Abstract
Aim: To further characterize the pharmacokinetics of Xtampza® ER. Subjects & methods: This was an open-label, randomized, active-controlled, five-treatment, five-period, naltrexone-blocked, cross-over study. Healthy subjects received five equivalent oxycodone doses: Xtampza ER (intact or crushed), OxyContin® (intact or crushed) or immediate-release (IR) oxycodone (crushed). Blood samples were collected to assess oxycodone concentrations. Results: Crushed and intact Xtampza ER resulted in lower peak plasma concentrations compared with crushed oxycodone IR; crushed and intact Xtampza ER were bioequivalent. Crushed OxyContin exhibited a rapid increase in plasma oxycodone and was bioequivalent to crushed oxycodone IR. Conclusion: This second pharmacokinetic study demonstrated that Xtampza ER maintains its ER properties after crushing, unlike OxyContin, which failed to retain its ER properties after crushing.
ANZCTR registration number: ACTRN12614000613606
Prescription opioids play an important role in the management of chronic pain. Extended-release (ER) opioid products offer several advantages over immediate-release (IR) formulations, including the convenience of less frequent dosing, decreased fluctuations in plasma levels, more consistent analgesia over the dosing period and less nighttime awakening due to pain [Citation1]. However, as ER opioids contain relatively large quantities of active drug per unit dose, these analgesics are often a target for abuse by physical manipulation and chemical extraction [Citation2]. Abusers frequently attempt to manipulate (e.g.,cut, crush, chew or dissolve) these formulations to get IR of the opioid [Citation2–4]. In addition to abuse, accidental or unintentional misuse of ER opioid analgesics occurs when patients or their caregivers manipulate such formulations, often to facilitate swallowing or to enable dosing using other routes of administration; this misuse has the potential for severe health consequences [Citation5].
Several approaches have been used to develop abuse-deterrent opioid formulations to mitigate misuse and abuse, the most common of which is alteration of the formulation’s physicochemical properties to prevent manipulation (e.g., crushing) and intravenous abuse (e.g., gelling on contact with water) [Citation6]. It has been shown that ‘crush-resistant’ tablets, the most notable of which is OxyContin® (oxycodone ER, Purdue Pharma L.P., CT, USA), are capable of reducing abuse by nonoral routes (i.e., nasal, injection); however, abuse of these products by the oral route is still reported [Citation3,Citation7]. Similarly, manipulation of tablets before oral administration, by methods such as chewing or dissolving in the mouth, is still reported for crush-resistant tablets, such as for reformulated OxyContin [Citation3,Citation7].
Xtampza® ER (Oxycodone DETERx®, Collegium Pharmaceutical, Inc., MA, USA) is an ER, microsphere-in-capsule, abuse-deterrent formulation of oxycodone. In this formulation, oxycodone is homogenously dispersed within hydrophobic, waxy microspheres; each microsphere acts as an independent, ER, abuse-deterrent drug delivery system. Xtampza ER was designed to resist extraction and to retain its ER characteristics following tampering with commonly available utensils. Previous in vitro [Citation8,Citation9] and in vivo [Citation8,Citation10] studies have assessed the effect of manipulation of Xtampza ER, demonstrating that despite aggressive manipulation, Xtampza ER microspheres retain their ER characteristics. An added benefit of Xtampza ER is the microsphere-in-capsule design. Each microsphere functions as an individual ER drug delivery system, allowing for multiple modes of administration (intact, sprinkled onto food and via nasogastric/gastric tubes) without losing the ER characteristics [Citation11]. This unique design may limit the misuse that occurs when patients, patient caregivers and healthcare providers manipulate ER abuse-deterrent formulation analgesics to facilitate swallowing or enable other modes of administration [Citation11].
This study further characterized the safety and pharmacokinetics (PK) of Xtampza ER when the capsule was taken intact compared with crushing the microspheres before administration. This second, confirmatory study was designed to replicate a previous study that compared the PK profile of Xtampza ER with the currently marketed OxyContin [Citation10].
Subjects & methods
Subjects were recruited at a clinical research unit (Frontage, NJ, USA) similar to a previous study [Citation10]. Subjects were either already part of the site’s database and were contacted by telephone or they were recruited using study-specific advertisements. Subjects were healthy males and females (aged 18–50 years) with no clinically significant abnormalities noted in medical history, physical examination, vital sign measurement, 12-lead electrocardiogram or clinical laboratory tests. Subjects were excluded if they had a history of alcohol and/or drug abuse or were regular users of tobacco products; had intolerance to venipuncture; had known allergies to any of the test products; or had a disorder that may have interfered with drug absorption, distribution, metabolism or excretion. Subjects were required to have a negative urine drug screen, saliva alcohol test and urine cotinine test at the screening visit and at admission to each treatment period. Subjects were restricted from using other prescription or nonprescription drugs (except acceptable forms of birth control and acetaminophen), herbal remedies or nutritional supplements during the study. Subjects were also told to avoid caffeine and alcohol for 24 h prior to admission for each treatment period and were told to abstain from food containing grapefruit, pomegranate, pomelo and poppy seeds from 1 week prior to treatment period 1 until the end of the study. Female subjects of childbearing potential were required to be nonpregnant and nonlactating, and had to use acceptable forms of contraception during the study and were required to have a negative serum pregnancy test at screening and a negative urine pregnancy test before dosing in each treatment period. All subjects provided written informed consent prior to participation in the study.
• Study design & treatment
This was an open-label, randomized, active-controlled, five-treatment, five-period, naltrexone-blocked cross-over comparison study conducted in accordance with the ethical principles of Good Clinical Practice, the World Medical Association Declaration of Helsinki (1989) and the International Conference on Harmonization Harmonized Tripartite Guideline. The study was conducted at a single center (NJ, USA). Study materials were reviewed and approved by an independent review board (IntegReview Ethics Review Board, TX, USA) as required by local regulations. The investigator ensured that the independent review board members complied with all federal, state and local regulations. This study consisted of a screening phase and an open-label treatment phase with one study drug treatment administered in each of five treatment periods. Subjects received single oral doses of the following treatments in a randomized order based on a 5 × 5 Williams square design: intact Xtampza ER 36 mg (contains the equivalent of 40 mg oxycodone hydrochloride), crushed Xtampza ER 36 mg, intact OxyContin 40 mg, crushed OxyContin 40 mg and crushed oxycodone IR 40 mg (two 20 mg tablets).
For each treatment period, subjects were admitted to the research clinic the day before study drug administration and received 50 mg of naltrexone (Mallinckrodt Pharmaceuticals, Inc., MO, USA) approximately 13 h (to determine tolerability) and 1 h (for safety) prior to study drug administration. Before study drug administration, subjects fasted for at least 10 h and then were served a standardized, high-fat, high-calorie breakfast [Citation12] 30 min prior to the scheduled administration of study drug. No additional food or drink was permitted for at least 4 h after dosing; water was permitted except for 1 h before and 1 h after dosing. Subjects were confined to the research clinic until the last blood sample was collected. A washout of at least 5 days was completed between each treatment period.
Crushed Xtampza ER and crushed oxycodone IR (KVK-Tech, Inc., PA, USA, administered as 2 × 20 mg tablets) were prepared using the same method. Crushed OxyContin was prepared using a different crushing method; however, the most aggressive method of reducing particle size was used for each respective product based on techniques described in a previous study [Citation8]. In brief, the most aggressive method of particle size reduction was previously determined in in vitro experiments by screening multiple household utensils, including those that could chop, grate, crush or grind both products [Citation8]. The contents of Xtampza ER capsules (i.e., the drug microspheres) were removed and subjected to manipulation. Intact Xtampza ER and intact OxyContin were administered as single 40-mg oxycodone hydrochloride equivalent dosages, respectively, with 240 ml of noncarbonated, room-temperatured water. Crushed products were poured into a dosing cup and administered to the subject followed by consumption of approximately half of the 240 ml of noncarbonated, room-temperatured water. The dosing cup was filled with the remaining portion of the 240 ml of water to rinse any remaining study drug from the dosing cup and was then consumed by the subject. Study staff conducted a visual oral cavity check to ensure that all study drugs had been consumed.
• Assessments
PK measures
During each Xtampza ER and OxyContin treatment period, blood samples were collected within 60 min before dosing, and at 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 7.0, 8.0, 9.0, 10.0, 12.0, 24.0 and 36.0 h postdose. During the oxycodone IR treatment period, all of the above blood samples were collected except for the 5.5-, 7.0-, 9.0-, 10.0- and 36-h postdose samples; in addition, a 16-h postdose sample was collected for the oxycodone IR treatment period. For each sample, approximately 3 ml of blood was collected. Plasma samples were sent to Celerion Laboratories (NE, USA) and analyzed using LC–MS/MS to determine oxycodone concentrations.
Safety assessment
Safety and tolerability assessments included incidence of treatment-emergent adverse events (AEs); vital sign measurements; oxygen saturation measurements; hematologic, biochemical and urinalysis laboratory parameters; pregnancy testing; and physical examinations.
• PK & statistical analysis
The safety population consisted of all subjects who received at least one dose of study drug and for whom there was at least one post-treatment safety observation. Safety and tolerability were analyzed using descriptive statistics. The PK population consisted of all subjects who completed at least two of the five treatment periods, who had sufficient data for the determination of PK parameters, who did not experience emesis within 12 h after study drug administration and who did not have a protocol deviation that could have compromised the integrity of the PK results. The following key PK parameters were calculated: area under the plasma concentration–time curve from zero to final time with a concentration ≥ the lower limit of quantitation (LLOQ; AUC0–t), area under the plasma concentration–time curve from zero to infinity (AUCinf), maximum observed plasma concentration (Cmax), time to reach maximum plasma concentration (Tmax), partial area under the plasma concentration–time curve (PAUC) from time zero to all blood sample time points and abuse quotient (AQ = Cmax/Tmax). Only those plasma concentrations ≥ LLOQ of 0.5 ng/ml were used in the analysis. Plasma concentrations less than the LLOQ were input as zero for the calculation of the descriptive statistics.
PK parameters were compared among treatments using an analysis of variance statistical model with sequence, treatment and period as the fixed effects and subject within sequence as a random effect, using the natural logarithms of the data. The analysis was performed using the SAS® (SAS Institute, Inc., NC, USA) PROC GLM procedure. CIs (90% CI) were constructed for the least squares geometric mean ratios (GMRs) of the three key PK parameters being compared using natural log-transformed data and the two one-sided t-tests procedure. The GMRs and associated 90% CIs were exponentiated back to the original scale. Bioequivalence was concluded if the 90% CI of the GMR for a specific comparison fell entirely within the 80–125% bioequivalence lower and upper boundaries. Tmax was analyzed using nonparametric analysis. Wilcoxon signed rank test was used to compare the test treatments with the reference treatments. The primary analyses were a comparison of crushed Xtampza ER versus crushed oxycodone IR and crushed OxyContin versus crushed oxycodone IR. Secondary analyses included comparisons of crushed versus intact Xtampza ER and crushed versus intact OxyContin.
Results
• Subjects
Of the 42 enrolled subjects (31 males and 11 females), 35 subjects received all planned study doses. Seven (16.7%) subjects discontinued from the study, one subject discontinued because of a positive cotinine test on admission to treatment period 5, three subjects withdrew consent and three subjects discontinued because of an AE. For the safety population, the mean (standard deviation) age was 30.5 (6.17) years and the mean (range) BMI was 26.71 (19.1–32.1) kg/m2. Baseline demographics for each treatment group are shown in .
Table 1. Baseline demographics and characteristics.
• Pharmacokinetics
After oral administration of crushed oxycodone IR, there was a rapid increase in oxycodone plasma concentration (); the Cmax of 78.1 ± 22.0 ng/ml was reached 1.5 h after administration (). In contrast, both intact and crushed Xtampza ER exhibited a gradual increase in plasma oxycodone concentration characteristic of an ER profile, with a Cmax of 56.9 ± 13.4 and 61.2 ± 13.1 ng/ml, respectively, at 3.5 h after administration. Intact OxyContin exhibited a characteristic ER profile, with a Cmax of 63.7 ± 14.8 ng/ml at 4.5 h after administration. However, after administration of crushed OxyContin, there was a rapid increase in plasma oxycodone concentration, with a Cmax of 79.9 ± 17.9 ng/ml at only 1.75 h postdose, which emulates the characteristic profile of crushed oxycodone IR.
Error bars indicate the positive standard deviation (negative standard deviation not shown).
ER: Extended release; IR: Immediate release.
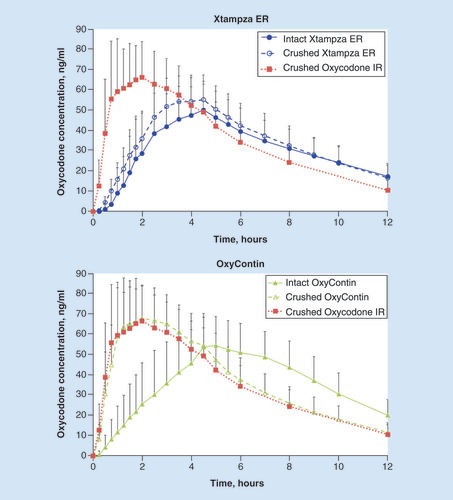
Table 2. Pharmacokinetic parameters.
Comparisons between intact and crushed Xtampza ER demonstrated bioequivalence (). In contrast, crushed OxyContin was not bioequivalent to the intact formulation. Furthermore, crushed OxyContin and crushed oxycodone IR were bioequivalent.
Table 3. Bioequivalence analysis.
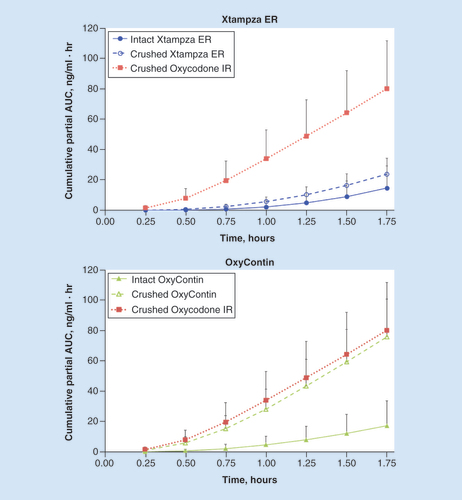
As anticipated, total plasma AUC values (AUCinf) were similar across the different treatments () because the same overall dose of drug was given for each formulation, whether intact or manipulated. The cumulative partial AUC values over the first 1.75 h after administration, which reflect early plasma exposure, were much lower for crushed and intact Xtampza ER than for oxycodone IR (). The cumulative PAUC values for intact OxyContin were similar to those values for intact and crushed Xtampza ER; however, the cumulative PAUC values for crushed OxyContin were similar to the values observed for crushed oxycodone IR.
Error bars indicate the positive standard deviation (negative standard deviation not shown).ER: Extended release; IR: Immediate release.
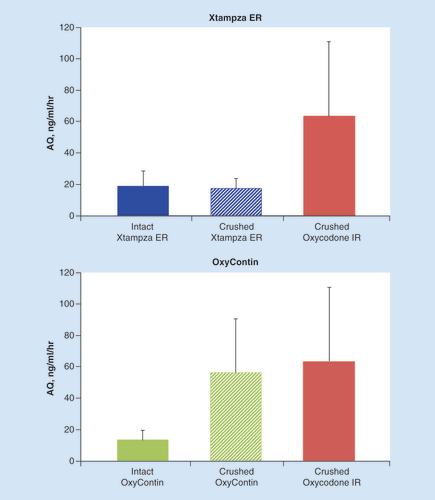
The abuse quotient score (AQ = Cmax/Tmax) can be used to assess the rate of plasma drug concentration increase; a higher AQ indicates a quicker rise in plasma drug concentration, which presumably results in the more rapidly achieved euphoria that abusers desire [Citation4]. Crushed oxycodone IR exhibited the quickest rise in plasma concentration, as indicated by the comparatively high mean AQ score, followed closely by crushed OxyContin (). In contrast, AQ scores were markedly lower for intact and crushed Xtampza ER, as well as intact OxyContin. The mean AQ scores for intact and crushed Xtampza ER were 2.9- and 3.1-fold lower than those of crushed OxyContin, respectively. The mean AQ score for intact OxyContin was 4.1-fold lower than that of crushed OxyContin.
Error bars indicate the positive standard deviation (negative standard deviation not shown).
AQ: Abuse quotient; ER: Extended release; IR: Immediate release.
• Safety
There were 48 AEs reported in 19 (45%) subjects; all AEs reported were mild in intensity. The most commonly reported AEs were headache (10 subjects, 23.8%), abdominal pain (3 subjects, 7.1%) and diarrhea (3 subjects, 7.1%). The frequency of AEs across treatment groups was similar (). AEs considered to be possibly related to treatment occurred in seven (16.7%) subjects across all treatments; these included headache, nausea, abdominal pain, diarrhea, fatigue and decreased appetite, which all occurred in two or fewer subjects per treatment group. There were no serious AEs reported. There were also no pregnancies reported. There were no clinically significant findings or trends noted from vital sign evaluations, blood oxygen saturation levels, clinical laboratory results or physical examinations.
Table 4. Adverse events reported in at least two subjects.
Discussion
This is the second comparative clinical PK study designed to further characterize the PK of intact and crushed Xtampza ER compared with that of OxyContin as well as oxycodone IR. Xtampza ER maintains its ER characteristics and does not result in increased plasma exposure after crushing. Intact and crushed Xtampza ER were bioequivalent. In contrast, OxyContin failed to retain its ER properties after crushing, resulting in increased Cmax, shorter Tmax and a higher AQ. After crushing, OxyContin was bioequivalent to crushed oxycodone IR and was not bioequivalent to intact OxyContin. These results were similar to previously published data on the comparative PK of Xtampza ER and OxyContin administered intact and after crushing and chewing [Citation8,Citation13], confirming the results from a similar previously published study [Citation10] and establishing the consistency of the PK of Xtampza ER (Supplementary Table) Supplementary Table, further demonstrating the ability of Xtampza ER to retain its ER characteristics after manipulation, unlike OxyContin [Citation8,Citation10,Citation13,Citation14]. Similar to previously published data [Citation10], the safety profiles of crushed and intact Xtampza ER were comparable. The reported AEs in both studies were mild to moderate in intensity, not clinically significant and were all common AEs associated with opioid administration.
The need for abuse-deterrent opioids is ever present, as 4.3 million people aged 12 and older reported abusing pain medications in 2014 [Citation15]. Even opioids reformulated as crush-resistant tablets are still being manipulated before ingestion [Citation3]. Despite sustained reductions in abuse and diversion of OxyContin in the first 5 years since its introduction [Citation16], particularly for abuse via nonoral routes (e.g., injection, snorting) [Citation3], oral abuse of OxyContin is still reported [Citation3,Citation7]. In a survey of oral abusers, 42% reported manipulation (e.g., chewing, dissolving in mouth) of crush-resistant tablet formulations prior to oral administration [Citation3]. Further, a review of Internet forums found 32 potentially feasible recipes to overcome the crush-resistant properties of OxyContin [Citation17]. Tampering methods to compromise the OxyContin formulation are not always sophisticated, and often chewing tablets before oral administration is used to attempt to elicit rapid onset of euphoria sought by drug abusers [Citation17]. It is thought that novice drug abusers often begin with oral administration and evolve their drug use habits to chewing, snorting and eventually injection, a progression that is associated with more dire outcomes [Citation18]. Thus, there is a need for a more robust abuse-deterrent opioid formulation that maintains its ER characteristics even when chewed, in hopes that the ‘abuse trajectory’ may not progress beyond the oral-abuse phase [Citation18].
Despite the inclusion of tamper-resistant properties, most opioids include language in the boxed warning against crushing and chewing opioids [Citation19–25]. Studies with Xtampza ER indicate that it retains its ER characteristics after crushing or chewing [Citation8,Citation10]; as a result, Xtampza ER is the first and only ER opioid formulation that does not include language in the boxed warning about potential dangers of crushing or chewing before ingestion [Citation26]. Based on published data [Citation8,Citation10,Citation13,Citation14], including this study, crushed OxyContin results in a rapid increase in serum oxycodone concentrations and thus, a higher AQ. This fact may reveal why oral abuse after manipulation remains prevalent for hard-to-crush tablet formulations. A sustained ER profile after chewing or crushing may be advantageous in reducing oral abuse after product manipulation, although epidemiologic data are not yet available to determine the impact on overall abuse at the societal level.
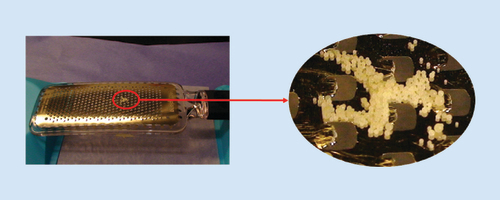
A unique advantage of Xtampza ER’s technology is the microsphere design, which may help eliminate potential paths of manipulation still available to other formulations. With a median diameter of approximately 300 μm, individual Xtampza ER microspheres are approximately 17-fold smaller than most solid, oral, monolithic dosage forms, including OxyContin, which typically has a diameter of >5 mm. The size of the microspheres precludes manipulation with many types of common household tools. For example, the size of the microspheres relative to a kitchen grater is illustrated in . This relatively small size, coupled with the physicochemical properties of the microspheres, results in the crush-resistant properties demonstrated in the present study.
Note the scale of the kitchen grater relative to the microsphere formulation.
ER: Extended release.
While manipulation of tablets is often done with the intent of abuse, some patients manipulate medications to accommodate a medical need. As many as 11 million Americans may experience swallowing difficulties, although this number may be higher – a survey of over 1000 patients indicated that when it comes to medications, as many as 81% of patients do not talk to their physician about their difficulties with swallowing [Citation5]. Patients often do not understand that manipulation of their ER analgesic can cause release of a fatal dose of opioid [Citation5]; reported rates of misuse by crushing to facilitate swallowing range between 21 and 29% [Citation27]. The physicochemical properties of Xtampza ER that limit its ability to be manipulated may help reduce the incidence of unintentional misuse by patients or their caregivers who manipulate analgesics to make them easier to swallow or to enable a different route of administration. In addition to these abuse-deterrent properties, Xtampza ER can be administered via multiple modes [Citation11], allowing clinicians to prescribe one analgesic that can provide treatment for patients who require different modes of administration (e.g., intact, sprinkled onto food, poured into a nasogastric/gastric tube).
In 2015, the US FDA published a guidance document outlining study categories recommended for the evaluation of abuse-deterrent opioid formulations [Citation28]. These categories include laboratory-based in vitro manipulation and extraction studies, PK evaluation of manipulated formulations and human clinical abuse potential studies. Our study fulfills category 2 criteria, demonstrating that crushing Xtampza ER has little to no effect on the PK profile. Combining these data with that of previous studies [Citation8–10,Citation13], Xtampza ER has labeling [Citation26] that includes data from all of the FDA-recommended categories. Data from in vitro studies have demonstrated that the formulation is difficult to prepare for injection [Citation9]. Data from the combination of in vitro and in vivo studies have shown that it is difficult to reduce the particle size of Xtampza ER microspheres [Citation8], the ER characteristics are maintained after manipulation [Citation10], and that Xtampza ER has lower drug liking after chewing [Citation29] and after intranasal administration [Citation30] when compared with IR oxycodone.
This study was successful in measuring the PK characteristics of crushed and intact oxycodone formulations, although it was limited by the lack of concurrent assessment of the subjective effects of abuse (e.g., drug liking). Subjective, drug liking assessments are considered to be the most sensitive measures of abuse potential. However, results from a previous study have demonstrated a lower abuse potential for both chewed and intact Xtampza ER after oral administration compared with crushed oxycodone IR [Citation13]. Another limitation of the study was crushing the products under controlled laboratory conditions. The approach used in this study followed FDA recommendations for premarket assessment of abuse-deterrent opioid products; a variety of household tools were screened in the in vitro studies, and the most effective methods identified for each respective product were used in subsequent PK and human abuse potential studies. Although this approach is intended to capture the worst case by using the best crushing method identified, it does not capture the potential variability in crushing that will likely occur in a ‘real-world’ setting. Last, this study is also limited by the lack of evidence linking the PK of Xtampza ER with rates of abuse; the impact of Xtampza ER as an abuse-deterrent formulation will not be fully established until long-term, postmarketing studies can assess rates of abuse in a real-world setting.
Conclusion
This is the second comparative clinical PK study demonstrating that Xtampza ER maintains its ER properties and does not result in increased plasma exposure after crushing. In contrast, OxyContin failed to retain its ER properties after crushing, resulting in bioequivalence to crushed oxycodone IR. As the first ER opioid formulation that does not include language in the boxed warning about the potential dangers of crushing or chewing before ingestion, Xtampza ER provides clinicians and patients with an ER, abuse-deterrent oxycodone formulation for the treatment of chronic pain that can be tailored to fit patients’ specific dosing needs.
Extended-release (ER) opioids have demonstrated efficacy at treating chronic pain; however, they are frequently abused because of the high per-unit dose of opioid.
Xtampza® ER (oxycodone DETERx®) is a microsphere-in-capsule, abuse-deterrent, ER oxycodone formulation designed to resist extraction and retain its ER characteristics after manipulation with common household tools.
This study assessed the safety and pharmacokinetics of Xtampza ER compared with OxyContin® (oxycodone ER) when dosed intact or crushed. Crushed immediate-release (IR) oxycodone was also used as a comparator.
Crushed and intact Xtampza ER were bioequivalent.
Crushed OxyContin exhibited a rapid increase in plasma oxycodone and was bioequivalent to crushed oxycodone IR.
Crushed oxycodone IR and OxyContin exhibited the highest mean abuse quotient (AQ) scores; Xtampza ER (intact and crushed) AQ scores were 2.9- and 3.1-fold below the AQ scores of oxycodone IR, respectively.
No serious adverse events were reported during this study.
This is the second comparative pharmacokinetic study demonstrating that Xtampza ER maintains its ER properties after crushing, unlike OxyContin, which failed to retain its ER properties after crushing.
Xtampza ER provides clinicians and patients with an ER, abuse-deterrent, oxycodone formulation for the treatment of chronic pain.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have follwed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Supplemental Table 1
Download PDF (13.9 KB)Financial & competing interests disclosure
MJ Brennan has received grants/consultant fees/speakers’ bureaus fees from Purdue, Teva, Astra Zeneca, Pfizer, Depomed, Shionogi, Collegium, Iroko, Cara and Daiichi Sankyo. He is a stockholder of Cara. A Marseilles, M O’Connor and AB Fleming are full-time employees of Collegium Pharmaceutical, Inc., and hold stock and/or stock options. EA Kopecky was an employee of Collegium Pharmaceutical, Inc., at the time of the study; he is currently employed at TEVA Pharmaceutical Industries Ltd. This study was funded and supported by Collegium Pharmaceutical, Inc., Canton, MA, USA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing assistance, funded by Collegium Pharmaceutical, Inc., was provided by KM Cameron of JB Ashtin, who developed the first draft based on an author-approved outline and implemented author revisions. The authors thank Christy Thompson and Michael DeGeorge for their critical review of the manuscript.
Additional information
Funding
References
- Nicholson B . Benefits of extended-release opioid analgesic formulations in the treatment of chronic pain . Pain Pract.9 ( 1 ), 71 – 81 ( 2009 ).
- Webster L . Update on abuse-resistant and abuse-deterrent approaches to opioid formulations . Pain Med.10 ( Suppl. 2 ), S124 – S133 ( 2009 ).
- Butler SF , BlackRA , FlemingAB . Relative abuse of crush-resistant prescription opioid tablets via alternative oral modes of administration . Pain Med.http://doi.org/10.1093/pm/pnx151 ( 2017 ) ( Epub ahead of print ).
- Moorman-Li R , MotyckaCA , IngeLD , CongdonJM , HobsonS , PokropskiB . A review of abuse-deterrent opioids for chronic nonmalignant pain . PT37 ( 7 ), 412 – 418 ( 2012 ).
- Pergolizzi JV Jr , TaylorRJr , NalamachuSet al. Challenges of treating patients with chronic pain with dysphagia (CPD): physician and patient perspectives . Curr. Med. Res. Opin.30 ( 2 ), 191 – 202 ( 2014 ).
- Hale ME , MoeD , BondM , GasiorM , MalamutR . Abuse-deterrent formulations of prescription opioid analgesics in the management of chronic noncancer pain . Pain Manag.6 ( 5 ), 497 – 508 ( 2016 ).
- Butler SF , CassidyTA , ChilcoatHet al. Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment . J. Pain14 ( 4 ), 351 – 358 ( 2013 ).
- Kopecky EA , FlemingAB , NoonanPKet al. Impact of physical manipulation on in vitro and in vivo release profiles of oxycodone DETERx(R): an extended-release, abuse-deterrent formulation . J. Opioid Manag.10 ( 4 ), 233 – 246 ( 2014 ).
- Fleming AB , ScungioTA , GrimaMP , MayockSP . In vitro assessment of the potential for abuse via the intravenous route of oxycodone DETERx(R) microspheres . J. Opioid Manag.12 ( 1 ), 57 – 65 ( 2016 ).
- Gudin J , Levy-CoopermanN , KopeckyEA , FlemingAB . Comparing the effect of tampering on the oral pharmacokinetic profiles of two extended-release oxycodone formulations with abuse-deterrent properties . Pain Med.16 ( 11 ), 2142 – 2151 ( 2015 ).
- McCarberg BH , KopeckyEA , O’ConnorMet al. An abuse-deterrent, microsphere-in-capsule formulation of extended-release oxycodone: alternative modes of administration to facilitate pain management in patients with dysphagia . Curr. Med. Res. Opin.32 ( 12 ), 1975 – 1982 ( 2016 ).
- Food and Drug Administration . Guidance for Industry: Food-Effect Bioavailability and Fed Bioequivalence Studies . CDER , MD, USA ( 2002 ).
- Kopecky EA , FlemingAB , Levy-CoopermanN , O’ConnorM , SellersEM . Oral human abuse potential of oxycodone DETERx® (Xtampza® ER) . J. Clin. Pharmacol.57 ( 4 ), 500 – 512 ( 2017 ).
- Harris S , ColucciS , PerrinoP , MandarinoD . Effects of various tampering methods on exposure to oxycodone in healthy subjects. Poster No. 78 . Presented at : The 74th Annual Scientific Meeting on the College on Problems of Drug Dependence . CA, USA , 9–14 June 2012 .
- Center for Behavior Health Statistics and Quality . Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15–4927, NSDUH Serioes H-50) ( 2015 ) www.samhsa.gov/data/sites/default/files/NSDUH-FRR1–2014/NSDUH-FRR1–2014.pdf .
- Severtson SG , EllisMS , KurtzSPet al. Sustained reduction of diversion and abuse after introduction of an abuse deterrent formulation of extended release oxycodone . Drug Alcohol Depend.168 , 219 – 229 ( 2016 ).
- McNaughton EC , CoplanPM , BlackRA , WeberSE , ChilcoatHD , ButlerSF . Monitoring of internet forums to evaluate reactions to the introduction of reformulated OxyContin to deter abuse . J. Med. Internet Res.16 ( 5 ), e119 ( 2014 ).
- Katz N , DartRC , BaileyE , TrudeauJ , OsgoodE , PaillardF . Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions . Am. J. Drug Alcohol Abuse37 ( 4 ), 205 – 217 ( 2011 ).
- OxyContin®, package insert . Purdue Pharma L.P. , CT, USA ( 2015 ). http://app.purduepharma.com/xmlpublishing/pi.aspx?id=o .
- Kadian® ER, package insert . Actavis Pharma, Inc. , NJ, USA ( 2014 ). www.allergan.com/assets/pdf/kadian_pi .
- Opana® ER, package insert . Endo Pharmaceuticals, Inc. , PA, USA ( 2014 ). www.endo.com/File%20Library/Products/Prescribing%20Information/OpanaER_prescribing_information_newformulation.html .
- MS Contin®, package insert . Purdue Pharma L.P. , CT, USA ( 2014 ). http://app.purduepharma.com/xmlpublishing/pi.aspx?id=ms .
- Exalgo®, package insert . Mallinckrodt Brand Pharmaceuticals, Inc. , MO, USA ( 2014 ). www2.mallinckrodt.com/workarea/downloadasset.aspx?id=2147483728 .
- Zohydro®, package insert . Pernix Therapeutics, LLC. , NJ, USA ( 2016 ). www.zohydroer.com/downloads/ZOHYDROERFullPrescribingInformation.pdf .
- Hysingla®, package insert . Purdue Pharma L.P. , CT, USA ( 2015 ). http://app.purduepharma.com/xmlpublishing/pi.aspx?id=h .
- Xtampza® ER, package insert . Collegium Pharmaceutical, Inc. , MA, USA ( 2016 ). www.xtampzaer.com/pdf/xtampza-pi.pdf .
- Vowles KE , McEnteeML , JulnesPS , FroheT , NeyJP , van der GoesDN . Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis . Pain156 ( 4 ), 569 – 576 ( 2015 ).
- Food and Drug Administration . CDER guidance for industry: abuse-deterrent opioids – evaluation and labeling . MD, USA ( 2015 ). www.fda.gov/downloads/Drugs/Guidances/UMC334743.pdf .
- Kopecky EA , FlemingAB , Levy-CoopermanN , O’ConnorM , SellersE . Oral human abuse potential of oxycodone DETERx® (Xtampza® ER) . J. Clin. Pharmacol.57 , 500 – 512 ( 2017 ).
- Webster LR , KopeckyEA , SmithMD , FlemingAB . A randomized, double-blind, double-dummy study to evaluate the intranasal human abuse potential and pharmacokinetics of a novel extended-release abuse-deterrent formulation of oxycodone . Pain Med.17 ( 6 ), 1112 – 1130 ( 2016 ).
