Abstract
Aim: We evaluated the clinical acceptance and feasibility of a pharmacist-guided personalized consult service following its transition from a mandatory (mPGx) to optional (oPGx) CYP2C9/VKORC1/CYP4F2 genotyping for warfarin. Methods: A total of 1105 patients were included. Clinical acceptance and feasibility outcomes were analyzed using bivariate and multivariable analyses. Results: After transitioning to optional genotyping, genotype testing was still ordered in a large segment of the eligible population (52.1%). Physician acceptance of pharmacist-recommended doses improved from 83.9% (mPGx) to 86.6% (oPGx; OR: 1.3; 95% CI: 1.1–1.5; p = 0.01) with a shorter median genotype result turnaround time (oPGX: 23.6 h vs mPGX: 25.1 h; p < 0.01). Conclusion: Ordering of genotype testing and provider acceptance of dosing recommendations remained high after transitioning to optional genotyping.
Warfarin remains a widely used anticoagulant for various indications, especially among elderly patients, despite the introduction of direct-acting oral anticoagulants [Citation1]. Warfarin dosing remains challenging, making its use a leading cause of drug-related emergency department visits [Citation2–4]. Researchers have continued to propose new methods to warfarin dosing to better maintain an International Normalization Ratio (INR) within therapeutic range, usually 2–3 for most indications. Traditional algorithms comprehensively include clinical factors, such as baseline INR, ethnicity, weight, age and concurrent medications to achieve a therapeutic INR with an appropriate warfarin dose. With the hope to further optimize anticoagulation control, genotype-based warfarin dosing has been utilized in recent years [Citation5–7].
Increasing evidence has indicated that genetic variation in CYP2C9, VKORC1 and, to a lesser extent, CYP4F2, play a significant role in predicting warfarin dose requirements. In 2007, the US FDA approved incorporation of CYP2C9 and VKORC1-based dosing information into the warfarin prescribing information [Citation8,Citation9]. However, recent randomized control trials have reported discordant results regarding the clinical utility of genotype-guided warfarin dosing [Citation10–24]. The Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for warfarin provides recommendations for genotype-guided warfarin dosing when genotype results are available but does not provide recommendations for when pharmacogenetic testing should be ordered. In addition to genotyping, several studies assessing the clinical utility of pharmacist-managed anticoagulation services in inpatient and outpatient settings have suggested pharmacist-managed anticoagulation services improve anticoagulation control and, in turn, reduce healthcare utilization compared with routine care [Citation25–30]. However, there remains an evidence gap regarding the operational feasibility of a pharmacist-driven anticoagulation consult service incorporating genotype-guided warfarin dosing.
In August 2012, the University of Illinois Hospital (UIH) implemented a pharmacist-guided personalized consult service (PGPCS) to manage warfarin dosing for patients newly initiated on warfarin. The PGPCS provides daily warfarin dosing recommendations based on genotype and clinical information. Initially, mandatory CYP2C9, VKORC1 and CYP4F2 genotyping was implemented for all patients initiated on warfarin to guide warfarin dosing recommendations. An enhanced electronic health record (EHR) with clinical decision support incorporation operationalized the mandatory PGPCS and was well-accepted by medical staff [Citation31]. Additionally, a previous analysis showed genotype-based warfarin dosing was associated with a reduction of drug-related adverse events [Citation32]. However, in response to discordant findings regarding the clinical benefit of genotype-based dosing stemming from the COAG and EU-PACT trials, the hospital transitioned the mandatory genotype testing as part of the PGPCS intervention to optional testing [Citation10,Citation11]. As the impact of transitioning from mandatory to optional genotyping on a pharmacogenetics consult service has not been previously evaluated, this study was designed to evaluate whether clinical acceptance and feasibility of a PGPCS was affected by transitioning from mandatory to optional genotyping. We hypothesized that operational feasibility was maintained following transitioning to optional genotyping.
Materials & methods
Description of the PGPCS & data collection
The detailed procedures of the PGPCS have been described previously [Citation31]. All study procedures were approved by the Institutional Review Board at the University of Illinois Chicago and conducted in accordance with the Declaration of Helsinki. Briefly, the PGPCS was implemented in a tertiary care university hospital. All patients newly initiated on warfarin starting August 2012 were managed by PGPCS. During the initial implementation phase (15 August 2012 through 15 April 2014), genotyping was automatically ordered by a clinical decision support tool for all patients newly starting warfarin without prior genotyping results. Warfarin genotyping was performed to detect CYP2C9 *2 (p.Arg144Cys, rs1799853), *3 (p.Ile359Leu, rs1057910), *5 (p.Asp360Glu, rs28371686), *6 (p.Lys23Argfs, rs9332131), *11 (p.Arg335Trp, rs28371685), *14 (p.Arg125His, rs72558189), *15 (p.Ser162null, rs72558190) and *16 (p.Thr299Ala, rs72558192), as well as VKORC1 -1639G >A (rs9923231) and CYP4F2 p.Val433Met (rs2108622) through a commercially available assay (GenMark Dx eSensor XT-8, GenMark Diagnostics, Inc., CA, USA). Once genotype information was made available in the EHR, a clinical pharmacist provided daily warfarin dose recommendations based on genotype and clinical information until patient discharge or target INR was achieved.
Genotype screening and pharmacogenomic (PGx) consulting were mandatory from August 2012 to April 2014. On 16 April 2014, mandatory genotyping was transitioned to optional genotyping in which healthcare providers ordered genotyping based on clinical judgement. Regardless of genotyping data availability, a PGPCS pharmacist provided dose recommendations for all patients based on demographics, clinical factors, medical history, baseline INR and genotype information when available using the warfarindosing.org dose estimation algorithm.
EHR data for patients initiating warfarin between 15 April 2012 and 15 December 2015 were captured using REDCap® and included demographics, laboratory results, procedures, medications and medical history.
Clinical acceptance & feasibility outcomes
The primary outcome of this study was physician compliance to PGPCS dosing recommendations, which was defined as the number of doses accepted by clinicians divided by the total number of recommendations made by the PGPCS service. Acceptance was defined as the daily dose ordered within ±0.5 mg range of the PGPCS dose recommendation. The goal compliance rate determined a priori was 70% in both periods.
Secondary feasibility outcomes were genotype result turnaround time (time from genotype test order to lab result release in the EHR) and time to dose recommendation. The goal of the PGPCS was to provide warfarin dose recommendations within 24 h of genotype order and prior to the time of the second warfarin dose. The frequency of meeting these feasibility goals were summarized using descriptive and multivariable analysis.
Statistical analysis
Baseline patient characteristics were compared between the mPGx and oPGx cohorts using Chi-square tests and Student’s t-tests for categorical and continuous variables, respectively.
Clinical acceptance and procedural feasibility were compared using multiple bivariate analyses. PGPCS dose recommendation acceptance rates during the oPGx period were compared with rates from the mPGx group using the Chi-square test. The level of concordance between ordered dose and recommended dose was determined using Pearson correlation coefficients. The primary outcome of PGPCS dose recommendation acceptance (i.e., ±0.5 mg) between the two periods was analyzed using multivariable logistic regression analysis adjusting for potential confounders including age, BMI, race, sex, indication and type of primary medical service. The secondary outcome, time to PGPCS recommendation, was not normally distributed. To summarize the data, median and inter quantile range were calculated in addition we performed nonparametric analyses using Mann–Whitney U test. Time to dose recommendation and time to genotype result in EHR were further analyzed using multivariable linear regression analyses. Additional secondary outcomes such as the number of times the genotype result and recommendation note were documented in the EHR prior to second dose and within 24 hr of the consult were summarized using bivariate analyses as well as using multivariable logistic regression analyses. To gain insight into the learning effects on the feasibility of the procedure, longitudinal changes in dose recommendation acceptance were assessed quarterly throughout the study period. The primary interest of this study was to compare the outcomes between the two settings, oPGx and mPGx. We also compared the outcomes between the patients who received PGx recommendation versus, those who did not in the oPGX cohort. The utilization of PGPCS and dose recommendation acceptance could also have been confounded by physician specialty. In order to address this issue, the primary outcome and utilization of genotype information were stratified by physician specialty categories.
Results
Baseline characteristics/demographic information
A total of 1105 patients were newly initiated on warfarin at UIH between August 2012 and December 2015, including 577 during the mPGx period and 528 during the oPGx period. There were more males in the oPGx relative to the mPGx cohort (53.4 vs 43.3%, p < 0.01). Slightly more patients in the oPGx cohort had a DVT indication (35.1 vs 30.5%; p < 0.01) and less were surgically treated compared with those in the mPGx cohort (7.9 vs 18.4%; p < 0.01). The primary medical service caring for patients differed between mPGx and oPGx. The oPGx period included less patients managed by the general medicine (29.7 vs 37.1%; p < 0.01) and orthopedics services, (12.3 vs 18.9%; p < 0.01) ().
Table 1. Baseline characteristics.
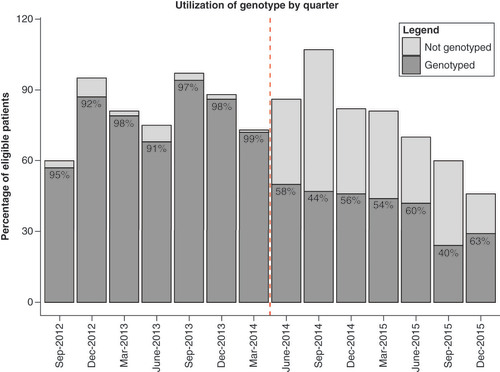
Interestingly, physicians still ordered genotyping for over half of eligible patients even after genotyping was changed to optional ( & ). Of the 528 patients newly initiated on warfarin during the oPGx period, genotyping was still ordered for 275 subjects (52.1%). In terms of baseline characteristics, the genotyped group included fewer black patients (genotyped: 52.6%, not genotyped: 65.1%; p = 0.05) with all other patient characteristics being similar between the two groups ().
Of the patients who received genotype-based warfarin dosing recommendations, the majority had genotypes associated with normal CYP2C9-mediated warfarin metabolism and CYP4F2-mediated vitamin K metabolism (mPGx: 82.8 and 68.9%; oPGx: 87.0 and 75.5%). Regarding the VKORC1 SNP, less than 10% of subjects in the mPGx and oPGx cohorts were highly sensitive to warfarin (9.4% of mPGx and 13.1% of oPGx) ().
Clinical acceptance & feasibility outcomes
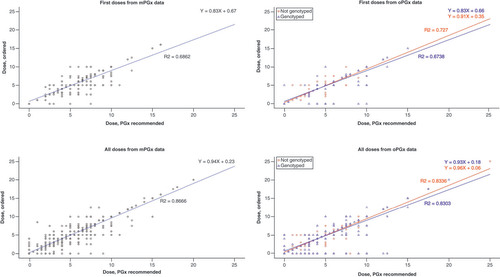
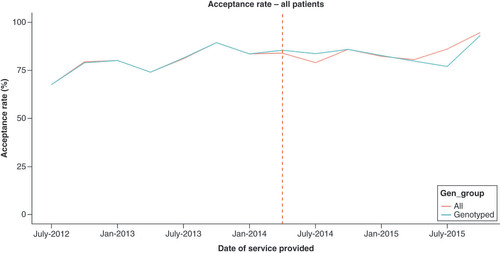
PGPCS dose recommendation acceptance rates increased following implementation of optional genotyping. During the mandatory phase, 72.9% of initial and 83.9% of all in-hospital PGPCS dose recommendations were accepted by physicians within a ±0.5 mg range, with increased acceptance rates of 80.9 and 86.6%, respectively, during the oPGx period (p < 0.01) (). Both crude and adjusted models showed improvement in dose recommendation acceptance during the oPGx period compared with mPGx. Initial dose acceptance (OR: 1.7; 95% CI: 1.2–2.4; p < 0.01) and all dose acceptance (OR: 1.3; 95% CI: 1.1–1.5; p < 0.01) increased during oPGx relative to mPGx after adjusting for potential confounders (). Longitudinal trends in acceptance of recommended dose by PGPCS showed that the acceptance rate increased after transitioning to optional genotyping ().
Table 2. Clinical acceptance and feasibility outcomes.
Table 3. Crude and adjusted analysis of operational outcome between mPGX and oPGx cohort.
Among the recommendations made during oPGx period, proposed doses on the basis of patient genotype showed higher acceptance rates (85.0% of initial and 86.9% of all in-hospital dose recommendations) compared with the recommendations not based on genotype information (76.3% of initial and 86.1% of all in-hospital dose recommendation) (). In adjusted analysis, acceptance of recommended dose following initial PGPCS assessment was higher in the genotype tested patients compared with non-genotyped patients (OR: 1.8; 95% CI: 1.1–3.0; p = 0.01); whereas, no significant differences were seen in acceptance of all dose recommendations (OR: 1.1; 95% CI: 0.8–1.4; p = 0.59) (). The correlation between dosing recommendations and daily doses remained high during the oPGx period (i.e., correlation coefficients of 0.69 for initial doses and 0.87 for all doses during mPGx vs 0.72 and 0.83, respectively, during oPGx). Similarly, the correlation between recommended and prescribed doses remained consistent within the oPGx cohort among those who were genotyped relative to those not genotyped ().
Table 4. Crude and adjusted analysis of operational outcomes between genotyped and nongenotyped subgroups during oPGx cohort.
Genotype turnaround time and time from PGPCS consult to dose recommendation are summarized in . Median genotype turnaround time following transition from mPGx to oPGx decreased slightly but significantly (25.1 [SD: 7.3] to 23.6 h [SD: 6.7], p < 0.01). Median time to pharmacist EHR dose recommendation documentation also decreased following transition to oPGx (5.5 to 3.3 h, p < 0.01). The significant decrease in genotype result turnaround time (β -1.2; 95% CI: -2.3, -0.2; p < 0.05) and time to dose recommendation documentation in EHR (β -4.2; 95% CI: -5.3, -3.2; p < 0.01) remained consistent on adjusted analysis. In the majority of instances, genotype results were available prior to the time of second dose administration in both periods (mPGx: 82.1%, oPGX: 93.7%). Significant improvement was seen on adjusted analysis for the proportion of genotype results available before the second dose (OR: 2.8; 95% CI: 1.6–5.0; p < 0.01). More than 95% of PGPCS dose recommendations were available within 24 h in both phases (96.4% of mPGx consults vs 98.6% of oPGx consults, p < 0.05) ( & ). No significant differences were observed in secondary outcomes for genotyped versus nongenotyped patients within the oPGx cohort.
Warfarin orders were most frequently placed by general medicine practitioners in both mPGx and oPGx periods (i.e., a total of 558 and 562 warfarin orders were made, respectively). PGPCS dose recommendation for a substantial amount of these orders were well accepted by general medicine physicians in both mandatory (84.9%) and optional phases (87.7%). Similarly, orthopedic physicians accepted most PGPCS recommended doses in both phases (mPGx: 82.6% and oPGx: 87.0%). The utilization of genotype information among physician specialty differed during the oPGx period (p = 0.03), with most genotyping orders placed by cardiologists and the least by orthopedic physicians (55.4 vs 45.8%). Most physician specialties including cardiology and general medicine specialties ordered genotyping for more than half of their warfarin orders during the optional period. Overall, improvements in dose acceptance rates were observed from mPGx to oPGx regardless of physician specialty, except for cardiology ().
Table 5. Utilization and acceptance of pharmacogenetic information by physician specialty.
Discussion
To our knowledge, this is the first study evaluating clinical acceptance and feasibility of a PGPCS transitioning to optional genotyping from mandatory genotyping in patients newly initiated on warfarin. These results show that clinicians still preferred to order genotyping for more than half of eligible patients even after the PGPCS service converted to optional genotyping. PGPCS dosing recommendations were well accepted, with improvement in feasibility outcomes with optional genotyping.
It was expected that genotype testing ordered in an optional setting would lead to a decrease in testing volume. However, despite transitioning to optional genotyping, clinicians still ordered genotype testing for a considerable segment of eligible patients (52.1%). Interestingly, following transition to optional genotyping, feasibility outcomes improved, which could be a potential indication of increased clinical utility of warfarin genotyping in optional testing settings. The concordance between PGPCS recommendations and warfarin dose prescribed by medical staff also improved during the oPGx period compared with the mPGx period. This might have been because physicians who selectively ordered pharmacogenomic testing were more willing to accept pharmacist-recommended doses based on genotype. Additionally, both secondary outcomes of genotype turnaround time and time to pharmacist dose recommendation documentation in the EHR also improved in the oPGx cohort. Decrease in the genotype orders during optional phase could have contributed to the shorter genotype turnaround time. Owing to considerable challenges of establishing an interdisciplinary system including obtaining genotype results in a timely manner, it is expected that such interdisciplinary interventions required time to be adopted and to improve efficiency [Citation31]. Improvements in PGPCS service feasibility during the oPGx period could also partially have been attributed due to a learning effect of the system to the new service implementation. This service implementation seems robust and promising given that service feasibility was not negatively influenced by changes in genotype ordering procedures.
Switching from mPGx to oPGx may also have been only one of multiple factors that led to improved operation and increased acceptance of the PGPCS. We performed a stratified analysis by primary medical service, a possible confounding factor, to assess if other influential factors were present. Provider decision to order genotype testing during the oPGx phase was not found to be associated with the physician specialty. Additionally, multivariable regression analyses adjusting for any baseline differences and potential confounders between the groups were also performed. Whether the statistical approaches employed in this study were sufficient to address unmeasured confounders is debatable, these results suggest that PGPCS acceptance and feasibility remained high despite changes to testing approach, and that such an optional testing approach can potentially be applied to other academic hospitals.
The involvement of clinical pharmacists and the use of their clinical knowledge in dose recommendation is also likely a determinant factor in the acceptance of the PGPCS service. Although, the clinical utility of genotype-guided warfarin dosing remains somewhat controversial [Citation10], preliminary results from UIH’s implementation experience suggest that a pharmacist-guided pharmacogenetics service may be key to improving warfarin therapy clinical outcomes [Citation32,Citation33]. In settings where financial or procedural burdens of pharmacogenetics service implementation and uncertainty of the clinical benefits of genotyping are considered barriers, this study suggests that pharmacogenetic testing implementation can be adopted as an optional procedure with the involvement and the expertise of clinical pharmacists.
One limitation to this study is the potential lack of generalizability. The data used were from one tertiary care hospital, which may reduce the generalizability of the implementation model to other types of clinical settings and health systems. Further investigation is also needed to elucidate factors impacting provider decisions in selecting and ordering genotype testing.
Conclusion
This study demonstrates that a PGPCS for warfarin was well accepted by medical staff, and operational feasibility improved after transitioning to an optional genotyping model. Improvement in clinical acceptance and feasibility outcomes of a PGPCS service, despite changes to genotyping approach, potentially indicates increased clinical utility of a pharmacist-guided consult service coupled with optional genotyping. Future studies should confirm the feasibility of such a model to other gene–drug pairs.
First of its kind study to evaluate clinician acceptance and feasibility when genotype testing for warfarin changed from a mandatory to an optional model.
Recognizing the importance of genotyping, UIH implemented a pharmacist guided personalized consult service (PGPCS) to guide warfarin dosing. PGPCS involved initial mandatory genotyping which was changed to an optional genotyping model due to inconsistent research on efficacy of mandatory genotyping. This study aimed to evaluate clinical acceptance and feasibility of a PGPCS service after its transition from mandatory to optional genotyping.
Physicians ordered genotyping for more than half of the eligible population even though PGPCS transitioned from a mandatory (mPGx) to optional genotyping (oPGx) model.
Providers ordered genotyping for 275 patients out of 528 individuals (52.1%) during the oPGx period even after genotyping changed from mandatory to optional.
Increase in clinician acceptance rate of recommended warfarin doses by the PGPCS when the service turned to an optional genotyping model.
Provider acceptance of warfarin dosing recommendation by PGPCS improved during oPGx period (83.9%–86.6%; p < 0.01). We hypothesized that physicians were more inclined in accepting the dosing recommendation because of the expertise of clinical pharmacists involved in driving this service.
Dosing recommendations based on genotype results had a higher acceptance rate by physicians during the optional genotyping model.
Physicians accepted about 87% of all genotype-guided dosing recommendations. A potential reason for this high acceptance rate may be that providers who chose to genotype patients were more willing to accept genotype-guided dosing.
Operational feasibility of the service improved during oPGx period.
There was a significant reduction in the median time from the genotype order to posting of the lab result (25.0 to 23.6 h, p < 0.01) and time from the consult to the making the dosing recommendation (9.83 to 5.72 h, p < 0.01). The reduction in time from the consult to the dosing recommendation can be explained by increased awareness and comfort of physicians with the service.
About 99% of the time the genotype-guided dose recommendation was available within 24 h of the consult and about 94% of the time the genotype results were available before the time for the second warfarin dose. Improvement in feasibility of the service could be partially attributed to increased staff efficiency over time.
Demonstrates the utility of a pharmacist driven clinical consult service with optional genotyping.
This study illustrates that pharmacogenetic testing can be operationalized in an optional model when coupled with the expertise of specialized clinical pharmacists.
Further studies are needed to evaluate the feasibility of such a service to guide with dosing of other gene–drug pairs.
Our future work will focus on assessing the impact of optional genotyping on patient related and clinical outcomes.
Our next step is to evaluate efficacy of genotyping in improving clinical outcomes of patients and whether changes in genotype setting of the service impacted patient outcomes.
Ethical conduct of research
The authors state that they have obtained appropriate Institutional Review Board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Zhu J , AlexanderGC , NazarianS , SegalJB , WuAW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010–2017. Pharmacotherapy38(9), 907–920 (2018).
- Cohen AL , BudnitzDS , WeidenbachKNet al. National Surveillance of Emergency Department visits for outpatient adverse drug events in children and adolescents. J. Pediatr.152(3), 416–421.e2 (2018).
- Kim MM , MetlayJ , CohenAet al. Hospitalization costs associated with warfarin-related bleeding events among older community-dwelling adults. Pharmacoepidemiol. Drug Saf.19(7), 731–736 (2010).
- Shehab N , LovegroveMC , GellerAI , RoseKO , WeidleNJ , BudnitzDS. US Emergency Department visits for outpatient adverse drug events, 2013–2014. JAMA316(20), 2115–2125 (2016).
- Williams DB , KarlRC. A simple technic for predicting daily maintenance dose of warfarin. Am. J. Surg.137(4), 572–576 (1979).
- Fennerty A , DolbenJ , ThomasPet al. Flexible induction dose regimen for warfarin and prediction of maintenance dose. Br. Med. J. Clin. Res. Ed.288(6426), 1268–1270 (1984).
- Doecke CJ , CoshDG , GallusAS. Standardised initial warfarin treatment: evaluation of initial treatment response and maintenance dose prediction by randomised trial, and risk factors for an excessive warfarin response. Aust. NZJ Med.21(3), 319–324 (1991).
- Dahal K , SharmaSP , FungEet al. Meta-analysis of randomized controlled trials of genotype-guided vs standard dosing of warfarin. Chest148(3), 701–710 (2015).
- pi_coumadin.pdf (2020). https://packageinserts.bms.com/pi/pi_coumadin.pdf
- Kimmel SE , FrenchB , KasnerSEet al. a pharmacogenetic versus a clinical algorithm for warfarin dosing. N. Engl. J. Med.369(24), 2283–2293 (2013).
- Pirmohamed M , BurnsideG , ErikssonNet al. A randomized trial of genotype-guided dosing of warfarin. N. Engl. J. Med.369(24), 2294–2303 (2013).
- Gage BF , BassAR , LinHet al. Effect of genotype-guided warfarin dosing on clinical events and anticoagulation control among patients undergoing hip or knee arthroplasty: the GIFT Randomized Clinical Trial. JAMA318(12), 1115 (2017).
- Syn NL , WongAL-A , LeeS-Cet al. Genotype-guided versus traditional clinical dosing of warfarin in patients of Asian ancestry: a randomized controlled trial. BMC Med.16(1), 104 (2018).
- Hillman MA , WilkeRA , YaleSHet al. A prospective, randomized pilot trial of model-based warfarin dose initiation using CYP2C9 genotype and clinical data. Clin. Med. Res.3(3), 137–145 (2005).
- Anderson JL , HorneBD , StevensSMet al. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II). Circulation125(16), 1997–2005 (2012).
- Burmester JK , BergRL , YaleSHet al. A randomized controlled trial of genotype-based Coumadin initiation. Genet. Med.13(6), 509–518 (2011).
- Pengo V , ZambonC-F , FogarPet al. A randomized trial of pharmacogenetic warfarin dosing in naïve patients with non-valvular atrial fibrillation. PLoS ONE10(12), e0145318 (2015).
- Wang M , LangX , CuiSet al. Clinical application of pharmacogenetic-based warfarin-dosing algorithm in patients of Han nationality after rheumatic valve replacement: a randomized and controlled trial. Int. J. Med. Sci.9(6), 472–479 (2012).
- Caraco Y , BlotnickS , MuszkatM. CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clin. Pharmacol. Ther.83(3), 460–470 (2008).
- Jonas DE , EvansJP , McLeodHLet al. Impact of genotype-guided dosing on anticoagulation visits for adults starting warfarin: a randomized controlled trial. Pharmacogenomics14(13), 1593–1603 (2013).
- Wen M-S , ChangK-C , LeeT-Het al. Pharmacogenetic dosing of warfarin in the Han-Chinese population: a randomized trial. Pharmacogenomics18(3), 245–253 (2017).
- Borgman MP , PendletonRC , McMillinGAet al. Prospective pilot trial of PerMIT versus standard anticoagulation service management of patients initiating oral anticoagulation. Thromb. Haemost.108(3), 561–569 (2012).
- Anderson JL , HorneBD , StevensSMet al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation116(22), 2563–2570 (2007).
- Huang S-W , ChenH-S , WangX-Qet al. Validation of VKORC1 and CYP2C9 genotypes on interindividual warfarin maintenance dose: a prospective study in Chinese patients. Pharmacogenet. Genomics19(3), 226–234 (2009).
- Downing A , MortimerM , HiersJ. Impact of a pharmacist-driven warfarin management protocol on achieving therapeutic international normalized r{atios. Am. J. Health Syst. Pharm.73(1 Suppl. 5), S69–S73 (2016).
- Damaske DL , BairdRW. Development and implementation of a pharmacist-managed inpatient warfarin protocol. Proc. Bayl. Univ. Med. Cent.18(4), 397–400 (2005).
- Dager WE , BranchJM , KingJHet al. Optimization of inpatient warfarin therapy: impact of daily consultation by a pharmacist-managed anticoagulation service. Ann. Pharmacother.34(5), 567–572 (2000).
- Manzoor BS , ChengW-H , LeeJC , UppuluriEM , NutescuEA. Quality of pharmacist-managed anticoagulation therapy in long-term ambulatory settings: a systematic review. Ann. Pharmacother.51(12), 1122–1137 (2017).
- Zhou S , ShengXY , XiangQ , WangZN , ZhouY , CuiYM. Comparing the effectiveness of pharmacist-managed warfarin anticoagulation with other models: a systematic review and meta-analysis. J. Clin. Pharm. Ther.41(6), 602–611 (2016).
- Saokaew S , PermsuwanU , ChaiyakunaprukN , NathisuwanS , SukonthasarnA. Effectiveness of pharmacist-participated warfarin therapy management: a systematic review and meta-analysis. J. Thromb. Haemost.8(11), 2418–2427 (2010).
- Nutescu EA , DrozdaK , BressAPet al. Feasibility of implementing a comprehensive warfarin pharmacogenetics service. Pharmacother. J. Hum. Pharmacol. Drug Ther.33(11), 1156–1164 (2013).
- Edith N , DuarteJ , ChengWet al. Abstract 16119: novel genotype guided personalized warfarin service improves outcomes in an ethnically diverse population. Circulation130(Suppl. 2), A16119–A16119 (2014).
- Kim K , GorD , WaltonSMet al. Novel pharmacist-guided pharmacogenetic service lowers warfarin-related hospitalizations. Value Health18(3), A130 (2015).