Abstract
Aim: To assess rates of no systemic treatment (NST), attrition across lines of therapy, and factors influencing treatment selection in patients with locally advanced or metastatic urothelial cancer (la/mUC). Methods: Systematic literature review to identify real-world studies reporting NST or attrition rates in la/mUC from 2017–2022 (including data reported since 2015). Results: Of 2439 publications screened, 29 reported NST rates, ranging from 40–74% in eight European-based studies, 14–60% in 12 US-based studies, and 9–63% in nine studies in other locations (meta-analysis estimate, 39%). Factors associated with NST or no second-line therapy included older age, female sex, poor performance status, poor renal function and distant metastases. Conclusion: A substantial proportion of patients with la/mUC do not receive guideline-recommended treatment.
Plain language summary
A review of how patients with bladder cancer are treated or not treated with anti-cancer drugs
People with advanced bladder cancer have a short survival. Bladder cancer is called advanced when it has spread outside of the urinary tract. Several drug treatments are available for people with advanced bladder cancer. However, sometimes people do not receive any drug treatment. We looked at published studies to see how many people with advanced bladder cancer did not receive any drug treatment and the reasons why. We also looked at how long people lived with or without drug treatment. We found that many people with advanced bladder cancer did not receive drug treatment. The number of people who received no drug treatment varied in studies from different countries. People who were older, were female, had poor health or kidney problems, or had cancer that had spread to other parts of the body were less likely to receive drug treatment. People who did not receive drug treatment lived for an average of 2 to 7 months, compared with 9 to 35 months for people who received drug treatment. More studies are needed to investigate the reasons why drug treatment is sometimes not used in people with advanced bladder cancer who could receive treatment, so that more people can benefit from available treatments.
This systematic literature review assessed real-world evidence for undertreatment rates, attrition rates and factors influencing treatment selection in patients with locally advanced or metastatic urothelial carcinoma (la/mUC).
Of 2439 studies reporting real-world evidence in la/mUC or bladder cancer that were screened, 29 reported the proportion of patients receiving no systemic treatment (NST), 47 reported the proportion of patients receiving no subsequent treatment after first-line (1L) treatment (ie, attrition rate) and six reported treatment rates after the approval of immunotherapy; in total, 66 studies reported either NST rates or attrition rates.
Several regions and countries were represented, including the USA, the European Union, Asia, the Middle East, the UK and Russia.
NST rates ranges from 40–74% in eight European-based studies, 14–60% in 12 US-based studies, and 9–63% in nine studies from other locations.
Rates of receipt of second-line treatment were lower in cisplatin-ineligible versus -eligible patients, and in those who received 1L immunotherapy versus chemotherapy.
Factors associated with NST or no subsequent treatment after 1L treatment included older age, female sex, poor performance status, poor renal function and distant metastases.
Median overall survival for patients with NST ranged from 2.0 to 6.9 months, compared with 9.2 to 34.5 months for patients who received systemic treatment.
Overall, our findings indicate that a substantial proportion of patients with la/mUC do not receive treatment according to guidelines, or at all.
Additional studies are needed to explore the factors influencing treatment selection to support optimal treatment sequencing in the era of 1L maintenance and targeted therapies in later lines, and allow most patients to benefit from available treatments.
Bladder cancer is the tenth most common cancer in the world, with both incidence and mortality rising over the past 2 decades [Citation1–3]. In 2020, 573,278 new cases and 212,536 deaths worldwide were attributed to bladder cancer [Citation3,Citation4]. Urothelial cancer (UC) accounts for 90% of the histological forms of bladder cancer [Citation5] and is characterized by the progressive accumulation of transitional cells of the mucosal epithelium lining the bladder, or the ureter or kidney (in the case of upper tract UC [UTUC]) [Citation6–8]. Among all UC cases, 25% are estimated to be muscle-invasive and 11% to be locally advanced or metastatic (la/mUC) at diagnosis [Citation5,Citation9]. The incidence of la/mUC varies by sex, ethnicity, geography and age; it commonly affects elderly populations (>70 years) and males (>65%) more than females [Citation10–13]. Regardless of patient characteristics, the prognosis for patients with la/mUC is poor, with a 5-year survival rate of <15%; metastatic UC specifically is considered incurable, with a 5-year survival rate of 4.6% in the USA [Citation5,Citation9].
The goal of treatment in la/mUC is to prevent disease progression, maintain health-related quality of life, help relieve cancer symptoms and extend life [Citation10,Citation14]. Treatment guidelines (European Society for Medical Oncology [ESMO], European Association of Urology [EAU] and National Comprehensive Cancer Network [NCCN]) recommend platinum-based chemotherapy for all platinum-eligible patients as first-line (1L) treatment [Citation15–17]. Cisplatin-based chemotherapy is the recommended 1L standard of care, with carboplatin-based chemotherapy recommended for patients who are not eligible for cisplatin [Citation15,Citation18,Citation19]. Roughly half of patients with mUC are ineligible for cisplatin; however, many are still eligible to receive carboplatin [Citation20]. In some countries, patients who are cisplatin ineligible and whose tumors express PD-L1, or who are platinum-ineligible, may receive 1L immunotherapy (IO). Avelumab is recommended as 1L maintenance for all patients with la/mUC that has not progressed with 1L platinum-containing chemotherapy [Citation21]. Available second-line (2L) treatments include IOs, antibody-drug conjugates, FGFR inhibitors and chemotherapies [Citation18]. For those in the end stages of the disease, palliative/supportive care is recommended (e.g., analgesia, antibiotics), and potentially palliative surgery or radiotherapy. Despite these guidelines, studies suggest that some patients with la/mUC receive no systemic treatment (NST) [Citation22].
While median survival with treatment is approximately 9 to 24 months, prognosis without treatment is poor and overall survival (OS) is extremely short [Citation12,Citation23–26]. While recent studies suggest that rates of NST and rates of not receiving a subsequent line of therapy (LOT) (i.e., attrition) are high [Citation12,Citation22,Citation26], underutilization of systemic therapy (ST) has not been comprehensively investigated and attrition rates are not well characterized. Questions remain regarding the drivers behind treatment decisions and why mortality rates remain high despite available treatments. The objectives of this study were to systematically characterize treatment patterns in la/mUC, focusing on rates of NST and subsequent LOT, in addition to associated clinical and sociodemographic factors and real-world OS.
Methods
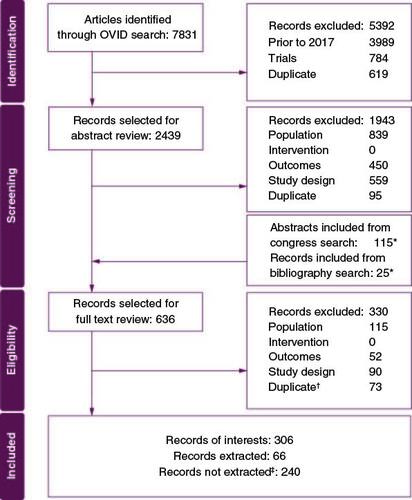
We conducted a systematic literature review (SLR) of real-world evidence studies reporting NST and/or attrition rates in la/mUC. The review, which was unregistered, was conducted in accordance with the methodological and reporting requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) using Population, Intervention, Comparator, Outcomes and Study Design (PICOS) criteria (Supplementary Table 1). The Cochrane Library, EconLit, Embase, MEDLINE and MEDLINE in-process databases were searched on 25 February 2022 (Supplementary Table 2). Conferences indexed in Embase were identified via electronic database search, and the following conferences were hand-searched for results from 2017 and later: American Society of Clinical Oncology (ASCO), ASCO Genitourinary (ASCO GU), ESMO, International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and EAU. The screening was conducted in two phases: title/abstract screening and full-text screening. All records were screened by two independent reviewers, with conflicts resolved by a third independent reviewer. Included studies were limited to those published in English, German, French, or Spanish, and studies published from 2017 to the search date (provided they included data from 2015 or later). A total of 306 records were selected for data extraction based on PICOS criteria. Records were not extracted unless they fulfilled the additional criteria of reported NST rates, attrition rates, reasons for NST (defined as the proportion of patients who received NST in a total study population, regardless of eligibility for ST) or reasons for non-receipt of subsequent therapy, and specification of a period when data were acquired. This resulted in the data extraction of 66 records ().
Figure 1. PRISMA diagram.
*Includes duplicates with title and abstract review.
†Includes duplicates between title and abstract review and manually added records from congress search* and bibliographic search.*
‡Records were not extracted unless they fulfilled the additional criteria for data extraction:
1. Reported NST rates, attrition rates, reasons for NST, or reasons for attrition.
2. Specification of the period when data were acquired.
NST: No systemic treatment
NST was described differently in the identified publications. Based on these descriptions, studies were categorized into three groups: group A did not specify whether patients received palliative surgery, palliative radiotherapy, or best supportive care (BSC); group B specified palliative/supportive care (including surgery, radiotherapy, or BSC) being used instead of 1L ST; and group C specified not receiving surgery, radiotherapy, or systemic therapy (Supplementary Table 3). Some records reported multiple categories of NST or attrition rates among different subgroups of patients based on the previous LOT received; therefore, they were separately reported as ‘subgroups’; 33 subgroups were included within the 29 studies reporting NST, and 63 subgroups were included within the 47 studies reporting attrition rates.
Risk of bias was assessed for the 29 studies reporting NST via a modified Newcastle-Ottawa Scale. The number of stars (1–9, 1 = biased, 9 = unbiased) was counted for each study. Our study showed a risk bias median of 7 stars and mean (standard deviation [SD]) of 6.7 (1.4) (Supplementary Table 4). For analysis of NST rates, two methods were used. The first was a traditional pooling method in which all patients with NST were pooled before dividing by the total population. Because the pooling approach does not account for the variance of the different estimates, we also employed classic meta-analysis estimates. By weighting the different proportions according to their precision (inverse of the variance), this approach gives a higher weight to proportions with a low uncertainty compared with proportions with high uncertainty or low sample size. A measure of the variance of the statistics was given by the inverse of the sum of the precisions of all studies. The analysis was performed using RStudio Version 2022.07.1., Meta package. Proportions are presented with 95% CI.
In the absence of statistics describing associations of patient characteristics with NST within records of interests, we performed Fisher's exact tests using MedCalc software. Temporal scores were calculated to reflect the recency of studies for the records reporting NST rates, as follows: a raw relevance score was derived for each study by dividing the number of study years after 2015 (i.e., relevant total span) by the total year span used in the study and re-ordering based on the higher raw relevance score, longer relevant total span, newer last year and newer start year. Finally, a temporal score was given to each study, with higher temporal scores reflecting more recent data collection.
Results
Evidence acquisition
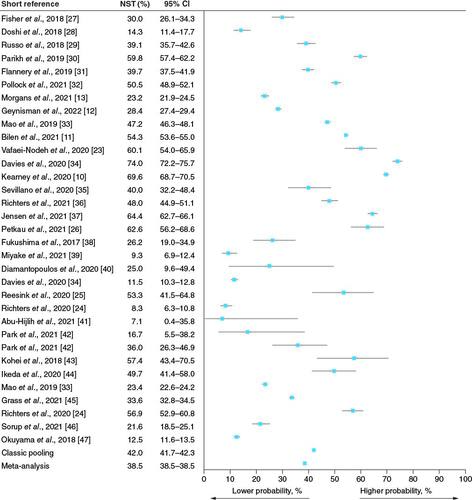
A total of 29 studies reported NST rates (median 39%; range 7–74%; pooled estimate 42%; meta-analysis estimate 39%; ), 47 studies reported the rate of subsequent treatment after 1L ST, and six studies reported treatment rates after the approval of IOs. In total, 66 unique studies reported either NST rates or attrition rates (Supplementary Table 4). In these heterogeneous studies, several regions were represented, including the USA, the European Union, Asia, the Middle East, the UK and Russia. Most studies identified were either retrospective observational studies (N = 32 studies) or analyses of large databases or registries (N = 30). Four studies were cross-sectional surveys or prospective ( & Supplementary Table 3). Overall, the SLR represented 112,687 patients with a range for individual study population size of 13 to 18,888.
Table 1. No systemic treatment summaries by individual study.
Rates of NST
Among 29 studies (33 subgroups in total because records reported multiple categories of NST or attrition rates among different subgroups of patients based on the previous LOT) reporting NST rates (), 19 subgroups were in Group A (no specification of whether patients received palliative surgery, palliative radiotherapy, or BSC) (median 47%; range 9–74%; pooled estimate 50%; meta-analysis estimate 49%), nine subgroups were in Group B (specified receipt of palliative/supportive care, including surgery, radiotherapy, or BSC, instead of 1L ST; median 25%; range 7–57%; pooled estimate 15%; meta-analysis estimate 12%), and five subgroups were in Group C (specified not receiving surgery, radiotherapy, or systemic therapy; median 23%; range 13–57%; pooled estimate 27%; meta-analysis estimate 24%; & Supplementary Figure 1 & Supplementary Table 3). A total of 13 studies (14 subgroups) from North America reported NST rates of 14 to 60%; eight studies (ten subgroups) from European countries reported NST rates of 8 to 74% (UK, Spain, the Netherlands, Denmark); and eight studies (nine subgroups) from the rest of the world reported NST rates ranging from 7 to 63% (Japan, Korea, Russia and Middle Eastern countries). Nine studies were retrospective analyses, whereas the rest were analyzed registries or large databases, such as the National Cancer Database, National Cancer Registration and Analysis Service, Netherlands Cancer Registry, Surveillance Epidemiology and End Results Program database, Danish national health registries, or hospital registries ().
Rates stratified by region & year of observation
North American studies mostly reported patients from NST Group A (no specification of whether patients received palliative surgery, palliative radiotherapy, or BSC), while studies from Europe and the rest of the world were split between NST groups (A). Patient characteristics differed slightly between studies to include special populations of mUC, mUTUC, or node-positive bladder cancer. The highest rates of NST (60–74%) were seen in Canada, Europe and Russia. NST rates between 14 and 59% were reported in US studies. Studies from other regions (Japan, Korea, Middle East) reported generally lower NST rates (7.1–36%), with only two studies reporting NST rates >40%.
Figure 3. Rates of NST by geography and time.
(A) NST rates by geography. Circle size is relative to reported NST rate. (B) NST rates by relative temporal score. Circle size reflects the population size. Circles with a red outline come from the same study [Citation12].
1L: First line; BSC: Best supportive care; la/mUC: Metastatic urothelial cancer; NST: No systemic treatment.
![Figure 3. Rates of NST by geography and time.(A) NST rates by geography. Circle size is relative to reported NST rate. (B) NST rates by relative temporal score. Circle size reflects the population size. Circles with a red outline come from the same study [Citation12].1L: First line; BSC: Best supportive care; la/mUC: Metastatic urothelial cancer; NST: No systemic treatment.](/cms/asset/7403ff74-6cc6-470a-a724-cda419bb0a84/ifon_a_2337492_f0003_c.jpg)
Among identified studies, we observed no clear changes in rates of NST over time (B). Of the recent Group A studies (B, right), two UK studies, one Danish study, one Canadian study, one Dutch study and one Russian study consistently showed NST rates of approximately 50–70%. The US study by Bilen et al. offered insights into yearly NST rates; reported rates were generally consistent from 2015 to 2019 and ranged from 48 to 58% (Supplementary Table 1 & B) [Citation11]. This was largely consistent with another, newer US study that reported an NST rate of 60% [Citation30]. Outliers of the recent Group A studies were two US studies with NST rates of 23% [Citation13] and 14% [Citation28], and one Japanese study reporting an NST rate of 9.3% (B [Citation39]).
Survival outcomes among patients with NST compared with those who received ST
Among the 29 studies reporting NST, eight studies reported survival/mortality for both treated and undertreated patients [Citation12,Citation23–27,Citation33,Citation44]. Median OS for patients with NST ranged from 2.0 to 6.9 months, compared with 9.2 to 34.5 months for patients who received ST. Results were statistically significant across all studies except one () [Citation44].
Figure 4. Overall survival with no systemic treatment or systemic treatment.
1L: First line; aUC: Advanced urothelial cancer; BSC: Best supportive care; IO: Immunotherapy; mBC: Metastatic bladder cancer; mUTUC: Metastatic upper urinary tract urothelial cancer; NE: Not evaluable; NR: Not reported; NST: No systemic treatment; OS: Overall survival; SEER: Surveillance Epidemiology and End Results Program; ST: Systemic treatment; w/: With; w/o: Without.
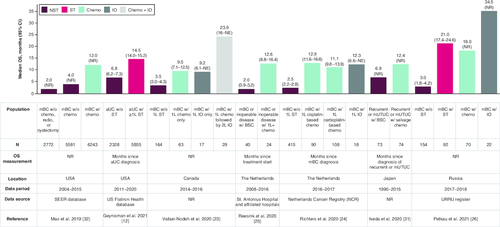
The OS of IO-treated patients was reported in three studies. Two studies found little difference in OS between patients treated with IO versus chemotherapy in 1L [Citation23,Citation24]. The third study reported longer OS for patients receiving IO compared with those receiving chemotherapy (LOT not reported; ) [Citation26].
Patient characteristics associated with NST
Reporting of patient characteristics varied across studies; only two of the six studies that recorded patient characteristics of undertreated patients also performed statistical analyses comparing with patients who received ST. A post hoc Fisher's exact test was performed to analyze the association of patient characteristics with NST for the remaining four studies. Age was the most commonly reported patient characteristic, and in five of five studies, it was significantly associated with NST (). Other factors associated with NST included known prognostic variables such as poor Eastern Cooperative Oncology Group performance status (ECOG PS), poor renal function, metastases outside the lymph nodes and comorbidities. Other patient characteristics that were associated with NST included non-White race or ethnicity, Medicaid dual eligibility and the primary tumor site in the bladder. One study reported a significant association between female sex and NST.
Table 2. Factors associated with no systemic treatment.
NST in subsequent LOT
Of 2439 records screened, 47 studies reported the rate of subsequent treatment (Supplementary Table 5; excluded studies and reasons are provided in Supplementary Table 6). Across the 47 included studies, patient subgroups were categorized based on reported type of 1L treatment or cisplatin eligibility at 1L; 2L treatment rates were reported for 63 subgroups who received 1L (median 43%; range 8–87%), third-line (3L) treatment rates were reported for 45 patient subgroups who received 2L treatment (30%; 8–77%), and treatment rates regardless of LOT were reported for 19 patient subgroups who received ST after IO (38%; 8–62%; Supplementary Table 7).
The median rate of 2L treatment among patients who received 1L chemotherapy was 47% (range 23–87%, as reported by 31 subgroups). Rates of 2L treatment were lower among patients who received 1L IO-based treatment (median 31% [range 8–57%], as reported by 8 subgroups; Supplementary Table 6). Additionally, in a study comparing treatment rates in patients who received IO-based versus non-IO-based 1L treatment, receipt of 1L IO was associated with non-receipt of 2L treatment (p < 0.001, post hoc Fisher's exact test) [Citation48].
The 2L treatment rates among patients who received cisplatin-based 1L treatment (n = 5 subgroups; median 45%; range 30–67%) were slightly higher than 2L rates among patients who received carboplatin-based 1L treatment (n = 4; 40%; 23–47%). In three studies comparing treatment rates in patients who received cisplatin-based versus carboplatin-based 1L therapy, receipt of a 1L carboplatin-based regimen was associated with non-receipt of 2L therapy in one study (p < 0.05, post hoc Fisher's exact test). In general, 2L treatment rates among 1L-cisplatin-eligible patients (n = 4; 49%; 46–50%) were slightly higher than 2L treatment rates among 1L-cisplatin ineligible patients (n = 3; 37%; 34–45%; ). In three studies comparing treatment rates in cisplatin-eligible versus -ineligible patients, cisplatin ineligibility was associated with non-receipt of 2L therapy in 2 studies (p < 0.001, post hoc Fisher's exact test) [Citation12,Citation13,Citation49]. Additionally, in a study comparing patients who received platinum-based therapy as 1L treatment in the USA versus Canada, the 2L treatment rate was higher in the USA than in Canada [Citation50].
Table 3. Factors associated with attrition.
Factors associated with not receiving subsequent therapy
Three subgroups within two studies analyzed characteristics associated with non-receipt of subsequent therapy. Significant associations (p < 0.05) for older age (1/2), female sex (1/2), poor ECOG PS (1/1), poor renal function (1/1), and stage IV at initial diagnosis (1/2) were associated with 1L to 2L attrition. From 2L to 3L, not receiving subsequent therapy was attributed to disease spreading outside the lymph nodes and more metastatic sites at the start of IO (1 subgroup from 1 study, all 1/1; ).
Overall, the reasons for non-receipt of 1L and subsequent treatment options as reported in six studies were: poor ECOG PS (2), unfit/frail (2), having died after 1L (2), having reached stable disease and did not require subsequent LOT (1), old age (1), progressive disease and refusal of further treatment (1) ().
Discussion
There is high variability in data on treatment patterns for patients with la/mUC, but it is clear that many patients are not receiving, or not continuing to receive, life-extending treatment.
In our review, rates of NST ranged from 7 to 74%, with a pooled estimate of 42.0% (95% CI 41.7–42.3%) and meta-analysis estimate of 38.5% (95% CI 38.5–38.5%; ). The range of NST rates differed among regions (A) and NST categories ( & Supplementary Figure 1 & Supplementary Table 3). While there was high heterogeneity in NST rates between studies, bias was low and many of the studies had very low variance in data and large sample sizes, which account for the narrow 95% CI. There was also clear heterogeneity among the identified studies in terms of sample size, study design, and years of data (B); however, no clear pattern was discernable with respect to patient population or study design and high or low rates of NST. Additionally, prior to the approval of 1L maintenance therapy, fewer than half of the patients who received 1L treatment received 2L treatment, and the numbers declined further for subsequent therapies. Ultimately, despite recommendations of clinical guidelines (NCCN, ESMO and EAU) [Citation15–17], the proportion of patients in the USA and the European Union who receive ST has remained largely unchanged since the 2000s, with the exception of 2 recent publications using Flatiron Health data [Citation12,Citation13], which may demonstrate a more pronounced impact of the regulatory approval of IOs starting in 2016 and other new novel agents, considering that the source data are exclusively from oncology centers, which may have different patterns of care [Citation52].
Treatment decisions are complex and may be informed by commonly reported concerns, including access to care, a patient's fitness/ability to withstand treatment, comorbidities and other contraindications to therapy, as well as patient preference [Citation53]. The findings of this SLR align with those of previous reports [Citation22,Citation54]; factors related to lack of fitness/ineligibility for ST were clear drivers in receiving NST and/or attrition. Likewise, age seems to be a common factor among the patients with NST; however, age alone should not preclude patients from receiving 1L treatment [Citation55–57].
Recent studies have identified non-disease-related factors that were associated with NST or non-receipt of subsequent therapy, including communication barriers, socioeconomic factors, marital status, sex and race/ethnicity [Citation22,Citation58]. In this review, NST or additional LOT from 1L or 2L treatment were associated with female sex, non-White race or ethnicity and Medicaid dual eligibility (in the USA). Of note, all identified studies reporting on factors associated with non-receipt of ST included female sex, although a significant association was noted in only one study. In a recent analysis of factors associated with receipt of ST in mUC in England, which was published after the date of this review, female sex was associated with a lower likelihood of receiving ST (odds ratio 0.72; 0.66–0.88) [Citation54]. A US study of ST and oncology referral data before 2014 reported that among patients with stage IV UC referred to a medical oncologist, older age, Hispanic or non-Hispanic Black race and unmarried status were negatively associated with subsequent chemotherapy receipt [Citation58].
While non-clinical factors were associated with rates of NST or receiving additional LOTs, there remains a lack of understanding of what role sociodemographic characteristics play in treatment decisions, so further exploration will be required. It is possible that physician referral patterns, preferences and potentially implicit biases influence treatment patterns and subsequent outcomes, highlighting the need to raise physician awareness. Interestingly, physicians who participated in two cross-sectional surveys believed that NST and subsequent treatment rates were lower than actual rates observed in the real-world studies that we identified [Citation53,Citation59]. Additionally, some patients may not be referred to a medical oncologist and, therefore, are not included in the data from studies of this nature (e.g., US Flatiron Health database), especially those patients with older age, more comorbidities and non-metastatic disease stage at diagnosis [Citation58].
The availability of new therapies and an evolving treatment paradigm means more treatment options are available for patients with la/mUC; thus, receipt of ST and optimal treatment selection in 1L is critical. Our findings suggest that initial treatment selection can influence OS; patients who receive cisplatin in 1L have improved survival and higher rates of subsequent treatment. Choosing the optimal 1L therapy includes objective evaluation of platinum eligibility using established guidelines, followed by a decision to choose between cisplatin and carboplatin for eligible patients. Ineligibility for cisplatin-based treatments is most commonly based on a glomerular filtration rate <60 ml/min and poor performance status (ECOG PS ≥2), although additional considerations may include neuropathy, hearing loss, advanced age and cardiovascular dysfunction [Citation12,Citation13,Citation60]. Because carboplatin treatment results in less toxicity, many patients who are ineligible for cisplatin may receive carboplatin [Citation15]. For those who are not eligible, consideration of available alternative treatments, as well as selecting the therapy that provides the highest potential for patients to move on to a subsequent LOT, is critical.
This SLR also analyzed the change in treatment patterns for la/mUC and found that the use of IO as 2L treatment among patients who received 1L platinum-based chemotherapy increased between 2017 (30%) and 2018 (71%) [Citation61]. IO use in 1L treatment also increased from 2017 (2%) to 2019 (19%) in Europe [Citation61], and from 2016 (15.2%) to 2020 (43.6%) in the USA [Citation12]. The proportion of patients who initiated any ST at the end of life doubled from 2015 (17.4%) to 2017 (34.8%), largely explained by the increased IO use, reflecting physicians' and patients' perceptions of IO's favorable risk-benefit profile [Citation30]. In patients with PD-1/PD-L1–negative la/mUC, the use of IO increased between 2017 (1%) to 2018 (10%), then decreased in 2019 (4%) [Citation61]. This could have been due to the European Medicines Agency restricting the 1L use of IOs to patients with tumors expressing high levels of PD-1/PD-L1 in 2018 following data demonstrating that patients with low expression of PD-L1 on tumors who were treated with 1L pembrolizumab or atezolizumab monotherapy had shorter survival [Citation62]. Future work is needed to further explore the clinical benefits of 1L IOs versus platinum-based chemotherapy following the failure of several phase 3 studies [Citation63–65]. For patients who are ineligible for platinum-based therapy, 1L IO remains a viable treatment option, and additional research will be needed based on the evolution of the treatment landscape. Nevertheless, a substantial group of patients do not receive ST. This is likely multifactorial rather than due to the available treatments; however, it underscores the need for optimization of treatment choices/sequences to allow patients to benefit from all of the available novel/innovative regimens.
Although this study was a systematic review, it was limited by the availability of published data. Given the heterogeneity of reporting and defining types of treatment/undertreatment, it is also possible that some studies may not have been captured because of a defined search terminology. Other inherent limitations of the reported studies included that some were only published as conference abstracts, most were not designed to capture the reasons for NST, and the data on attrition also incorporated other reasons such as death or no progression. It was not possible to determine whether patients with NST had worse outcomes because of lack of treatment or because they had a poorer prognosis (i.e., more comorbidities, poor performance/frailty). There also were insufficient studies reporting mUTUC subgroups to analyze this patient population, which may differ from patients with la/mUC arising from the bladder. Additionally, because the majority of these studies were retrospective, poor outcomes with NST could also be due to immortal time bias because patients who received 1L treatment survived long enough to receive treatment. Finally, the study was limited by patient characteristics that were reported or were captured in the databases/original studies (e.g., US Flatiron Health database characteristics are based on data from oncology centers only). There may have been additional factors contributing to NST or attrition that were not reported.
Conclusion
The findings of this SLR suggest that a substantial proportion of patients with la/mUC do not receive ST and that therapeutic decisions in la/mUC may be influenced by patient age, performance status and other clinical and non-clinical factors. Systematic evaluation of the evidence on la/mUC with NST shows that mortality is extremely high. The results of this study suggest that there may be patients who are eligible for ST but do not receive it; therefore, they are not provided the opportunity for potential survival benefits. Additionally, among patients who receive 1L treatment, many do not receive subsequent ST despite available options. The choice of further ST is influenced by several factors, including the type of initial treatment received. There is a need for patient-focused, evidenced-based 1L treatment selection to optimize sequencing over multiple LOTs given the availability of promising treatments that offer improved survival and tolerable side effect profiles [Citation10,Citation11].
Previous presentations
Previously presented at: the ESMO Congress 2022 and at ISPOR Europe 2022 [Citation66].
Supplementary Figure S1 and Tables S1-S7
Download Zip (887.2 KB)Financial & competing interests disclosure
This study was sponsored by Merck (CrossRef Funder ID: 10.13039/100009945), and was previously conducted under an alliance between Merck and Pfizer. M Kearney is an employee of Merck. L Zhang, E Hubscher, M Musat, S Harricharan, and T Wilke are employees of Cytel, Inc, which served as a consultant on the project. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Editorial support was provided by Katherine Quiroz-Figueroa of Clinical Thinking and was funded by Merck and Pfizer.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 70(1), 7–30 (2020).
- Fitzmaurice C, Abate D, Abbasi N et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 5(12), 1749–1768 (2019).
- Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
- Chen D, Ye Y, Guo S, Yao K. Progress in the research and targeted therapy of ErbB/HER receptors in urothelial bladder cancer. Front. Mol. Biosci. 8, 800945 (2021).
- Hepp Z, Shah SN, Smoyer K, Vadagam P. Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: a systematic literature review and gap analysis. J. Manag. Care. Spec. Pharm. 27(2), 240–255 (2021).
- Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J. Clin. 70(5), 404–423 (2020).
- Grisay G, Pierrard J, Confente C, Seront E. Future strategies involving immune checkpoint inhibitors in advanced urothelial carcinoma. Curr. Treat. Options Oncol. 22(1), 7 (2020).
- Amin MB, Mckenney JK, Paner GP et al. ICUD-EAU international consultation on bladder cancer 2012: pathology. Eur. Urol. 63(1), 16–35 (2013).
- Park I, Lee JL. Systemic treatment for advanced urothelial cancer: an update on recent clinical trials and current treatment options. Korean J. Intern. Med. 35(4), 834–853 (2020).
- Kearney M, Knott C, Lamy FX, Harnett J, Amin A, Verpillat P. Treatment patterns and clinical outcomes in patients with locally advanced or metastatic urothelial cancer in England: results of a longitudinal observational cohort study. Value Health. 23(Suppl. 2), S483 (2020).
- Bilen Ma XA, Wong A, Peng J, Robinson S, Bhanegaonkar A. Real-world treatment patterns and clinical outcomes in patients with metastatic urothelial carcinoma (mUC) receiving first-line (1L) treatment: results from IMPACT UC. Ann. Oncol. 32(Suppl. 5), S713 (2021).
- Geynisman DM, Broughton E, Hao Y, Zhang Y, Le T, Huo S. Real-world treatment patterns and clinical outcomes among patients with advanced urothelial carcinoma in the United States. Urol. Oncol. 40(5), 195e191–195e111 (2022).
- Morgans AK, Galsky MD, Hepp Z et al. 704P Treatment patterns among patients with advanced urothelial carcinoma (aUC) in the USA. Ann. Oncol. 32, S714–S715 (2021).
- Witjes JA, Bruins HM, Carrión A et al. EAU guideline on muscle-invasive and metastatic bladder cancer (2023). https://uroweb.org/guidelines/muscle-invasive-and-metastatic-bladder-cancer
- Cathomas R, Lorch A, Bruins HM et al. The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur. Urol. 81(1), 95–103 (2022).
- NCCN Clinical Practice Guidelines in Oncology. Bladder Cancer, Version 3.2023 (2023). https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf
- Powles T, Bellmunt J, Comperat E et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33(3), 244–258 (2022).
- De Santis M, Bellmunt J, Mead G et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J. Clin. Oncol. 30(2), 191–199 (2012).
- Galsky MD, Chen GJ, Oh WK et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann. Oncol. 23(2), 406–410 (2012).
- Galsky MD, Hahn NM, Rosenberg J et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J. Clin. Oncol. 29(17), 2432–2438 (2011).
- Powles T, Park SH, Voog E et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 383(13), 1218–1230 (2020).
- Swami U, Grivas P, Pal SK, Agarwal N. Utilization of systemic therapy for treatment of advanced urothelial carcinoma: lessons from real world experience. Cancer Treat. Res. Commun. 27, 100325 (2021).
- Vafaei-Nodeh S, Beigi A, Huang L, Mimmack G, Sun SZ, Ko JJ. Survival outcomes associated with different palliative systemic therapies (PSTs) in patients with metastatic bladder cancer (mBC). J. Clin. Oncol. 38(6), abstract 450 (2020).
- Richters A, Mehra N, Meijer RP et al. Utilization of systemic treatment for metastatic bladder cancer in everyday practice: results of a nation-wide population-based cohort study. Cancer Treat Res. Commun. 25, 100266 (2020).
- Reesink DJ, Van De Garde EMW, Peters BJM et al. Treatment patterns and clinical outcomes of chemotherapy treatment in patients with muscle-invasive or metastatic bladder cancer in the Netherlands. Sci. Rep. 10(1), 15822 (2020).
- Petkau V, Alekseeva G, Zukov R et al. The association of access to systemic therapy and overall survival in metastatic bladder cancer in Russia: an analysis of URRU register. J. Clin. Oncol. 39(28), abstract 88 (2021).
- Fisher MD, Shenolikar R, Miller PJ, Fenton M, Walker MS. Treatment patterns and outcomes in stage iv bladder cancer in a community oncology setting: 2008–2015. Clin. Genitourin. Cancer. 16(6), e1171–e1179 (2018).
- Doshi G, Bhanegaonkar A, Bharmal M et al. PCN339 - SPEAR-BLADDER (study informing treatment pathway decision in bladder cancer): timing of treatment following first-line therapy in patients with locally advanced and metastatic urothelial cancer in the community oncology setting in the United States. Value Health. 21, S72 (2018).
- Russo L, Esposito D, Lamy F-X et al. Characteristics, treatment patterns and safety events from 4 cohorts of advanced or metastatic cancer patients based on health care claims data. J. Clin. Oncol. 36(Suppl. 15), e13603 (2018).
- Parikh RB, Galsky MD, Gyawali B et al. Trends in checkpoint inhibitor therapy for advanced urothelial cell carcinoma at the end of life: insights from real-world practice. Oncologist. 24(6), e397–e399 (2019).
- Flannery K, Boyd M, Black-Shinn J, Robert N, Kamat AM. Outcomes in patients with metastatic bladder cancer in the USA: a retrospective electronic medical record study. Future Oncol. 15(12), 1323–1334 (2019).
- Pollock G, Hsu CH, Batai K, Lee BR, Chipollini J. Postoperative and survival outcomes after cytoreductive surgery in the treatment of metastatic upper tract urothelial carcinoma. Urology 153, 244–249 (2021).
- Mao W, Ma B, Huang X et al. Which treatment is best for patients with AJCC stage IV bladder cancer? Int. Urol. Nephrol. 51(7), 1145–1156 (2019).
- Davies FJ, Knott C, Kerr C, Adedokun L, Lockhat DM. Utilising Public Health England datasets to establish a standing cohort of patients with metastatic bladder cancer: initial results and algorithm defining disease progression. Value Health. 23(Suppl. 2), S480–S481 (2020).
- Sevillano E, Rodriguez-Moreno JF, Barquin A et al. Clinical outcome in terms of survival and treatment response of patients with metastatic urothelial carcinoma harboring FGFR alterations compared to wild-type tumors. J. Clin. Oncol. 38(Suppl. 15), e17042 (2020).
- Richters A, Boormans JL, van der Heijden MS et al. Overall survival of patients receiving cisplatin or carboplatin for primary metastatic urothelial carcinoma of the bladder: a contemporary Dutch nationwide cohort study. Eur. Urol. Focus 8(14), 995–1002 (2022).
- Jensen JB, Hauberg DS, Duus Hjortsoe M, Madsen ME, Olsen J, Agerbaek M. Treatment pattern and overall survival among patients with locally advanced or metastatic urothelial carcinoma: results from a complete nationwide unselected real-world registry study in Denmark from 2010 to 2017. Ann. Oncol. 32(5), S716 (2021).
- Fukushima H, Nakanishi Y, Kataoka M, Tobisu K-I, Koga F. Prognostic significance of serum γ-glutamyltransferase in advanced urothelial carcinoma patients. J. Urol. 197(4), e433–434 (2017).
- Miyake M, Shimizu T, Nishimura N et al. Response to pembrolizumab after dose-reduced cisplatin plus gemcitabine chemotherapy is inferior to that after carboplatin plus gemcitabine chemotherapy in cisplatin-unfit patients with advanced urothelial carcinoma. Clin. Genitourin. Cancer. 20(2), 196e191–196e199 (2022).
- Diamantopoulos LN, Khaki AR, Sonpavde GP et al. Central nervous system metastasis in patients with urothelial carcinoma: institutional experience and a comprehensive review of the literature. Clin. Genitourin. Cancer. 18(3), e266–276 (2020).
- Abu-Hijlih RA, Salah S, Al-Ibraheem A et al. Bladder cancer in young adults: disease and treatment characteristics of patients treated at tertiary cancer center. J. Clin. Oncol. 39(Suppl. 15), e16521 (2021).
- Park K, Nam JK, Koo BJ et al. Clinical characteristics, treatment delivery, and cisplatin eligibility in Korean patients initially diagnosed with urothelial carcinoma. Ewha Med. J. 44(3), 63–69 (2021).
- Kohei N, Sugiyama K, Chihara I et al. Impact of relative dose intensity in gemcitabine-cisplatin chemotherapy for metastatic urothelial carcinoma. SAGE Open Med. 6, 2050312118783011 (2018).
- Ikeda M, Matsumoto K, Hirayama T et al. Oncologic outcomes of salvage chemotherapy in patients with recurrent or metastatic lesions after radical nephroureterectomy: a multi-institutional retrospective study. Chemotherapy 65(5–6), 134–140 (2020).
- Grass GD, Awasthi S, Jain R et al. A population-based assessment of local therapy for de novo oligometastatic urothelial carcinoma of the bladder. Int. J. Radiat. Oncol. Biol. Physics. 111(3), E259 (2021).
- Sørup S, Darvalics B, Kearney M et al. Treatment patterns, clinical outcomes, and healthcare resource use among patients with stage IV urothelial cancer: findings from a nationwide cohort study in Denmark. Value Health. 24(Suppl. 1), S65 (2021).
- Okuyama A, Higashi T. Patterns of cancer treatment in different age groups in Japan: an analysis of hospital-based cancer registry data, 2012–2015. Jpn J. Clin. Oncol. 48(5), 417–425 (2018).
- Aguilar KM, Grivas P, Seal B et al. Adoption of immune checkpoint inhibitors (ICI) for unresectable locally advanced or metastatic urothelial carcinoma (u/mUC): a retrospective assessment of community oncology practices. J. Clin. Oncol. 39 (Suppl. 28), 293 (2021).
- Katayama S, Kobayashi Y, Takamoto A et al. Impact of paclitaxel, cisplatin, and gemcitabine as first-line chemotherapy in cisplatin-fit and -unfit patients with advanced/metastatic urothelial carcinoma. Urol. Oncol. Semin. Original Invest. 39(10), 731.e725–731.e732 (2021).
- Klein A, Parikh R, Kurosky S, Esterberg E, Kaye JA. PCN311 Clinical characteristics, treatment patterns, and healthcare resource utilization in patients with advanced urothelial carcinoma after progression on platinum-based therapy: a medical record review study in the United States and Canada. Value Health. 22, S115 (2019).
- Gomez de Liano Lista A, van Dijk N, de Velasco Oria de Rueda G et al. Clinical outcome after progressing to frontline and second-line anti-PD-1/PD-L1 in advanced urothelial cancer. Eur. Urol. 77(2), 269–276 (2020).
- Motzer RJ, Escudier B, Mcdermott DF et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J. Immunother. Cancer. 8(2), e000891 (2020).
- Gupta S, Su C, Bhanegaonkar A et al. Disease management and frontline treatment of locally advanced or metastatic urothelial (la/mUC) carcinoma: The U.S. physician PARADIGM study. J. Clin. Oncol. 39 (Suppl. 28), 293 (2022).
- Knott C, Kearney M, Mahmoudpour H, Verpillat P. 1750P Factors associated with receipt of systemic treatment (tx) for metastatic urothelial carcinoma (mUC) in England. Ann. Oncol. 33(Suppl. 7), S1338 (2022).
- Gironés Sarrió R, Antonio Rebollo M, Molina Garrido MJ et al. General recommendations paper on the management of older patients with cancer: the SEOM geriatric oncology task force's position statement. Clin. Transl. Oncol. 20(10), 1246–1251 (2018).
- Jodon G, Fischer SM, Kessler ER. Treatment of urothelial cancer in elderly patients: focus on immune checkpoint inhibitors. Drugs Aging 35(5), 409–421 (2018).
- Mohile SG, Velarde C, Hurria A et al. Geriatric assessment-guided care processes for older adults: a Delphi consensus of geriatric oncology experts. J. Natl Compr. Cancer Network. 13(9), 1120–1130 (2015).
- Aly A, Johnson C, Doleh Y, Shenolikar R, Botteman MF, Hussain A. Medical oncology referral and systemic therapy of patients with advanced stage urothelial carcinoma. J. Comp. Effectiveness Res. 9(13), 945–957 (2020).
- Hupe MC, Merseburger AS, De Wit M, Rexer H, Gschwend JE, Krege S. Practice pattern of systemic therapy for urothelial cancer in Germany - a survey of the German cancer society. Aktuelle Urol. 49(4), 346–354 (2018).
- Galsky MD, Ma E, Shah-Manek B et al. Cisplatin ineligibility for patients with metastatic urothelial carcinoma: a survey of clinical practice perspectives among US oncologists. Bladder Cancer. 5, 281–288 (2019).
- Jindal K, Mendoza L, Moehler T, Anger C. Adoption of immune checkpoint inhibitors (ICIs) in bladder cancer: findings from a real-world study in EU5. Ann. Oncol. 31, S595 (2020).
- Gourd E. EMA restricts use of anti-PD-1 drugs for bladder cancer. Lancet Oncol. 19(7), e341 (2018).
- Crist M, Iyer G, Hsu M, Huang WC, Balar AV. Pembrolizumab in the treatment of locally advanced or metastatic urothelial carcinoma: clinical trial evidence and experience. Ther. Adv. Urol. 11, 1756287219839285 (2019).
- Powles T, Csőszi T, Özgüroğlu M et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 22(7), 931–945 (2021).
- Stenehjem DD, Tran D, Nkrumah MA, Gupta S. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. Onco. Targets Ther. 11, 5973–5989 (2018).
- ESMO Congress and ISPOR Europe (2022).