?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim: To evaluate temporal changes in metastatic colorectal cancer (mCRC), incidence, and use of chemotherapy treatment by age group using real-world data (RWD) from the USA. Methods: A retrospective, observational study describing temporal trends in mCRC incidence and FOLFOXIRI treatment by age group using a nationwide database of commercially and Medicare Advantage-insured patients from 2010 to 2019. Results: Incidence of mCRC increased by 22.1 and 14.9% in the 18–49 and 50–64 years cohorts, respectively, and decreased by 21.6% in the ≥65 years cohort. Overall, younger patients were more likely to receive FOLFOXIRI treatment versus older patients. Conclusion: The shifting age distribution of mCRC should be considered when recommending screening and treatment. Further research is needed to inform age-specific treatment guidelines.
Plain language summary
What is this article about? This article reports the results of a study that used a US database of commercially and Medicare Advantage-insured adults to evaluate how the number of adults with metastatic colorectal cancer (mCRC) in three age groups (18–49 years, 50–64 years and 65 years and over) changed from 2010 to 2019. The study also looked at the use of an aggressive chemotherapy treatment, known as 5-fluorouracil, oxaliplatin, leucovorin calcium and irinotecan (FOLFOXIRI), by age group. What were the results? Overall, 23,970 adults with mCRC were included in the study. From 2010 to 2019, the number of adults with mCRC increased by 22.1% among those aged 18–49 years, increased by 14.9% among those aged 50–64 years, and decreased by 21.6% among those aged 65 years and over. There were some differences between age groups; a higher percentage of younger patients (18–49 years) were Hispanic or Asian, and from the South compared with the older age groups. In comparison, those aged 65 years and over were more likely to be from the West and Northeast of the USA. The study also found that a higher proportion of those aged 18–49 years received FOLFOXIRI (8.4%) compared with adults aged 50–64 years (4.4%) and 65 years and over (1.9%). What do the results of the study mean? Healthcare providers should be aware that early-onset mCRC is becoming more common and consider this when recommending screening and treatment.
A rise in early-onset metastatic colorectal cancer (mCRC) has been observed in high-income countries, in contrast with the steady decline in late-onset mCRC due to improved screening.
Contemporary data are required to better understand temporal changes in mCRC incidence by age group in the USA.
This retrospective, observational cohort study used a database of commercially and Medicare Advantage-insured patients enrolled between 2010 and 2019 to describe temporal trends in mCRC incidence, patient characteristics, and treatment regimens by age group.
Among patients aged 18–49 years, the standardized incidence rate (IR) for mCRC increased by 22.1% in 2010 to 2019, with an annual percentage change (APC) of 2.8%; in the 50–64 years cohort, the standardized IR increased by 14.9%, with an APC of 2.3%; in the ≥65 years cohort, the standardized IR decreased by 21.6%, with an APC of −2.1%.
Patient characteristics varied between age groups, with a higher proportion of patients with early-onset mCRC being Hispanic or Asian, from the Southern USA and having commercial rather than Medicare Advantage insurance compared with patients with late-onset mCRC.
Exploratory analyses suggested that a higher proportion of patients with early-onset mCRC received more aggressive multiagent chemotherapy compared with patients with late-onset mCRC.
These findings add to the accumulating evidence of a rise in the incidence of early-onset mCRC and increasing use of aggressive chemotherapy regimens in these patients without a confirmed corresponding survival benefit.
The shifting age distribution and patient characteristics in mCRC should be considered when developing appropriate treatment guidelines.
Colorectal cancer (CRC) is the second most common cancer diagnosis and cause of cancer-related death in the USA [Citation1]. There has been a substantial increase in the incidence of early-onset CRC (defined as onset at <50 years of age) over the past few decades, with early-onset CRC now accounting for 10.0% of new CRC cases [Citation2,Citation3]. It is estimated that 25.0% of rectal cancers and 10.0–12.0% of colon cancers will be diagnosed as early onset in the next 10 years [Citation2–4]. Importantly, the shifting age distribution of CRC is likely to partly reflect improved screening and earlier disease detection, and, in the USA, a broadening of the recommended age for CRC screening to 45 years for persons with average risk for early- or late-onset disease [Citation3,Citation5].
Early-onset CRC is more commonly characterized by advanced disease stage at diagnosis reflected by symptomatic presentation, increased biologic aggressiveness, and longer time to diagnosis compared with late-onset CRC [Citation3,Citation6,Citation7]. The advanced disease stage at diagnosis is thought to be a main contributor to the worse outcomes observed in patients with early-onset CRC compared with late-onset CRC [Citation3,Citation8].
Metastatic CRC (mCRC), also referred to as advanced CRC, is defined as cancer that has spread outside the original colorectal mass. About 20.0% of newly diagnosed patients with CRC have de novo mCRC [Citation9,Citation10], and up to 50.0% of patients with initially localized CRC will eventually develop metastases [Citation11]. Patients with mCRC usually have poor prognosis, with a 5-year survival rate of 15.0% compared with 91.0% in patients with localized (defined as no sign of spread outside the colon or rectum) CRC [Citation1]. Importantly, evidence is emerging that the incidence of early-onset mCRC is increasing [Citation6,Citation12–15].
The treatment of mCRC in elderly patients presents different challenges to those associated with early-onset disease that are related to balancing benefit with a perceived higher risk of developing toxicity. However, despite the worse prognosis of patients with early-onset CRC compared with late-onset CRC [Citation3], until recently neither CRC nor mCRC treatment recommendations differed by chronological age group, but do so by important measures that are considered related to biological age such as physical fitness and function [Citation16–19]. A multidisciplinary international group (Delphi Initiative for Early-Onset Colorectal Cancer [DIRECt]) have now developed the first evidence-based consensus recommendations for early-onset CRC [Citation20]. These guidelines provide a new tool for clinicians treating patients with early-onset CRC, however, evidence is emerging that, in day-to-day practice, clinicians may administer more aggressive multiagent chemotherapy regimens (e.g. oxaliplatin- or irinotecan-based) to younger patients with no clear superiority in terms of efficacy relative to administration in older patients [Citation19,Citation21–24]. Some studies suggest that multiagent chemotherapy with backbone FOLFOXIRI (5-fluorouracil, oxaliplatin, leucovorin calcium, and irinotecan [triplet chemotherapy]) may be more effective than doublet regimens such as FOLFOX (5-fluorouracil, oxaliplatin, and leucovorin calcium) or FOLFIRI (5-fluorouracil, leucovorin calcium and irinotecan) plus bevacizumab, however it is also associated with increased toxicity, highlighting the need for contemporary data on FOLFOXIRI use and associated real-world outcomes by both biological and chronological age group [Citation25–28].
This retrospective study evaluated the temporal trends in the incidence of mCRC, and the characteristics and treatments of patients with mCRC in a US database of commercial health insurance claims and those from the US Medicare Advantage federal health insurance program for people aged ≥65 years (or those with certain disabilities/morbidities).
Materials & methods
Study design & data source
This observational cohort study used data from patients included in Optum®‘s de-identified Clinformatics® Data Mart Database (CDM) from 1 July 2009, through 31 December 2019. This dataset contains health insurance claims data from large commercial and Medicare Advantage health plans for approximately 17–19 million annually covered lives and approximately 68 million unique lives over 12 years (2007–2019), with most membership coverage in the South (44%) compared with the Midwest (23%), West (22%), Northeast (10%) and other/unknown (1%) USA regions. Data include information on patient's use of inpatient and outpatient services, along with prescription drug and enrollment into a health insurance plan [Citation29]. No Institutional Review Board was required for this study, as it used de-identified data. The study adhered to the guidelines set forth by the Declaration of Helsinki in 2013 [Citation30].
Study population
Among the study population, patients who were newly diagnosed with mCRC between 1 January 2010 and 31 December 2019 (study period), were eligible for inclusion. To be defined as a patient with mCRC during the study period, patients were required to have a primary diagnosis of CRC (International Classification of Diseases 9th revision Clinical Modification [ICD-9-CM] diagnostic codes: 153.xx, 154.xx; ICD-10-CM: C18–C21) and a metastasis diagnosis (ICD-9-CM diagnostic codes: 196–198; ICD-10-CM: C77–C79) from at least one inpatient visit or from two different outpatient visits at least 7 days apart. The date of first metastasis diagnosis was considered as the index date. Patients were 18 years or older at the time of the index date and had data for at least 6 months before the index date (baseline period), and were followed until the last day of insurance enrollment, death, or end of study period, whichever occurred first. Patients were excluded if they had any diagnosis of other primary cancer or metastasis within 6 months before the index date.
Outcomes
The primary outcomes were annual crude incidence rates (IR), age-specific IRs, and standardized age-specific IRs from 2010 to 2019. The crude IR was defined as the number of patients with newly diagnosed mCRC divided by the total number of adult patients who had a full year of insurance enrollment in each calendar year (total enrollees). The age-specific IR was defined as the number of patients with newly diagnosed mCRC divided by the number of total enrollees in each age group: 18–49 years, 50–64 years and ≥65 years. The standardized IR was defined as the sum of age-specific IRs multiplied by the proportion of the US 2010 population.
An exploratory analysis was conducted to describe the use of FOLFOXIRI by age group in patients with mCRC during the follow-up period. The use of FOLFOXIRI was identified from pharmacy claims collected after the index date and until the end of follow-up that indicated treatment with FOLFOXIRI within a 21-day regimen window, beginning at the first claim for the FOLFOXIRI agent.
Covariates
Baseline covariates were extracted using data from the 6-month period preceding the index date. Patient demographics, including age at diagnosis (18–49 years, 50–64 years, ≥65 years), sex, race/ethnicity, USA region (South, Midwest, West, Northeast), and insurance plan (Medicare Advantage and commercial), were reported. Charlson Comorbidity Index [Citation31] and comorbidities prevalent in patients with mCRC, including hypertension, infectious diseases, anemia, gastric perforation, electrolyte disorder, existing wound (including bone fractures, wound-healing complications and open wounds), digestive system complications, history of bleeding and respiratory disease [Citation32], were also reported.
Statistical analyses
Patient demographics and comorbidity burden were described in the overall cohort and stratified by age group (18–49, 50–64 and ≥65 years). Annual crude IRs, age-specific IRs, and standardized age-specific IRs were reported for the overall cohort and by age group in each calendar year from 2010 to 2019. Annual percent change (APC) was used to characterize trends in mCRC rates over time. With this approach, the cancer rates were assumed to change at a constant percentage of the rate of the previous year. To estimate the APC for the annual US population-standardized IRs from 2010 to 2019, the following regression model was used [Citation33]:
where Ry was the rate in year y.
Results
Patient disposition & characteristics
Among the 230,293 patients with CRC, 23,970 patients with mCRC met inclusion criteria for this study (). Overall, 8.1% of patients were 18–49 years of age; 22.9% were 50–64 years of age and 69.0% were ≥65 years of age (). The mean age (standard deviation) was 69.6 years (13.0) in the overall cohort, 42.8 years (5.7) in the 18–49 years cohort, 57.7 years (4.2) in the 50–64 years cohort and 76.7 years (7.2) in the ≥65 years cohort.
Table 1. Demographic and clinical characteristics.
A majority of patients were male in the 18–49 years (56.0%), 50–64 years (58.0%) and overall cohorts (51.2%), whereas there was a higher proportion of females in the ≥65 years cohort (51.6%). There was a higher proportion of Hispanic and Asian patients in the 18–49 years cohort compared with other cohorts. A higher proportion of patients were from the South in the 18–49 years cohort (44.4%) and 50–64 years cohort (47.5%) compared with the ≥65 years cohort (37.6%), whereas a higher proportion of patients in the ≥65 years cohort were from the West and Northeast regions of the USA (24.4 and 14.0%, respectively) versus the 18–49 years cohort (18.0 and 9.5%, respectively) and 50–64 years cohort (16.8 and 8.1%, respectively). Most patients in the ≥65 years cohort (91.5%) had Medicare Advantage. A higher proportion of patients in the ≥65 years cohort compared with the 18–49 years and 50–64 years cohorts had a higher comorbidity burden (assessed by Charlson Comorbidity Index ≥4; 20.8 vs 5.5 and 9.9%, respectively), and hypertension (58.3 vs 19.4 and 38.5%, respectively).
Temporal trends in the incidence of mCRC
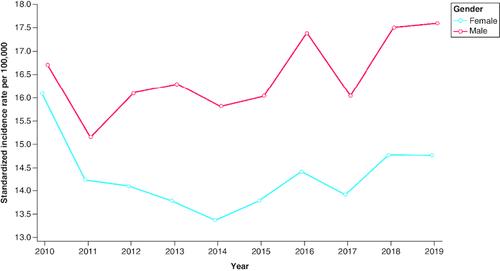
The crude and standardized IRs of mCRC during the study period (2010–2019) are shown in . In the ≥65 years cohort, the crude IRs were 54.1 and 46.1 per 100,000 in 2010 and 2019, respectively, and the standardized IR decreased by 21.6% from 64.4 in 2010 to 50.5 per 100,000 in 2019. In the 18–49 years and 50–64 years cohorts, respectively, the crude IRs were 3.6 and 20.0 per 100,000 in 2010 and 4.1 and 23.5 per 100,000 in 2019. In the 18–49 years cohort, the standardized IR increased by 22.1% from 3.3 per 100,000 in 2010 to 4.0 per 100,000 in 2019. In the 50–64 years cohort, the standardized IR increased by 14.9% from 20.1 per 100,000 in 2010 to 23.1 per 100,000 in 2019. The APC for standardized IRs during the study period was 2.8% in the 18–49 years cohort, 2.3% in the 50–64 years cohort, and -2.1% in the ≥65 years cohort. The temporal trend of standardized IRs and APCs from 2010 to 2019 is shown in . The standardized IRs were higher for males compared with females across the study period ( & ). No clear pattern of change in standardized IRs by sex was observed over time.
Table 2. Crude and standardized incidence rates of metastatic colorectal cancer by age group from 2010 to 2019.
Figure 2. Standardized incidence rates (per 100,000) and annual percentage change for metastatic colorectal cancer by age group from 2010 to 2019.
APC: Annual percentage change.
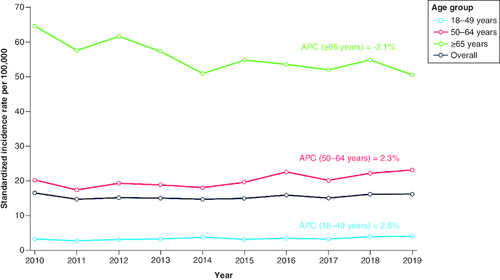
Table 3. Crude and standardized incidence rates of metastatic colorectal cancer by sex from 2010 to 2019.
Figure 3. Standardized incidence rates (per 100,000) for metastatic colorectal cancer by sex from 2010 to 2019.
Standard US population is based on the 2010 census. Patients with unknown sex were excluded from the denominator population.
The exploratory analysis of the use of the triplet FOLFOXIRI regimen highlighted differences by age group, with a FOLFOXIRI use after the diagnosis of metastasis reported for 8.4% (n = 163/1942) of patients aged 18–49 years, 4.4% of patients (n = 242/5484) aged 50–64 and 1.9% of patients (n = 320/16,544) aged ≥65 years.
Discussion
This retrospective observational study of commercial and Medicare Advantage health insurance claims data showed that the incidence of mCRC increased in patients aged <65 years and decreased in patients aged ≥65 years between 2010 and 2019 in the USA. Demographic and clinical characteristics, including race, region of the USA, insurance plan, comorbidity burden and hypertension, varied between age groups. A higher proportion of patients aged 18–49 years were treated with FOLFOXIRI compared with older patients.
The annual percentage increase in the incidence of mCRC was highest in patients aged 18–49 years, with a decrease observed in patients aged ≥65 years. Of note, the population included in CDM claims covers a proportion of the commercially insured and Medicare Advantage population in all 50 US states and Washington DC [Citation34], and is considered to be representative of the broader US commercially insured population in terms of age and sex [Citation35]. These findings align with accumulating evidence of a steady rise in the incidence of early-onset CRC in the USA and several European countries, with a contrasting decline of late-onset CRC [Citation3,Citation36], plus the well-documented evidence that advanced disease stage is more common in patients aged <50 years versus ≥50 years [Citation3]. Of note, the increasing incidence of early-onset CRC in the USA was first identified using Surveillance, Epidemiology and End Results (SEER) data from 1974 to 2013 [Citation37]. According to SEER data from nine registries covering approximately 9.4% of the US population (based on the 2010 census), there was a 63.0% increase in the age-adjusted incidence of early-onset CRC between 1988 and 2015 in the USA [Citation3,Citation38].
Similar trends in early-onset CRC have been demonstrated worldwide [Citation36,Citation39]. In a study of national and regional cancer registries in Europe that included 187,918 patients, the incidence of CRC increased by 7.9% per year from 2004 to 2016 in patients aged 20–29 years, by 4.9% per year from 2005 to 2016 in patients aged 30–39 years, and by 1.6% per year from 2004 to 2016 in patients aged 40–49 years [Citation36]. In a study published in 2019 involving 36 countries on five continents, the incidence of early-onset CRC increased significantly (p < 0.05) during the last 10 years of available data in 19 countries, nine of which also reported stable or declining incidence of CRC in patients aged ≥50 years (Australia, Canada, Denmark, Germany, New Zealand, Slovenia, Sweden, the UK and the USA) [Citation39]. A narrative review and two systematic reviews collated evidence of this global trend of increasing incidence of early-onset CRC and decreasing incidence of late-onset CRC [Citation3,Citation19,Citation40].
A recent study of SEER data from 2000 to 2016 highlighted that the largest increase in the incidence of early-onset CRC between 2000 to 2002 and 2014 to 2016 was among patients with metastatic adenocarcinomas [Citation15]. The study reported a 49.3% increase in the incidence of metastatic colon adenocarcinoma among patients aged 30–39 years. For metastatic rectal adenocarcinoma, the increase in incidence was 133.3% among patients aged 20–29 years, 97.4% among patients aged 30–39 years, and 47.8% among patients aged 40–49 years. Our study showed a similar shifting age distribution in the incidence of mCRC and builds upon the existing literature by using contemporary data (2010–2019), focusing on mCRC, and including a US patient population with commercial or Medicare Advantage insurance.
SEER data also show that treatment patterns in CRC have changed over time [Citation41]. From 1990 to 2005, there was an increased adoption of chemotherapy and radiation therapy in patients with CRC, followed by a slight decline in 2010, and the therapy received varied by age, comorbidities, and year of diagnosis [Citation41]. It has been shown that patients with early-onset mCRC received more aggressive first-line chemotherapy compared with older patients [Citation19,Citation21,Citation22]. Our study points to a higher proportion of patients with early-onset mCRC receiving treatment with FOLFOXIRI compared with patients with late-onset mCRC. Intensive schedules of adjuvant systemic chemotherapy and multiagent regimens that are associated with increased toxicities may be preferred in young, fit patients with good performance status, especially as patients with early-onset CRC tend to present with more advanced disease stage at diagnosis [Citation3]. However, such aggressive treatments have not shown any significant survival benefit in young patients with early-onset mCRC compared with older patients [Citation19,Citation21,Citation24]. Overall, there is an urgent need for further research and guidance on care for patients with mCRC based on fitness and performance status, both of which may be age-dependent.
The analyses in this study were descriptive and no conclusions can be drawn regarding causal relationships or statistical inference. Additionally, these findings cannot be generalized to patients outside of this US commercial and Medicare Advantage health insurance claims setting. To mitigate the influence of different age distributions in the study population on the incidence calculations, the 2010 US population was used and standardized IRs were calculated to facilitate annual comparisons. Finally, patients with mCRC were identified using ICD codes collected for reimbursement and not for research purposes, with potential ascertainment bias due to underreporting, misclassification and coding for ruling out a suspected disease rather than determining the actual presence of the disease. The availability of demographic and clinical variables in the database was limited. For example, potentially valuable data on body mass index and tumor sidedness were not available, and this was a limitation of the analysis in this study. A further limitation of the study was that the use of approved immunotherapies in the population of younger patients with mCRC was not evaluated. Although the focus of this analysis was on the increasingly common use of aggressive chemotherapy regimens in this population, the use of immunotherapy in the same population is a valuable topic for future analysis, particularly if combined with information on microsatellite stability status, which was not available in the claims data used.
Conclusion
In conclusion, this retrospective, observational study using a large, nationwide US claims database provided evidence of the increasing incidence of early-onset mCRC during the past decade, with varied patient demographic and clinical characteristics between age groups. The shifting age distribution and patient characteristics in mCRC should be considered when recommending early screening and selecting appropriate treatments; this is particularly important in view of the emerging evidence that patients with early-onset mCRC tend to receive more aggressive treatment in clinical practice compared with older patients. Further research is needed to inform age-specific treatment guidelines.
Author contributions
All authors made substantial contributions to the conception or design of the work or to the acquisition, analysis, or interpretation of data for the work. All authors were involved in drafting the work or revising it critically for important intellectual content. All authors provided final approval of the version to be published. All authors provided agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical conduct of research
As dictated by Title 45 Code of Federal Regulations (45 CFR 46.101(b)(4)) (available at www.govinfo.gov/content/pkg/CFR-2011-title45-vol1/pdf/CFR-2011-title45-vol1.pdf), this analysis of the Optum®'s de-identified Clinformatics® Data Mart Database was conducted under an exemption from Institutional Review Board oversight for US-based studies using de-identified healthcare records. The research reported in this paper adhered to guidelines set forth by the Declaration of Helsinki as revised in 2013.
Financial & competing interests disclosure
This study was funded by Bayer. The authors are all employees of Bayer HealthCare Pharmaceuticals. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing and editorial support were provided by Matthew Reynolds and Silvia Pregnolato of OPEN Health Communications, London, UK, with financial support from Bayer.
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 72(1), 7–33 (2022).
- Zaborowski AM, Abdile A, Adamina M et al. Characteristics of early-onset vs late-onset colorectal cancer: a review. JAMA Surg. 156(9), 865–874 (2021).
- Sinicrope FA. Increasing incidence of early-onset colorectal cancer. N. Engl. J. Med. 386(16), 1547–1558 (2022).
- Bailey CE, Hu CY, You YN et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 150(1), 17–22 (2015).
- Wolf AMD, Fontham ETH, Church TR et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 68(4), 250–281 (2018).
- Chen FW, Sundaram V, Chew TA, Ladabaum U. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin. Gastroenterol. Hepatol. 15(5), 728–737; e723 (2017).
- Mueller M, Schneider MA, Deplazes B, Cabalzar-Wondberg D, Rickenbacher A, Turina M. Colorectal cancer of the young displays distinct features of aggressive tumor biology: a single-center cohort study. World J. Gastrointest. Surg. 13(2), 164–175 (2021).
- Fu J, Yang J, Tan Y et al. Young patients (≤35 years old) with colorectal cancer have worse outcomes due to more advanced disease: a 30-year retrospective review. Medicine (Baltimore). 93(23), e135 (2014).
- Van Der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, De Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis 32(5), 457–465 (2015).
- Ansa BE, Coughlin SS, Alema-Mensah E, Smith SA. Evaluation of colorectal cancer incidence trends in the United States (2000–2014). J. Clin. Med. 7(2), 22 (2018).
- Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J. Clin. 72(4), 372–401 (2022).
- O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Livingston EH, Ko CY. Do young colon cancer patients have worse outcomes? World J. Surg. 28(6), 558–562 (2004).
- Willauer AN, Liu Y, Pereira AAL et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 125(12), 2002–2010 (2019).
- Saraste D, Järås J, Martling A. Population-based analysis of outcomes with early-age colorectal cancer. Br. J. Surg. 107(3), 301–309 (2020).
- Montminy EM, Zhou M, Maniscalco L et al. Shifts in the proportion of distant stage early-onset colorectal adenocarcinoma in the United States. Cancer Epidemiol. Biomarkers Prev. 31(2), 334–341 (2022).
- Benson ABR, Venook AP, Cederquist L et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw. 15(3), 370–398 (2017).
- Van CE, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 25 (Suppl. 3), iii1–9 (2014).
- Yoshino T, Arnold D, Taniguchi H et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 29(1), 44–70 (2018).
- Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol. Oncol. 13(2), 109–131 (2019).
- Cavestro GM, Mannucci A, Balaguer F et al. Delphi Initiative for Early-Onset Colorectal Cancer (DIRECt) International Management Guidelines. Clin Gastroenterol Hepatol. 21(3), 581–603; e533 (2023).
- Kneuertz PJ, Chang GJ, Hu CY et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg. 150(5), 402–409 (2015).
- Lund CM, Vistisen KK, Dehlendorff C, Rønholt F, Johansen JS, Nielsen DL. Age-dependent differences in first-line chemotherapy in patients with metastatic colorectal cancer: the DISCO study. Acta. Oncol. 57(11), 1445–1454 (2018).
- Kolarich A, George TJ Jr, Hughes SJ et al. Rectal cancer patients younger than 50 years lack a survival benefit from NCCN guideline-directed treatment for stage II and III disease. Cancer 124(17), 3510–3519 (2018).
- Lipsyc-Sharf M, Zhang S, Ou FS et al. Survival in young-onset metastatic colorectal cancer: findings from Cancer and Leukemia Group B (Alliance)/SWOG 80405. J. Natl Cancer Inst. 114(3), 427–435 (2022).
- Cremolini C, Antoniotti C, Rossini D et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 21(4), 497–507 (2020).
- Falcone A, Ricci S, Brunetti I et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 25(13), 1670–1676 (2007).
- Jindal V, Gupta R, Sahu KK, Rahi MS, Stender MJ, Jalyesimi IA. Doublet (FOLFOX or FOLFIRI) versus triplet (FOLFOXIRI) backbone chemotherapy regimen as first-line treatment of metastatic colorectal cancer: a meta-analysis and systematic review. J. Clin. Oncol. 39(Suppl. 15), 3593 (2021).
- Marques RP, Duarte GS, Sterrantino C et al. Triplet (FOLFOXIRI) versus doublet (FOLFOX or FOLFIRI) backbone chemotherapy as first-line treatment of metastatic colorectal cancer: a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 118, 54–62 (2017).
- Optum®. Optum® Clinformatics® Data Mart (2017). www.optum.com/content/dam/optum/resources/productSheets/Clinformatics_for_Data_Mart.pdf ( Accessed 31 October 2022).
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. JAMA 310(20), 2191–2194 (2013).
- Patel AK, Duh MS, Barghout V et al. Real-world treatment patterns among patients with colorectal cancer treated with trifluridine/tipiracil and regorafenib. Clin. Colorectal Cancer. 17(3), e531–e539 (2018).
- Fu AZ, Zhao Z, Gao S, Barber B, Liu GG. Comorbid conditions in patients with metastatic colorectal cancer. World J. Oncol. 2(5), 225–231 (2011).
- National Cancer Institute. Annual percent change (APC) and confidence interval (2022). https://surveillance.cancer.gov/help/joinpoint/setting-parameters/method-and-parameters-tab/apc-aapc-tau-confidence-intervals/estimate-average-percent-change-apc-and-confidence-interval ( Accessed 31 October 2022).
- Optum®. Optum® claims data (2022). www.optum.com/business/solutions/life-sciences/real-world-data/claims-data.html ( Accessed 31 October 2022).
- Kiang MV, Humphreys K, Cullen MR, Basu S. Opioid prescribing patterns among medical providers in the United States, 2003-17: retrospective, observational study. BMJ. 368, l6968 (2020).
- Vuik FE, Nieuwenburg SA, Bardou M et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 68(10), 1820–1826 (2019).
- Siegel RL, Fedewa SA, Anderson WF et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J. Natl Cancer Inst. 109(8), djw322 (2017).
- SEER Program. Incidence —SEER research data, 9 registries, Nov 2019 sub (1975–2017). (2019).
- Siegel RL, Torre LA, Soerjomataram I et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 68(12), 2179–2185 (2019).
- Done JZ, Fang SH. Young-onset colorectal cancer: a review. World J. Gastrointest. Oncol. 13(8), 856–866 (2021).
- Murphy CC, Harlan LC, Lund JL, Lynch CF, Geiger AM. Patterns of colorectal cancer care in the United States: 1990–2010. J. Natl Cancer Inst. 107(10), djv198 (2015).
- Murphy CC, Sanoff HK, Stitzenberg KB, Baron JA, Lund JL, Sandler RS. Patterns of sociodemographic and clinicopathologic characteristics of stages II and III colorectal cancer patients by age: examining potential mechanisms of young-onset disease. J. Cancer Epidemiol. 2017, 4024580 (2017).