Abstract
The complex nature of depression is mirrored by difficulties in tailoring its treatment. Key underlying mechanisms of this mental disorder include elevated inflammation and a dysregulated hypothalamic–pituitary–adrenal (HPA) axis. More recently, the endocannabinoid system has been proposed as another important component in the pathogenesis of depression, and strong evidence suggests that all three systems communicate with each other. A growing number of genetic studies have investigated polymorphisms in depression in each of these systems separately. However, no study to date has looked at these genes in conjunction. In this article we will review the crosstalk between the endocannabinoid system, immune system and HPA axis; and discuss the evidence of gene polymorphisms and their relation to the risk of depression and its treatment. We propose future directions where genes of these three systems are considered from a joint perspective to improve prediction of treatment response, taking into account potentially overlooked genetic variations.
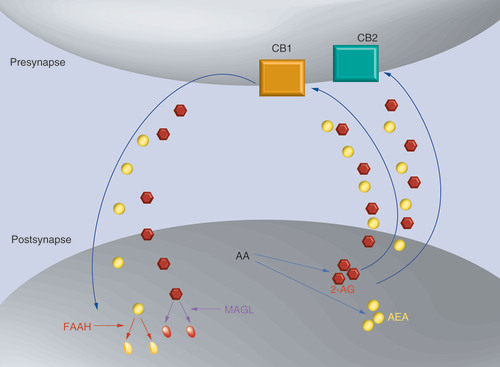
2-AG and AEA are synthesized on demand from AA. Endocannabinoids travel from the postsynapse to the presynapse and bind to CB1 and CB2 receptors. Endocannabinoids are transported back to the postsynapse (mechanism unknown – elusive transporter) where they are degraded by their enzymes, MAGL and FAAH, respectively.
2-AG: 2-arachidonoylglycerol; AA: Arachidonic acid; AEA: Anandamide; CB1: Cannabinoid receptor type 1; CB2: Cannabinoid receptor type 2.
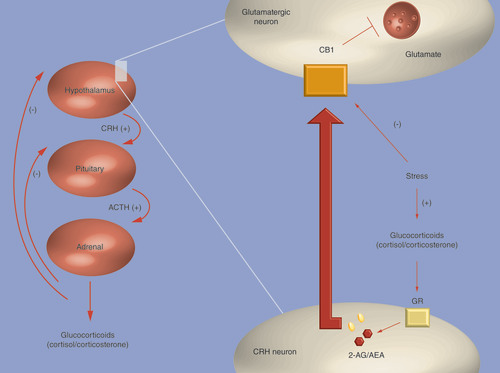
The hypothalamus–pituitary–adrenal axis is shown on the left, and the role of endocannabinoid system in the negative feedback loop is shown on the right. Activation of membrane-associated GR on CRH neurons leads to an increase in AEA and 2-AG. AEA and 2-AG travel across the synaptic cleft to CB1 receptors on the postsynaptic glutamatergic neurons, subsequently inhibiting their activity, and therefore suppressing the release of CRH. In chronic stress, there is a downregulation of CB1 receptors.
2-AG: 2-arachidonoylglycerol; ACTH: Adrenocorticotropic hormone; AEA: Anandamide; CB1: Cannabinoid receptor type 1; CRH: Corticotrophin-releasing hormone; GR: Glucocorticoid receptor.
The severity of depression continuously prompts researchers to elucidate its underlying mechanisms. Several systems have been considered, starting with the monoaminergic one, central to most current antidepressant treatments Citation[1]. Considerations about elevated inflammation Citation[2–6] and altered stress responses manifested by a dysregulated activity of the hypothalamic–pituitary–adrenal (HPA) axis then followed Citation[7–9]. More recently, studies have found that depression can also occur with alterations of the endogenous cannabinoid/endocannabinoid (eCB) system, a homeostatic signaling system with neuromodulatory properties Citation[10–13]. Genetic approaches have looked at gene polymorphisms in each of the aforementioned systems, although no studies have evaluated these from a joint perspective. This review will focus on the pharmacogenomics of the eCB and the immune systems, and that of the HPA axis. We will start with a brief description of the eCB system and its links to depression, we then describe the putative role of the immune system and the HPA axis in relation to this mental disorder, discussing their possible connections with the eCB system. This will be followed by current findings on gene polymorphisms and how they relate to prognosis. Lastly we propose how future research could explore in more depth these three systems in conjunction to further improve our understanding and treatment of depression.
Endocannabinoid system: receptors & ligands
The exploration of biological mechanisms underlying the actions of cannabis, used as a mood enhancer, led to the discovery of the eCB system in animals and humans Citation[11,14]. Two main eCBs are known, namely 2-arachidonoylglycerol (2-AG) and anandamide (AEA), with related pharmacological properties to the major psychoactive phytocannabinoid, Δ-9-tetrahydrocannabinol. The eCBs act mainly on two different receptors, cannabinoid receptor type 1 (CB1) and 2 (CB2; ), but also to a lesser extent on TRPV1 and on other G-protein-coupled receptors Citation[15]. While AEA binds to CB1 with high affinity and weak efficacy, 2-AG has lower affinity but is more potent at inducing inhibitory signaling of target neurons Citation[16]. 2-AG and AEA are produced on demand, as a response to elevated intracellular calcium Citation[16], from arachidonic-acid-containing phospholipids and are degraded postsynaptically by MAGL and FAAH enzymes, respectively Citation[17]. As will be described below, both levels and polymorphisms of receptors and enzymes have been found to be linked to depression.
eCB links to depression
The eCB system, with neuromodulatory properties, has been implicated in mood regulation through its influence on emotional reward and cognitive behaviors as well as its links with the stress response Citation[10,18]. Typically, depression manifests with pathophysiological alterations in signaling pathways in areas associated with emotional regulation. CB1 receptors are commonly found in brain regions involved in stress processing and depression, and are mostly present on GABAergic and glutamatergic synapses Citation[18–20]. In particular, they have been described on glutamatergic neurons in the paraventricular nucleus (PVN) of the hypothalamus, which takes part in modulating the stress response via the HPA axis Citation[18,21]. CB2 receptors have been detected in the brain and are commonly found in the peripheral immune system Citation[13]. Interestingly, both the immune system Citation[3,5] and the HPA axis Citation[7,22,23] have been described as dysregulated in depression, and will be described in further detail below. CB1 receptors have been shown to selectively regulate behavioral and neuroendocrine stress responses in mice, via different glutamatergic neuronal subpopulations within principal forebrain neurons and cortex Citation[20]. Being the most abundant G-protein-coupled receptors in the brain, CB1 receptors help to maintain homeostasis of neural networks, via retrograde signaling Citation[24]. When this homeostasis is disrupted, changes in mood and behavior follow Citation[13,25]. For example, preclinical studies have shown that genetically deleting CB1 receptors in mice resulted in depressive-like behavior Citation[12,26]. Furthermore, preclinical studies have shown that genetic deletion of CB1 receptors as well as their pharmacological blockade upregulated plasma corticosterone levels in both basal and stress conditions. These observations are indicative of a dysregulated HPA axis, which is also seen in depression Citation[27]. In humans, pharmacological blockade of the CB1 receptor has been associated with depressogenic effects. For instance, rimonabant, an inverse CB1 agonist, developed as an antiobesity drug, had to be removed from the market as its side effects included depression and suicide Citation[28]. Interestingly, while rimonabant showed similar antidepressant properties as desipramine in a preclinical model, these two agents had opposite effects on corticosterone levels with rimonabant increasing corticosterone levels Citation[27]. This observation led authors to suggest that CB1 inverse agonists may be more suitable for melancholic depression, which manifests with hypoactivity of the HPA axis. Moreover, the antidepressant properties but not the neuroendocrine effects of desipramine were absent in CB1 knockout mice, further suggesting the role of this receptor in behavioral adaptation to stress response Citation[27]. The other main eCB receptor, CB2, has received particular interest as a pharmacological target in pain and immune modulation Citation[29], with growing evidence from animal studies showing that CB2 receptors play a role in emotional regulation Citation[30,31]. For instance, mice that overexpress CB2 receptors showed decreased depressive-like behaviors when exposed to stressful situations compared with the control group Citation[30]. Furthermore, pharmacological administration of a CB2 agonist produced antidepressant-like effects in rats, as observed by reduced immobility and mechanical hypersensitivity Citation[31]. In addition to receptors, enzymes regulating eCBs also showed links to depression. As such, pharmacological inhibition of FAAH has shown antidepressant-like effects in animal studies Citation[32–34], and also augmented the antidepressant properties of imipramine Citation[33]. Further support for the roles of an eCB deficiency comes from clinical studies showing that depressed patients had significantly lower circulating levels of 2-AG than controls. Interestingly, levels of 2-AG decreased progressively with the duration of the disorder Citation[25].
Communication between the eCB & immune systems
Several observations indicate a complex communication between the eCB and immune systems. As mentioned above, CB1 receptors are present in astrocytes and also in microglia, with CB2 receptors being largely expressed in several cells of the peripheral immune system, including lymphocytes and macrophages, and in glial cells and microglia in areas of the brain including the cerebral cortex, the limbic system and the cerebellum Citation[13,35,36]. Activation of these receptors under cell culture conditions has been shown to regulate specific immune-related functions carried by these cells. However, the overall functionality of both CB1 and CB2 receptors in vivo is still to be defined. Circulating eCBs are affected by, and also affect, the immune response. For example, 2-AG was shown to be produced by immune cells in response to injuries and, through its inhibitory function, it then reduced the inflammatory response of these cells Citation[37]. Microglial cell activation and excitotoxicity were shown to be reduced by 2-AG, highlighting the immunosuppressant effect of these molecules Citation[36]. Furthermore, IL-1β, a cytokine shown to be elevated in depression Citation[22,38] has been shown to inhibit the function of CB1 receptors on both glutamatergic and GABAergic neurons in the rat striatum Citation[39]. In addition to IL-1, levels of circulating protein and mRNA encoding for IL-6 and TNF-α, also described as elevated in depression, were reduced by administering synthetic cannabinoids in a preclinical model of multiple sclerosis treatment Citation[40]. Interestingly, antidepressant treatments are also effective in decreasing circulating cytokine levels Citation[41,42]. These findings suggest common convergent physiological pathways shared between the eCB and immune systems that are yet to be explored in relation to the treatment of depression. Of relevance here, celecoxib, an anti-inflammatory drug that inhibits COX-2, improved depressive symptoms and reduced inflammation when added to antidepressant medication Citation[43]. COX-2 is capable of degrading AEA and 2-AG. At the same time, 2-AG has been shown to reduce gene expression and circulating levels of COX-2 in response to proinflammatory and excitotoxic stimuli in the hippocampus of rats Citation[17,44]. Furthermore, pharmacological administration of substrate-selective COX-2 inhibitors has shown to increase eCB activity and reduce anxiety behaviors in mice Citation[45], again highlighting a link between the eCB and immune systems.
The strength and prevalence of the crosstalk between these systems becomes stronger when we consider enzymes. Elevated levels of MAGL have been associated with inflammation and increased levels of FAAH with both inflammation and mood disorders. Pharmacological inhibition of both enzymes have shown improvement of these disorders Citation[32]. Interestingly, reduced FAAH levels have been shown to produce antidepressant-like effects in animal models Citation[32]. Furthermore, decreasing FAAH levels pharmacologically has been linked with reduction in mRNA expression of lipopolysaccharide-induced proinflammatory cytokines in the hypothalamus of rats Citation[46]. In light of these observations, studies investigating the role of pharmacological inhibition of FAAH in clinical settings, which are yet to be carried out, appear promising.
Communication between the eCB system & the HPA axis
Strong evidence highlights the role of eCB signaling in HPA axis regulation, including effects during basal conditions and also situations of acute and repeated stress response Citation[18,21,47,48]. To better understand the mechanisms through which this crosstalk occurs we will first briefly summarize some of the ways by which a dysregulated HPA axis is thought to contribute to depression. The HPA axis regulates the stress response by controlling the secretions of corticotrophin-releasing hormone (CRH), adrenocorticotropic hormone and glucocorticoids (cortisol in humans), and is tightly controlled by a negative feedback inhibition loop that involves two receptors: the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR). When levels of glucocorticoids are up, this feedback loop signals the axis to downregulate itself (see , left). In depression, glucocorticoids may not bind to their receptors, leading to hyperactivity of the HPA axis and increased levels of circulating glucocorticoids Citation[7]. Interestingly, activation of membrane-associated GR on CRH neurons in the PVN leads to an increase in both AEA and 2-AG. AEA and 2-AG travel across the synaptic cleft to CB1 receptors on the postsynaptic glutamatergic neurons, subsequently inhibiting their activity, and therefore suppressing the release of CRH. This process, known as fast feedback loop, is part of the HPA negative feedback inhibition loop Citation[18,21]. Repeated exposure to stress has been shown to downregulate CB1 receptors in glutamatergic synapses on parvocellular neuroendocrine cells in the PVN of the hypothalamus in rats. This mechanism was mediated by increased levels of corticosterone and intracellular GR activation. Specifically, CB1 receptor downregulation has been shown to reduce the negative feedback loop inhibition within the HPA axis (see ) Citation[48,49]. The eCB system also regulates the HPA basal activity via CB1 receptors in the basolateral nucleus of the amygdala, with evidence coming from studies demonstrating that administration of CB1 receptor antagonists resulted in elevated HPA axis activity Citation[18,27]. In situations of an acute stress response, an increased activity of the HPA axis has been linked with increased FAAH levels and decreased AEA in the amygdala Citation[18]. Interestingly, in animal models, pharmacological administration of FAAH inhibitors or CB1 agonists in the basolateral nucleus of the amygdala attenuated the stress response and reduced HPA axis Citation[50].
Gene polymorphisms & treatment response
▪ Endocannabinoid system
CNR1 & CNR2 genes
Several studies have looked at the genes coding for CB1 and CB2, namely CNR1 and CNR2, in relation to depression. Specifically, they investigated SNPs as well as haplotype blocks of the aforementioned genes and trinucleotide repeats in the 3´-UTR of the CNR1 gene Citation[51–55]. Two CNR1 mRNA splice variants have been described that modify protein expression and lead to the formation of two additional variants of the CB1 receptor, CB1a and CB1b Citation[56]. They are structurally different, therefore having different binding properties to eCBs, whereby AEA binds with lower affinity and 2-AG acts as an inverse agonist for both receptors, CB1a and CB1b Citation[19]. While no studies have looked at splice variants of CB1 receptors in relation to depression, several SNPs of CNR1 and CNR2 genes have been investigated: CNR1 rs1049353 Citation[52,53,57,58], CNR1 rs806371 Citation[52], CNR2 Q63R Citation[59] and CNR2 rs2501431 Citation[51]. Some of the described SNPs are synonymous, and while they do not affect the final amino acid incorporated, they may influence mRNA translation, structure and stability. Some also occur with other SNPs in linkage disequilibrium, in the form of haplotype blocks. Interestingly, evidence suggests that CNR1 gene higher haplotype frequency consisting of minor allele C at rs806368, major allele G at rs1049353 and major allele T at rs806371 (C–G–T) as well as two long alleles with more than 16 AAT´ trinucleotides in the CNR1 gene were associated with lower susceptibility for depression Citation[52,60]. Several polymorphisms have been related to serotonin-based treatment response Citation[51–53,61]. For instance, homozygosity for the major T alleles at rs806368 and rs806371 of CNR1 was associated with increased risk of no remission following treatment compared with carriers of the minor C and G alleles, respectively Citation[52]. Homozygosity for the major A allele of rs2501431 in the CNR2 gene has been found to be associated with increased severity of depressive symptoms in the follow-up of patients who underwent 12 weeks of antidepressant treatment Citation[51]. In another study, haplotype block 1 of the CNR1 gene (rs806368–rs1049353–rs806371, combination C–G–G [minor–major–minor]alleles), was associated with higher remission rates following treatment Citation[52]. While homozygosity for the major G allele of CNR1 rs1049353 in males predicted better antidepressant response, in another study the presence of the major allele predicted worse response in females, particularly in those with high comorbid anxiety Citation[51,53]. Furthermore, fMRI studies have shown that CNR1 rs1049353 major allele has been implicated in weaker striatal response to happy faces, a symptom commonly found in depression Citation[53]. These findings suggest that functional polymorphisms in eCB system genes are, at least to some extent, specific to symptoms of depression rather than diagnosis. To further confirm this hypothesis, haplotype ATTCAAATTC at rs806379 (A major allele), rs1535255 (T major allele), rs2023239 (T major allele), rs806369 (C major allele), rs1049353 (A minor allele), rs4707436 (A minor allele), rs12720071 (A major allele), rs806368 (T major allele), rs806366 (T major allele) and rs7766029 (C minor allele), was positively associated with neuroticism and depression and negatively with agreeableness whereas the opposite results were found for haplotype ATTTGGATCT. Furthermore, the same study demonstrated SNP–SNP interactions such as rs806379–rs7766029, rs806379–rs4707436, rs806379–rs1049353, rs806379–rs806369 and rs806379–rs806366 to be associated with both neuroticism and agreeableness, and rs1535255–rs12720071 as well as rs2023239–rs12720071 to be linked with agreeableness only Citation[54]. As reported in previous studies, high neuroticism and low agreeableness are part of a phenotypic profile of depression and can affect an individual‘s way of interpreting events in a less favorable way, putting them at risk of depression Citation[54,62,63]. Interestingly, individuals who experienced physical abuse in childhood were more likely to develop symptoms of anhedonia if they had rs1049353 GG genotype compared with AG and AA Citation[58]. By contrast, in a study comparing parasuicidal patients and healthy controls, AA genotype carriers of rs1049353 (minor alleles) were only found among patients; however, this constituted only 5.2% of patients Citation[64]. These findings suggest that genetic polymorphisms may influence trajectories in which depression develops and that personality variations may play a part in this process. Indeed, individuals who developed anhedonia went on to develop lifetime depression Citation[58]. As such, it is plausible to assume that these individuals who experienced physical abuse in childhood processed similar experiences in different ways and developed different behavioral responses, depending on their particular genotype. In line with this thought process, homozygosity for the A allele of rs1049353 in the CNR1 gene may put carriers of this genotype at risk of parasuicide by affecting the way they process negative life events Citation[64]. In addition, recent negative life events have been shown to interact with the minor T allele of rs7766029 in the CNR1 gene in predicting depression Citation[54]. These data could inform the path of personalized medicine by looking at symptoms and personality traits present in depression as individual variables, together with their association with genetic polymorphisms, which would allow for the more effective tailoring of treatments.
FAAH gene
Extensive research has investigated genetic polymorphisms in FAAH in various disorders that have been linked to depression, including addictive behaviors, post-traumatic stress disorder, metabolic syndrome, diabetes and obesity Citation[65–68]. As such, rs2295633 C ancestral allele was significantly associated with post-traumatic stress disorder diagnosis in war veterans who suffered combat-related penetrating traumatic brain injury Citation[65]. Furthermore, heterozygosity of FAAH gene at rs324420 C/A showed a significant association with risks of methamphetamine dependence in Malaysian and Chinese populations Citation[68]. Minor allele at rs324420 was associated with obesity and cardiovascular risk factors, with noncarriers of the allele A385 having a bigger decrease of insulin postdiet Citation[66], and also associated with a decreased risk of metabolic syndrome Citation[67]. Further studies have reported mixed results for rs324420 association with depression. For instance, major allele at rs324420 showed increased emotional reactivity to threat stimuli Citation[69]. By contrast, another study reported A minor allele carriers to have increased emotional responsiveness towards unpleasant pictures Citation[70]. Finally, a genotypic association was found between heterozygous AC rs324420 and depression Citation[57]. Having the minor allele at rs324420 has been shown to reduce the enzymatic activity of FAAH by approximately 50%, leading to increased AEA levels Citation[69]. Of relevance here, strong evidence from animal studies indicates that elevated levels of AEA and FAAH inhibition produce antidepressant-like effects Citation[32]. In light of pharmacological evidence and inconclusive genetic findings, a thorough investigation of the role of FAAH gene polymorphisms in depression warrants further efforts.
▪ Immune system
While the research literature investigating gene polymorphisms of the immune system in depression treatment is extensive, the results remain inconclusive Citation[71]. We will briefly describe two genes: IL-1β and COX-2. IL-1β is of particular interest owing to its multiple cellular properties Citation[3,72] and its links with several mental disorders Citation[73]. COX-2 was selected because of its extensive involvement in the metabolism of eCBs as described above Citation[17,43,45].
IL-1β gene
Several polymorphisms of the IL-1β gene have been associated with depression and antidepressant treatment response. Individuals homozygous for the T allele of the -511C/T polymorphism (rs16944) have been shown to have delayed onset of depression in geriatric samples compared with C allele carriers Citation[74]. In another study, homozygosity for the G allele at rs16944 and A allele at rs1143627 were linked with major recurrent depression Citation[75]. Interestingly, there was a genotypic association between no symptom remission following antidepressant treatment and homozygosity for the G alleles at rs16944 and rs1143643 Citation[73]. Interestingly, early onset of depression was not associated with any variants at rs1143627, rs13032029, rs1143623, rs1143634 and rs3917368 when analyzed individually and in haplotype Citation[76]. Gene polymorphisms of IL-1β have also shown to be linked with responses of the HPA axis and eCB system. Following the administration of dexamethasone (in the test commonly used to evaluate the HPA axis negative feedback mechanism), carriers of minor allele T at rs1143633 and major allele G at rs16944 showed increased cortisol levels Citation[77]. Homozygosity for the rs16944 G allele and rs1143643 G allele were linked with decreased activity of the limbic system and anterior cingulate cortex to facial expressions of various emotions, similar to that observed for CNR1 gene polymorphisms and response to emotional stimuli Citation[53,73]. Findings from the aforementioned studies further suggest links between the immune system with both HPA axis and eCB systems. This crosstalk could suggest that all three systems are part of the same process that underpins the pathophysiology of depression.
▪ COX-2 gene
While COX-2 polymorphisms have been extensively studied in relation to several conditions Citation[78,79], very few have looked at mental health disorders, including neurodegenerative ones. While no associations with risk of Alzheimer‘s disease were found for -765G/C (rs20417) and 8473T/C (rs5275), the -1195G/A (rs689466) polymorphism was found to be associated with risk, with the major A allele representing a protective factor Citation[80]. Specifically in relation to depression, individuals with rs4648308 AG genotype were shown to be at increased risk of developing depression following IFN-α treatment for hepatitis C Citation[81]. Similarly, the -765G major allele and the G-765G genotype have been shown to be more prevalent in those who are at increased risk of recurrent depression Citation[82]. No allelic and genotypic associations were found between rs4648276, rs2066826 or rs689466 and treatment resistance, response or remission in a multicenter resistant depression study Citation[83]. Similarly, no significant associations were found for rs5275 or rs20417 Citation[84].
▪ HPA axis
A number of studies have looked at polymorphisms of different genes involved in the HPA axis, starting with the GR, which regulates the axis activity. The GR protein interacts with several cochaperone molecules. One of these, FK506 binding protein 5 (FKBP5), tightly regulates GR sensitivity Citation[85] and has emerged from several genetic association studies as an important candidate gene for depression pathogenesis. We will focus on polymorphisms of both GR and FKBP5.
GR gene
Several SNPs of the GR gene that affect HPA axis function and relate to depression have been described. Several alleles have been found to alter the function of the GR. Among these, associations have been reported for N363S minor G allele (rs6195), minor allele at A3669G (rs6198) and minor A allele at ER22/23EK, sometimes referred as R23K (rs6189 and rs6190) Citation[86]. In particular, BclI minor G allele and ER22/23EK minor A allele have been associated with unipolar depression. In addition, the ER22/23EK polymorphism (GAG AGG>GAA AAG) was also associated with a decreased risk of dementia in healthy individuals Citation[87]. Moreover, carriers of the minor allele at ER22/23EK have been shown to have higher expression of the active GR translational variant GR-α and showed reduced glucocorticoid sensitivity, as measured by the dexamethasone suppression test. An enhanced sensitivity to glucocorticoids, also demonstrated by the dexamethasone suppression test, was seen in carriers of minor alleles BclI C/G and N363S A/G polymorphisms. In addition, N363S minor allele has been associated with increased BMI and low-density lipoprotein cholesterol levels, as well as increased risk of cardiovascular disease. Finally, A3669G minor allele, also referred as 9β A/G, has been associated with increased expression and stabilization of the dominant negative splice variant GR-α Citation[88]. The prevalence of depression in patients with chronic coronary heart disease increased from noncarriers to heterozygotes to homozygotes for one haplotype that includes the minor allele of the 9β A/G polymorphism. This association in a gene-dosage-dependent manner might be a vulnerability factor for depression Citation[89]. A different haplotype, in the regulatory region of the GR, was associated with increased risk of hospital admission for depressive disorders in a study that followed participants over 35 years Citation[90]. Two further haplotypes, covering the entire coding region and 3´-UTR of the GR have been described as associated with bipolar disorder Citation[91]. Finally, a gene–environment interaction between the GR polymorphisms 22/23EK and 9β heterozygotes and childhood adversity was described as resulting in an increased risk of clinically relevant depressive symptoms Citation[92].
FKBP5
Genetic polymorphisms of the FKBP5 gene have been described in major depression and antidepressant treatment. FKBP5 rs1360780 minor T allele has been shown to be associated with lower FKBP5 mRNA expression following GR stimulation with dexamethasone in depressed patients compared with healthy controls. Interestingly, HPA axis dysregulation, manifested in elevated levels of cortisol and adrenocorticotropic hormone following dexamethasone stimulation, was only present in depressed individuals with minor allele and not in healthy controls with the same polymorphism. These findings suggest that GR sensitivity in major depression is genotype dependent Citation[93]. Preclinical studies have shown that antidepressant treatment led to a reduction of FKBP5 mRNA gene expression levels previously increased by chronic mild stress Citation[94], with clinical findings also showing that antidepressant response is associated with FKBP5 mRNA decrease Citation[95]. Several polymorphisms of this gene have been associated with treatment response in depression. For instance, individuals with minor A allele at rs4713916 were more likely to remit and respond to treatment Citation[96,97]. Carriers of minor T allele rs1360780 in the Caucasian sample and CC (major allele) genotype in the mixed ethnicity sample showed better response to antidepressant treatment as did carriers of the minor C allele at rs3800373 Citation[98,99]. Two other studies, however, showed that variants of FKPB5 at rs3800373 and rs1360780 were not associated with antidepressant response, and C allele of rs3800373 showed a trend towards worse response Citation[100,101]. These contradictory findings may be due to variation between samples in regards to age. Of particular relevance, homozygosity for the T allele has been shown to have a more predictive validity on treatment response when combined with SNPs from the glutamate receptor, GRIK4, and the serotonin receptor, HTR2A Citation[102]. Similarly, we would expect that a joint evaluation of SNPs in genes of the HPA axis, the eCB and the immune systems would increase our predictive power of treatment response.
Future perspective
Considering that most studies looking at gene polymorphisms within the HPA axis, the eCB and the immune system in relation to depression have looked at SNPs, future research would benefit from considering other types of genetic variation such as copy number polymorphisms, deletions, mRNA splice variants of existing genes and trinucleotide polymorphisms, particularly because of their ability to produce functional cellular changes. Little attention has been given to enzymes that take part in eCB synthesis processes, such as DAGL and NAPE-PLD Citation[103]. Exploration of these enzymes in pharmacogenetics could provide further information to improve our approaches of personalized medicine in depression. Furthermore, it would be important to explore CNR2 and COX-2 gene polymorphisms in more depth, especially considering the distribution and function of CB2 receptors as well as the role of the COX-2 enzyme in the metabolism of eCBs. While gathering large samples can be challenging it should certainly be pursued in efforts to better understand and treat the complex condition that is depression. Given that evaluating a combination of gene polymorphisms predicted antidepressant response better than considering each SNP alone Citation[102], investigating gene polymorphisms of the HPA axis, eCB and immune systems together, in an interactive way, could be a way forward in the pharmacogenomics of depression. This is particularly relevant owing to the interplay between these systems suggesting that they may be a part of a bigger pathophysiological mechanism underlying depression.
The endocannabinoid system & its links to depression
▪ The endocannabinoid (eCB) system is a neuromodulatory system; its deficiency has been linked with depression in preclinical and clinical studies.
Communication between the eCB & immune systems
▪ eCB receptors CB1 and CB2 are expressed in the brain and immune system, and regulate immune-related functions.
▪ Levels of mRNA for proinflammatory cytokines elevated in depression are reduced by both synthetic cannabinoids and antidepressants.
▪ COX-2 can degrade anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and pharmacological inhibition of COX-2 reduces depression and anxiety. Similarly, pharmacological inhibition of FAAH improves mood and also reduces inflammation.
▪ Inflammation induces 2-AG release, which reduces immune response, including COX-2 levels.
Communication between the eCB system & the hypothalamic–pituitary–adrenal axis
▪ The eCB system takes part in the regulation of basal activity and acute stress of the hypothalamic–pituitary–adrenal (HPA) axis.
▪ eCB signaling is involved in the regulation of the fast feedback loop, part of the HPA negative feedback loop via glucocorticoid signaling.
▪ Chronic exposure to stress dampens eCB signaling and its ability to effectively take part in HPA axis regulation.
Gene polymorphisms & treatment response
▪ eCB system
– Risk of depression was associated with allelic and/or haplotypic variations of CNR1 and FAAH genes.
– TT homozygosity of CNR1 at rs806368 and rs806371 was associated with increased risk of no remission following treatment.
– Haplotype block 1 of the CNR1 gene (rs806368–rs1049353–rs806371, which is a combination C–G–G [minor-major-minor] alleles), was associated with higher remission rates following treatment.
– There is evidence of genotype-dependent variation in treatment response by gender.
– There is evidence that individually considered clinical symptoms, personality traits, responses to environmental stimuli, risk and severity of depression are associated with SNPs and haplotype blocks of the CNR1 gene.
▪ Immune system
– IL-1β T homozygous-511 patients showed milder symptoms of depression, were more likely to have delayed onset of depression (geriatric sample) and responded better to antidepressants than C carriers.
– No symptom remission following antidepressant treatment was observed in individuals who were homozygous for the G allele of rs16944 and rs1143643 in the IL-1β gene.
– IL-1β gene polymorphisms are associated with increased HPA activity and emotional reactivity.
– No allelic or genotypic associations were found between COX-2 rs4648276, rs2066826, rs689466, rs5275 or rs20417 and treatment resistance, response or remission; however, polymorphisms associated with risk of developing depression following IFN-α treatment for hepatitis C and recurrent depression were reported.
▪ HPA axis
– Glucocorticoid receptor (GR) ER22/23EK minor allele carriers have reduced glucocorticoid sensitivity and increased risk of unipolar depression.
– Minor alleles of BclI and N363S polymorphisms are associated with enhanced sensitivity to glucocorticoids, and the Bcll minor allele is associated with unipolar depression.
– Allelic association was found between 9βA/G and depression in patients with chronic coronary heart disease; gene–environment interaction between 22/23EK and 9β polymorphisms and childhood adversity increased risk of developing depressive symptoms.
– FKBP5 rs4713916 and rs352428 are more likely to respond to antidepressants.
– Homozygosity for TT at rs1360780 has more predictive validity on treatment response when combined with SNPs from the glutamate receptor, GRIK4, and the serotonin receptor, HTR2A.
Future perspective
▪ Future studies should explore genetic variation in haplotypes, mRNA splice variants, genetic deletions, trinucleotide repeats and copy numbers. More research should look at gene polymorphisms in enzymes that take part in eCB synthesis and degradation as well as the CNR2 gene.
▪ Large-scale studies should focus on investigating gene polymorphism interactions of the HPA axis, eCB and immune systems jointly.
Acknowledgements
The authors are grateful to N Bray for his critical reading of this manuscript. Special thanks to MB Elias who helped with the figures.
Financial & competing interests disclosure
PA Zunszain is funded by the South London and Maudsley NHS Trust and King‘s College Hospital NIHR Biomedical Research Centre for Mental Health. PA Zunszain has received research funding and speakers fees from companies interested in developing antidepressants or anti-inflammatory strategies for the treatment of depression, such as Janssen Pharmaceuticals and Servier, but the data reviewed in this paper is unrelated to this funding. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Anderson IM . Pharmacological treatment of unipolar depression. Curr. Top. Behav. Neurosci.14 , 263–289 (2013).
- Haroon E , RaisonCL, MillerAH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology37(1) , 137–162 (2012)
- Zunszain PA , HepgulN, ParianteCM. Inflammation and depression. Curr. Top. Behav. Neurosci.14 , 135–151 (2013).
- Danese A , MoffittTE, ParianteCM, AmblerA, PoultonR, CaspiA. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry65(4) , 409–415 (2008).
- Patas K , PenninxBW, BusBA et al. Association between serum brain-derived neurotrophic factor and plasma interleukin-6 in major depressive disorder with melancholic features. Brain. Behav. Immun. 36 , 71–79 (2013).
- Miller AH , HaroonE, RaisonCL, FelgerJC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress. Anxiety30(4) , 297–306 (2013).
- Pariante CM , LightmanSL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci.31(9) , 464–468 (2008).
- Stetler C , MillerGE. Depression and hypothalamic–pituitary–adrenal activation: a quantitative summary of four decades of research. Psychosom. Med.73(2) , 114–126 (2011).
- Lopez-Duran NL , KovacsM, GeorgeCJ. Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology34(9) , 1272–1283 (2009).
- Gorzalka BB , HillMN. Putative role of endocannabinoid signaling in the etiology of depression and actions of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry35(7) , 1575–1585 (2011).
- Mechoulam R , ParkerLA. The endocannabinoid system and the brain. Annu. Rev. Psychol.64 , 21–47 (2013).
- Aso E , OzaitaA, Serra MÀ, Maldonado R. Genes differentially expressed in CB1 knockout mice: involvement in the depressive-like phenotype. Eur. Neuropsychopharmacol.21(1) , 11–22 (2011).
- Micale V , Di Marzo V, Sulcova A, Wotjak CT, Drago F. Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacol. Ther.138(1) , 18–37 (2013).
- Denson TF , EarleywineM. Decreased depression in marijuana users. Addict. Behav.31(4) , 738–742 (2006).
- De Petrocellis L , Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J. Neuroimmune Pharmacol.5(1) , 103–121 (2010).
- Pertwee RG . The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol.153(2) , 199–215 (2008).
- Rouzer CA , MarnettLJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev.111(10) , 5899–5921 (2011).
- Hill MN , TaskerJG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic–pituitary–adrenal axis. Neuroscience204 , 5–16 (2012).
- Hillard CJ , WeinlanderKM, StuhrKL. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience204 , 207–229 (2012).
- Steiner MA , MarsicanoG, WotjakCT, LutzB. Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses [Internet]. Psychoneuroendocrinology33(8) , 1165–1170 (2008).
- Evanson NK , TaskerJG, HillMN, HillardCJ, HermanJP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology151(10) , 4811–4819 (2010).
- Zunszain PA , AnackerC, CattaneoA, CarvalhoLA, ParianteCM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry35(3) , 722–729 (2011).
- Anacker C , ZunszainPA, CarvalhoLA, ParianteCM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology36(3) , 415–425 (2011).
- Castillo PE , YountsTJ, ChávezAE, HashimotodaniY. Endocannabinoid signaling and synaptic function. Neuron76(1) , 70–81 (2012).
- Hill MN , MillerGE, HoWS, GorzalkaBB, HillardCJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry41(2) , 48–53 (2008).
- Valverde O , TorrensM. CB1 receptor-deficient mice as a model for depression. Neuroscience204 , 193–206 (2012).
- Steiner MA , MarsicanoG, NestlerEJ, HolsboerF, LutzB, WotjakCT. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology33(1) , 54–67 (2008).
- Topol EJ , BousserMG, FoxKA et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet 376(9740) , 517–523 (2010).
- Riether D . Selective cannabinoid receptor 2 modulators: a patent review 2009 – present. Expert Opin. Ther. Pat.22(5) , 495–510 (2012).
- García-Gutiérrez MS , Pérez-OrtizJM, Gutiérrez-AdánA, ManzanaresJ. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. Br. J. Pharmacol.160(7) , 1773–1784 (2010).
- Hu B , DoodsH, TreedeRD, CeciA. Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain143(3) , 206–212 (2009).
- Petrosino S , Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr. Opin. Investig. Drugs11(1) , 51–62 (2010).
- Adamczyk P , GołdaA, McCrearyAC, FilipM, PrzegalińskiE. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J. Physiol. Pharmacol.59(2) , 217–228 (2008).
- Vinod KY , XieS, PsychoyosD, HungundBL, CooperTB, Tejani-ButtSM. Dysfunction in fatty acid amide hydrolase is associated with depressive-like behavior in Wistar Kyoto rats. PLoS ONE7(5) , e36743 (2012).
- Bisogno T , Di Marzo V. Cannabinoid receptors and endocannabinoids: role in neuroinflammatory and neurodegenerative disorders. CNS Neurol. Disord. Drug Targets9(5) , 564–573 (2010).
- Stella N . Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia58(9) , 1017–1030 (2010).
- Alhouayek M , MasquelierJ, MuccioliGG. Controlling 2-arachidonoylglycerol metabolism as an anti-inflammatory strategy. Drug Discov. Today19(3) , 295–304 (2014).
- Dowlati Y , HerrmannN, SwardfagerW et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67(5) , 446–457 (2010).
- De Chiara V , MottaC, RossiS et al. Interleukin-1β alters the sensitivity of cannabinoid CB1 receptors controlling glutamate transmission in the striatum. Neuroscience 250 , 232–239 (2013).
- Croxford JL , MillerSD. Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R+WIN55,212. J. Clin. Invest.111(8) , 1231–1240 (2003).
- Janssen DG , CaniatoRN, VersterJC, BauneBT. A psychoneuroimmunological review on cytokines involved in antidepressant treatment response. Hum. Psychopharmacol.25(3) , 201–215 (2010).
- Hannestad J , DellaGioiaN, BlochM. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology36(12) , 2452–2459 (2011).
- Maciel IS , SilvaRB, MorroneFB, CalixtoJB, CamposMM. Synergistic effects of celecoxib and bupropion in a model of chronic inflammation-related depression in mice. PLoS ONE8(9) , e77227 (2013).
- Zhang J , ChenC. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J. Biol. Chem.283(33) , 22601–22611 (2008).
- Hermanson DJ , HartleyND, Gamble-GeorgeJ et al. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat. Neurosci. 16(9) , 1291–1298 (2013).
- Hill M , KerrDM, BurkeNN et al. Pharmacological inhibition of endocannabinoid degradation modulates the expression of inflammatory mediators in the hypothalamus following an immunological stressor. Neuroscience 204 , 53–63 (2012).
- Hill MN . Introduction to the special issue on stress, emotional behavior, and the endocannabinoid system: a decade of research. Neuroscience204 , 1–4 (2012).
- Steiner MA , WotjakCT. Role of the endocannabinoid system in regulation of the hypothalamic–pituitary–adrenocortical axis. Prog. Brain Res.170(8) , 397–432 (2008).
- Wamsteeker JI , KuzmiskiJB, BainsJS. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J. Neurosci.30(33) , 11188–11196 (2010).
- Hill MN , McLaughlinRJ, MorrishAC et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic–pituitary–adrenal axis. Neuropsychopharmacology 34(13) , 2733–2745 (2009).
- Mitjans M , GastóC, CatalánR, FañanásL, AriasB. Genetic variability in the endocannabinoid system and 12-week clinical response to citalopram treatment: the role of the CNR1, CNR2 and FAAH genes. J. Psychopharmacol.26(10) , 1391–1398 (2012).
- Mitjans M , SerrettiA, FabbriC et al. Screening genetic variability at the CNR1 gene in both major depression etiology and clinical response to citalopram treatment. Psychopharmacology (Berl.) 227(3) , 509–519 (2013).
- Domschke K , DannlowskiU, OhrmannP et al. Cannabinoid receptor 1 (CNR1) gene: impact on antidepressant treatment response and emotion processing in major depression. Eur. Neuropsychopharmacol. 18(10) , 751–759 (2008).
- Juhasz G , ChaseD, PeggE et al. CNR1 gene is associated with high neuroticism and low agreeableness and interacts with recent negative life events to predict current depressive symptoms. Neuropsychopharmacology34(8) , 2019–2027 (2009).
- Rossi S , ButtariF, StuderV et al. The (AAT)n repeat of the cannabinoid CB1 receptor gene influences disease progression in relapsing multiple sclerosis. Mult. Scler. 17(3) , 281–288 (2011).
- Palermo FA , AngeliniM, CottoneE et al. Involvement of endocannabinoid CB1 receptor in the modulation of stress responses related to xenoestrogen exposure. Ann. NY Acad. Sci. 1163 , 504–507 (2009).
- Monteleone P , BifulcoM, MainaG et al. Investigation of CNR1 and FAAH endocannabinoid gene polymorphisms in bipolar disorder and major depression. Pharmacol. Res. 61(5) , 400–404 (2010).
- Agrawal A , NelsonEC, LittlefieldAK et al. Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Arch. Gen. Psychiatry 69(7) , 732–740 (2012).
- Onaivi ES , IshiguroH, GongJP et al. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS ONE 3(2) , e1640 (2008).
- Barrero FJ , AmpueroI, MoralesB et al. Depression in Parkinson‘s disease is related to a genetic polymorphism of the cannabinoid receptor gene (CNR1). Pharmacogenomics J. 5(2) , 135–141 (2005).
- Lazary J , LazaryA, GondaX et al. Promoter variants of the cannabinoid receptor 1 gene (CNR1) in interaction with 5-HTTLPR affect the anxious phenotype. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150B(8) , 1118–1127 (2009).
- Kotov R , GamezW, SchmidtF, WatsonD. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol. Bull.136(5) , 768–821 (2010).
- Wiersma JE , van Oppen P, van Schaik DJ, van der Does AJ, Beekman AT, Penninx BW. Psychological characteristics of chronic depression: a longitudinal cohort study. J. Clin. Psychiatry72(3) , 288–294 (2011).
- Yildiz M , KaraM, BozdemirM et al. Parasuicide patients in the emergency department and their relationship with cannabinoid gene polymorphism. Translational Psychiatry 2(1) , e75 (2012).
- Pardini M , KruegerF, KoenigsM et al. Fatty-acid amide hydrolase polymorphisms and post-traumatic stress disorder after penetrating brain injury. Transl. Psychiatry 2 , e75 (2012).
- De Luis DA , SagradoMG, AllerR, IzaolaO, CondeR, RomeroE. C358A missense polymorphism of the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) and insulin resistance in patients with diabetes mellitus Type 2. Diabetes Res. Clin. Pract.88(1) , 76–80 (2010).
- Zeng J , LiJ, HuangG. 385 C/A polymorphism of the fatty acid amide hydrolase gene is associated with metabolic syndrome in the Chinese Han population. Arch. Med. Sci.7(3) , 423–427 (2011).
- Sim MS , HatimA, ReynoldsGP, MohamedZ. Association of a functional FAAH polymorphism with methamphetamine-induced symptoms and dependence in a Malaysian population. Pharmacogenomics14(5) , 505–514 (2013).
- Hariri AR , GorkaA, HydeLW et al. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol. Psychiatry 66(1) , 9–16 (2009).
- Conzelmann A , ReifA, JacobC et al. A polymorphism in the gene of the endocannabinoid-degrading enzyme FAAH (FAAH C385A) is associated with emotional-motivational reactivity. Psychopharmacol. (Berl.) 224(4) , 573–579 (2012).
- Bufalino C , HepgulN, AgugliaE, ParianteCM. The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav. Immun.31 , 31–47 (2013).
- Zunszain PA , AnackerC, CattaneoA et al. Interleukin-1β: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 37(4) , 939–949 (2012).
- Baune BT , DannlowskiU, DomschkeK et al. The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol. Psychiatry 67(6) , 543–549 (2010).
- Hwang JP , TsaiSJ, HongCJ, YangCH, HsuCD, LiouYJ. Interleukin-1 beta -511C/T genetic polymorphism is associated with age of onset of geriatric depression. Neuromol. Med.11(4) , 322–327 (2009).
- Borkowska P , KuciaK, RzezniczekS et al. Interleukin-1beta promoter (-31T/C and -511C/T) polymorphisms in major recurrent depression. J. Mol. Neurosci. 44(1) , 12–16 (2011).
- Misener VL , GomezL, WiggKG et al. Tagging SNP association study of the IL-1beta gene (IL1B) and childhood-onset mood disorders. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150B(5) , 653–659 (2009).
- Sasayama D , HoriH, IijimaY et al. Modulation of cortisol responses to the DEX/CRH test by polymorphisms of the interleukin-1beta gene in healthy adults. Behav. Brain Funct. 7 , 23 (2011).
- Shao N , FengN, WangY, MiY, LiT, HuaL. Systematic review and meta-analysis of COX-2 expression and polymorphisms in prostate cancer. Mol. Biol. Rep.39(12) , 10997–11004 (2012).
- Dong J , DaiJ, ZhangM, HuZ, ShenH. Potentially functional COX-2-1195G>A polymorphism increases the risk of digestive system cancers: a meta-analysis. J. Gastroenterol. Hepatol.25(6) , 1042–1050 (2010).
- Tang W , HeM, YangB, WeiK, YinM, ZhangL. Association study of polymorphisms in the cyclooxygenase-2 gene and Alzheimer‘s disease risk in Chinese. Neurol. Sci.34(5) , 695–699 (2013).
- Su KP , HuangSY, PengCY et al. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol. Psychiatry 67(6) , 550–557 (2010).
- Gałecki P , FlorkowskiA, BieńkiewiczM, SzemrajJ. Functional polymorphism of cyclooxygenase-2 gene (G-765C) in depressive patients. Neuropsychobiology.62(2) , 116–120 (2010).
- Serretti A , ChiesaA, CalatiR et al. No influence of PTGS2 polymorphisms on response and remission to antidepressants in major depression. Psychiatry Res. 188(1) , 166–169 (2011).
- Mendlewicz J , CrisafulliC, CalatiR et al. Influence of COX-2 and OXTR polymorphisms on treatment outcome in treatment resistant depression. Neurosci. Lett. 516(1) , 85–88 (2012).
- Binder EB . The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology34(Suppl. 1) , S186–S195 (2009).
- Claes S . Glucocorticoid receptor polymorphisms in major depression. Ann. NY Acad. Sci.1179 , 216–228 (2009).
- Spijker AT , van Rossum EF. Glucocorticoid receptor polymorphisms in major depression. Focus on glucocorticoid sensitivity and neurocognitive functioning. Ann. NY Acad. Sci.1179 , 199–215 (2009).
- Manenschijn L , van den Akker EL, Lamberts SW, van Rossum EF. Clinical features associated with glucocorticoid receptor polymorphisms. An overview. Ann. NY Acad. Sci.1179 , 179–198 (2009).
- Otte C , WüstS, ZhaoS, PawlikowskaL, KwokPY, WhooleyMA. Glucocorticoid receptor gene and depression in patients with coronary heart disease: the Heart and Soul Study – 2009 Curt Richter Award Winner. Psychoneuroendocrinology34(10) , 1574–1581 (2009).
- Lahti J , RäikkönenK, BruceS et al. Glucocorticoid receptor gene haplotype predicts increased risk of hospital admission for depressive disorders in the Helsinki birth cohort study. J. Psychiatr. Res. 45(9) , 1160–1164 (2011).
- Ceulemans S , De Zutter S, Heyrman L et al. Evidence for the involvement of the glucocorticoid receptor gene in bipolar disorder in an isolated northern Swedish population. Bipolar Disord.13(7–8) , 614–623 (2011).
- Bet PM , PenninxBW, BochdanovitsZ et al. Glucocorticoid receptor gene polymorphisms and childhood adversity are associated with depression: new evidence for a gene–environment interaction. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150B(5) , 660–669 (2009).
- Menke A , KlengelT, RubelJ et al. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes Brain Behav. 12(3) , 289–296 (2013).
- Guidotti G , CalabreseF, AnackerC, RacagniG, ParianteCM, RivaMA. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment. Neuropsychopharmacology38(4) , 616–627 (2013).
- Cattaneo A , GennarelliM, UherR et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline “predictors” and longitudinal “targets”. Neuropsychopharmacology 38(3) , 377–385 (2013).
- Lekman M , LajeG, CharneyD et al. The FKBP5-gene in depression and treatment response – an association study in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Cohort. Biol. Psychiatry 63(12) , 1103–1110 (2008).
- Zou YF , WangF, FengXL et al. Meta-analysis of FKBP5 gene polymorphisms association with treatment response in patients with mood disorders. Neurosci. Lett. 484(1) , 56–61 (2010).
- Kirchheiner J , LorchR, LebedevaE et al. Genetic variants in FKBP5 affecting response to antidepressant drug treatment. Pharmacogenomics 9(7) , 841–846 (2008).
- Niitsu T , FabbriC, BentiniF, SerrettiA. Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry45 , 183–194 (2013).
- Sarginson JE , LazzeroniLC, RyanHS, SchatzbergAF, MurphyGM. FKBP5 polymorphisms and antidepressant response in geriatric depression. Am. J. Med. Genet. B. Neuropsychiatr. Genet.153B(2) , 554–560 (2010).
- Brent D , MelhemN, FerrellR et al. Association of FKBP5 polymorphisms with suicidal events in the Treatment of Resistant Depression in Adolescents (TORDIA) study. Am. J. Psychiatry 167(2) , 190–197 (2010).
- Horstmann S , LucaeS, MenkeA et al. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology 35(3) , 727–740 (2010).
- Di Marzo V . The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res.60(2) , 77–84 (2009).
