diagnosis.
This real-world study evaluated the effectiveness (recurrent VTE) and safety (major bleeding [MB] and clinically relevant non-major bleeding [CRNMB] events) of apixaban, low molecular weight heparin (LMWH), and warfarin among patients with VTE and with common (prostate, breast, lung) or high-risk (multiple myeloma, and pancreatic) tumor type.
This study pooled medical and pharmacy claims from four commercial databases (Optum, Humana, PharMetrics Plus and MarketScan) and the Medicare Fee-for-Service database.
A total of 30,586 patients with VTE and cancer met all eligibility criteria. From this overall cancer population, patients with lung cancer (19.9%), breast cancer (17.2%), prostate cancer (13.1%), pancreatic cancer (6.1%) and multiple myeloma (4.7%) were selected for this analysis.
Among patients with VTE, treatment effects were generally not significantly different between patients with or without a specific tumor.
Apixaban patients (versus LMWH and warfarin) generally had lower rates of recurrent VTE, MB, and CRNMB.
Most interactions between tumor type and treatments were not significant; however, significant interactions between some treatments and lung and pancreatic cancer on recurrent VTE, MB, and CRNMB were observed.
Though some tumor specific treatment effects were observed, the effectiveness and safety of apixaban, LMWH and warfarin was generally consistent between patients with or without a specific tumor.
Further research is needed to better understand effects of anticoagulants in patients with VTE and different tumor types.
Author contributions
All authors contributed equally in the development of this manuscript.
Financial disclosure
This study was sponsored by Pfizer and Bristol-Myers Squibb. TC received research support from Pfizer Inc. and Bristol-Myers Squibb Company. S Shah and V Noxon are employees of STATinMED, LLC who were paid consultants to Pfizer and Bristol Myers Squibb in connection with the development of this manuscript. AD Dhamane is an employee and shareholder of Bristol Myers Squibb, one of the study sponsors. DM Hines, T Alfred and X Luo are employees and shareholders of Pfizer, one of the study sponsors. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Since this study did not involve the collection, use, or transmittal of individually identifiable data, it was deemed exempt from Institutional Review Board review by Solutions IRB. Both the datasets and the security of the offices where analysis was completed (and where the datasets are kept) meet the requirements of the Health Insurance Portability and Accountability Act of 1996. Solutions IRB determined this study to be EXEMPT from the Office for Human Research Protections (OHRP)’s Regulations for the Protection of Human Subjects (45 CFR 46) under Exemption 4: Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. The HIPAA Authorization Waiver was granted in accordance with the specifications of 45 CFR 164.512(i). This project was conducted in full accordance with all applicable laws and regulations, and adhered to the project plan that was reviewed by Solutions Institutional Review Board.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Supplemental Text 1
Download MS Excel (29.6 KB)Supplemental Text 2
Download MS Excel (41.1 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/fon-2022-1254
Data sharing statement
Data for these analyses were made available to the authors through third-party licenses from commercial data providers in the US and Medicare data, which are available from the Centers for Medicare and Medicaid through ResDAC (www.resdac.org/). As such, the authors cannot provide the raw data themselves. Other researchers could access these data by purchasing third party licenses through these commercial data providers. Inclusion criteria specified in the Methods section would allow other researchers to identify the same cohort of patients we used for these analyses.
References
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 5(3), 632–634 (2007).
- Gussoni G, Frasson S, La Regina M, Di Micco P, Monreal M. Three-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registry. Thromb. Res. 131(1), 24–30 (2013).
- Lyman GH, Culakova E, Poniewierski MS, Kuderer NM. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb. Res. 164(Suppl. 1), S112–S118 (2018).
- Key NS, Khorana AA, Kuderer NM et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 38(5), 496–520 (2020).
- Donnellan E, Khorana AA. Cancer and venous thromboembolic disease: A Review. Oncologist. 22(2), 199–207 (2017).
- Khorana AA, Mackman N, Falanga A et al. Cancer-associated venous thromboembolism. Nat. Rev. Dis. Primers. 8(1), 11 (2022).
- Heit JA. Epidemiology of venous thromboembolism. Nat. Rev. Cardiol. 12(8), 464–474 (2015).
- Roberti R, Iannone LF, Palleria C et al. Direct oral anticoagulants: from randomized clinical trials to real-world clinical practice. Front. Pharmacol. 12, 684638 (2021).
- Lee AY, Levine MN, Baker RI et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 349(2), 146–153 (2003).
- Gu Z-C, Yan Y-D, Yang S-Y et al. Direct versus conventional anticoagulants for treatment of cancer associated thrombosis: a pooled and interaction analysis between observational studies and randomized clinical trials. Ann. Transl. Med. 8(4), 95 (2020).
- Douros A, Filliter C, Azoulay L, Tagalakis V. Effectiveness and safety of direct oral anticoagulants in patients with cancer associated venous thromboembolism. Thromb. Res. 202, 128–133 (2021).
- McBane RD 2nd , Wysokinski WE, Le-Rademacher JG et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J. Thromb. Haemost. 18(2), 411–21 (2020).
- Mulder FI, Bosch F, Young AM et al. Direct oral anticoagulants for cancer-associated venous thromboembolism: a systematic review and meta-analysis. Blood 136(12), 1433–1441 (2020).
- Carrier M, Abou-Nassar K, Mallick R et al. Apixaban to prevent venous thromboembolism in patients with cancer. N. Engl. J. Med. 380(8), 711–719 (2019).
- Agnelli G, Becattini C, Meyer G et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N. Engl. J. Med. 382(17), 1599–607 (2020).
- Riaz IB, Fuentes HE, Naqvi SAA et al. Direct oral anticoagulants compared with dalteparin for treatment of cancer-associated thrombosis: a living, interactive systematic review and network meta-analysis. Mayo Clin. Proc. 97(2), 308–324 (2022).
- Mahé I, Chidiac J, Bertoletti L et al. The clinical course of venous thromboembolism may differ according to cancer site. Am. J. Med. 130(3), 337–47 (2017).
- Agnelli G, Muñoz A, Franco L et al. Apixaban and dalteparin for the treatment of venous thromboembolism in patients with different sites of cancer. Thromb. Haemost. 122(5), 796–807 (2022).
- Lecumberri R, Ruiz-Artacho P, Tzoran I et al. Outcome of cancer-associated venous thromboembolism is more favorable among patients with hematologic malignancies than in those with solid tumors. Thromb. Haemost. 122(9), 1594–1602 (2022).
- Mulder FI, van Es N, Kraaijpoel N et al. Edoxaban for treatment of venous thromboembolism in patient groups with different types of cancer: Results from the Hokusai VTE Cancer study. Thromb. Res. 185, 13–19 (2020).
- Rungjirajittranon T, Owattanapanich W, Chinthammitr Y, Ruchutrakool T, Suwanawiboon B. Direct oral anticoagulants versus low-molecular-weight heparin for acute treatment of venous thromboembolism in patients with gastrointestinal cancer: a systematic review and meta-analysis. Thromb. J. 20(1), 41 (2022).
- Raskob GE, van Es N, Verhamme P et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N. Engl. J. Med. 378(7), 615–624 (2018).
- Young AM, Marshall A, Thirlwall J et al. Comparison of an oral Factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J. Clin. Oncol. 36(20), 2017–2023 (2018).
- Cohen AT, Keshishian A, Lee T et al. Effectiveness and safety of apixaban, LMWH, and warfarin among high-risk subgroups of VTE patients with active cancer. Curr. Med. Res. Opin. 37(9), 1467–1482 (2021).
- Sorigue M, Cañamero E, Siguenza P, Nomdedeu M, López-Núñez JJ. Recent developments and persisting challenges in the prevention and treatment of venous thromboembolism in patients with hematological malignancies. Leuk. Lymphoma 61(6), 1277–1291 (2020).
- Lyman GH, Carrier M, Ay C et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood. Adv. 5(4), 927–974 (2021).
- Living Interactive Systematic Reviews . Direct oral anti-coagulants compared to dalteparin for treatment of cancer associated thrombosis: a living, interactive systematic review and network meta-analysis (2022). https://cat.network-meta-analysis.com
- Planquette B, Bertoletti L, Charles-Nelson A et al. Rivaroxaban vs dalteparin in cancer-associated thromboembolism: a randomized trial. Chest 161(3), 781–790 (2022).
- National Cancer Institute Cancer Stat Facts: Common Cancer Sites (2022). https://seer.cancer.gov/statfacts/html/common.html
- Kraaijpoel N, Carrier M. How I treat cancer-associated venous thromboembolism. Blood 133(4), 291–298 (2019).
- Cohen A, Noxon V, Dhamane A et al. Effectiveness and safety of anticoagulants among venous thromboembolism cancer patients with and without brain cancer. Thromb. Res. 226, 117–126 (2023).
- Cornell RF, Goldhaber SZ, Engelhardt BG et al. Primary prevention of venous thromboembolism with apixaban for multiple myeloma patients receiving immunomodulatory agents. Br. J. Haematol. 190(4), 555–561 (2020).
- Farge D, Frere C, Connors JM et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet. Oncol. 23(7), e334–e347 (2022).
- Thapa N, Shatzel J, Deloughery TG, Olson SR. Direct oral anticoagulants in gastrointestinal malignancies: is the convenience worth the risk? J. Gastrointest. Oncol. 10(4), 807–809 (2019).
- Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Subcommittee on Control of Anticoagulation . Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J. Thromb. Haemost. 13(11), 2119–2126 (2015).
