Abstract
Adhesive Dermally-Applied Microarray (ADAM) is a device for intracutaneous drug administration consisting of a 3 cm2 disposable array of drug-coated titanium microprojections on an adhesive backing. It is applied using a low cost, reusable, handheld applicator. Microprojections penetrate the stratum corneum, delivering drug proximal to capillaries with limited likelihood of pain. The pharmacokinetics of zolmitriptan delivery using ADAM was evaluated in 20 healthy volunteers. Median tmax was <20 min, comparable to subcutaneous sumatriptan. Absorption was faster than for oral zolmitriptan, with higher exposure in the first 2 h. Most adverse events were consistent with those seen in previous triptan trials. Application site reactions were generally mild and resolved within 24 h. ADAM zolmitriptan shows a promising pharmacokinetic profile for migraine treatment.
Migraine is a common cause of disability, and despite the availability of multiple treatment options, considerable unmet need persists [Citation1]. For moderate-to-severe migraine, treatment guidelines recommend the first-line use of triptans [Citation2–4]. Optimal migraine treatment provides rapid pain relief with good palatability; currently available formulations and routes of administration for migraine medications do not fully meet these criteria.
Although orally administered medications are user-friendly, they have some drawbacks [Citation5]. Drug absorption is typically not rapid and may be further hindered by gastrointestinal dysmotility, resulting in inconsistent treatment effects. Moreover, as many patients experience nausea and vomiting in association with migraine, they may experience difficulty in swallowing or retaining oral formulations. Delayed gastric emptying in migraine may be further exacerbated by triptans, which can prolong gastric emptying time by activating 5-HT1 receptors on gastric myenteric neurons [Citation6–8]. Therefore, in many patients, rapidly acting nonoral formulations may be preferable.
Available nonoral migraine medications include injectable and intranasal formulations of sumatriptan and zolmitriptan nasal spray. Among these, subcutaneously injected sumatriptan is most rapidly absorbed with a time to maximum concentration (tmax) of 10–12 min [Citation9]. For sumatriptan nasal powder and nasal spray, the average tmax are 45 min [Citation10] and approximately 2 h [Citation11], respectively. The tmax for zolmitriptan nasal spray is 1.5–2.5 h [Citation12]. While providing more rapid absorption than orally administered triptans, these formulations have limitations that include needle aversion and poor palatability of nasal formulations. Here we describe an investigational device that circumvents these limitations by providing intracutaneous delivery of zolmitriptan.
The Adhesive Dermally-Applied Microarray
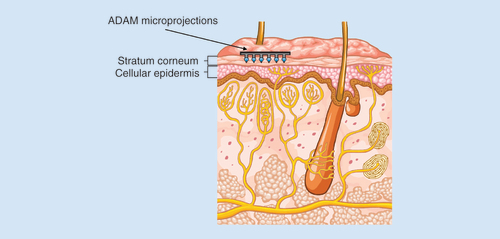
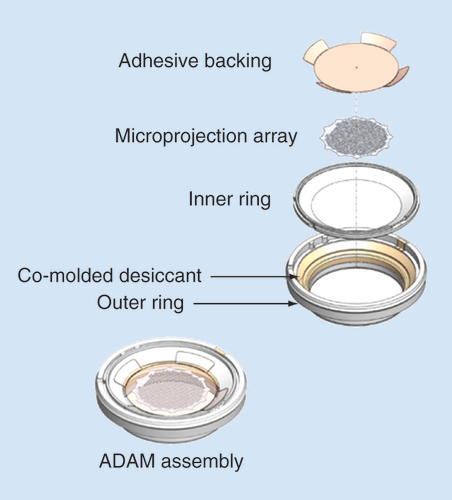
The Adhesive Dermally-Applied Microarray (ADAM) is an investigational product for intracutaneous drug delivery allowing rapid absorption into the bloodstream. It consists of a disposable adhesive backing, with an array of drug (zolmitriptan)-coated titanium microprojections mounted on the skin-facing surface (); and a ready to use low-cost reusable handheld applicator that ensures that each microprojection array is applied to the skin with a defined application force, ensuring consistent delivery at the site of administration ().
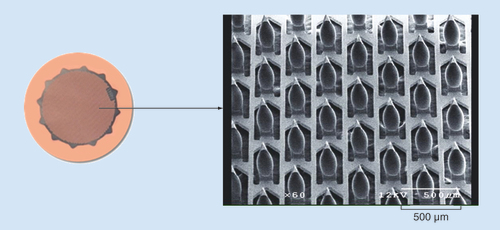
Left panel depicts the assembled ADAM. Right shows a scanning electron micrograph of the zolmitriptan-coated microprojections.
ADAM: Adhesive Dermally-Applied Microarray.
The microprojection array is 3 cm2 in diameter and consists of 1987 titanium microprojections, each 340 μm in length. The microprojections are coated with drug formulation applied as a solution, which is then dried. The array is attached to a 5 cm2 adhesive backing (). The microprojection array is mounted inside a polycarbonate plastic ring with a co-molded desiccant (). The complete patch in ring assembly is packaged in a nitrogen-purged foil cup, which is stored at room temperature.
ADAM: Adhesive Dermally-Applied Microarray.
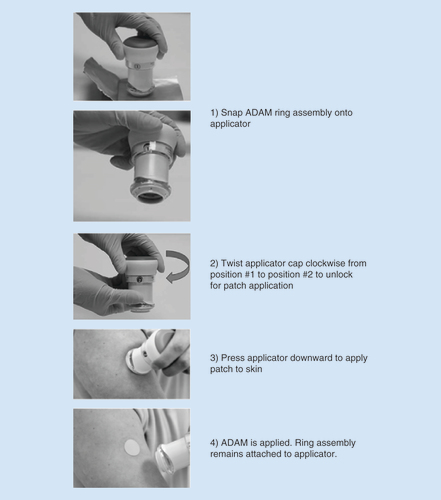
The user prepares the ADAM for application by simply pressing the handheld reusable applicator onto the ring assembly (). The applicator is unlocked by a quick twist of the cap from the #1 position to #2 position (). The user then applies the ADAM by just pressing the applicator/ring assembly onto the skin site. The applicator releases its piston when sufficient mild force is applied. The piston detaches the ADAM from the ring and easily applies it to the skin. The applicator is designed to ensure that the same energy is applied across multiple uses and is user independent.
Following application, the microprojections penetrate the stratum corneum proximal to the microcapillaries in skin. The shallow depth of penetration and the minuteness of the size of the microprojections limit the likelihood of stimulating sensory nerve endings (). The zolmitriptan coating is rapidly reconstituted from the microprojections by the interstitial fluid in the skin and is available for absorption. The ADAM is removed after 30 min and is disposed.
Pharmacokinetic evaluation of zolmitriptan delivery using ADAM
• Methods
The pharmacokinetics (PK) of zolmitriptan delivery using ADAM was evaluated in a single center, open-label, ascending dose and tolerability Phase I study in healthy volunteers, aged 18–60 years (n = 20). The study was reviewed and approved by The Alfred Hospital Ethics Committee and all participants provided informed consent.
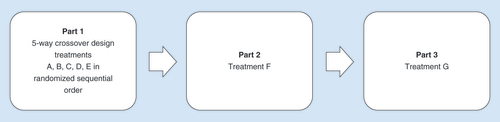
The study was a five-way crossover study with treatments administered in a randomized order using a randomization table (). The study treatments are outlined in . In treatment groups A, B, C and F varying doses of zolmitriptan were administered using the 5 cm2 (3 cm2 microarray) ADAM system and a 0.26 J applicator. In treatment groups B and F, two ADAMs of identical zolmitriptan dose (0.48 and 1.9 mg, respectively) were administered simultaneously. In treatment group G, the 3.8 mg dose was administered using 10 cm2 ADAM and a 0.52 J applicator. Treatment D consisted of a single zolmitriptan tablet, and treatment E consisted of a subcutaneous injection of sumatriptan.
Table 1. Study treatments.
No concomitant prescription medications were allowed other than hormone replacement therapy, proton pump inhibitors, antihistamines or intermittently used NSAIDs.
Participants were required to stay in the clinic overnight in a fasting state, taking only liquids after 8:00 PM. Fasting was continued until 2 h after dosing. The ADAMs were removed 30 min after application. PK sampling occurred at 0, 2, 5, 10, 15, 20, 30, 60 min and 2, 4, 8, 12 and 24 h after dosing.
Plasma concentrations of zolmitriptan and its active metabolite, N-desmethyl zolmitriptan, a 5-HTIB/1D receptor agonist that is two- to five-times more potent than zolmitriptan in animal models [Citation13], were determined by HPLC electrospray ionization-tandem mass spectrometry. All PK calculations and descriptive and inferential statistical analyses were performed using EXCEL and SPSS v22.0 software. Pairwise comparisons of AUC2hr were performed using ANOVA paired t-tests (SAS v9). The relative exposure and relative Cmax of ADAM zolmitriptan were compared with zolmitriptan tablets using the following formula:
Adverse events (AEs) were collected until post-study procedures for the seventh treatment were completed or until early withdrawal. All AEs considered treatment-related were followed until the event or its sequelae resolved or stabilized. Visual assessments of the application sites were made at 30 min and 24 h after removal of the ADAM. Erythema, edema, patch-related superficial punctate bruising and bleeding were scored according to severity on a scale of 0 to 3, with 0 indicating no findings and 3 indicating a severe finding.
All datasets on which the conclusions of the report rely are available on request.
Results
A total of 44 participants were screened and 20 were enrolled. Treatment was completed in 19, 20, 20, 19, 20, 20 and 20 participants in groups A, B, C, D, E, F and G, respectively. Participant demographics and baseline characteristics are provided in .
Table 2. Participant demographics and baseline characteristics.
The zolmitriptan (groups A, B, C, D, F, G) and sumatriptan (group E) concentration–time curves are shown in . The median (range) tmax for group E was 10 (5–20) min. Absorption in the ADAM zolmitriptan groups was rapid at all doses; tmax was comparable to sumatriptan subcutaneous injection, with peak median plasma concentration occurring within 20 min of application (). For the ADAM zolmitriptan 1.9 mg and above groups, Cmax and exposure at 2 h (AUC2hrs) were considerably higher than for zolmitriptan 2.5 mg oral tablets. However, overall exposure (AUCinf) was lower in all ADAM zolmitriptan groups. Pairwise comparisons of AUC2hrs are presented in . The AUC2hrs were significantly different among all groups except groups B and D. The mean exposure was proportional to the dose for single ADAM administration. There was less conversion and less exposure to the N-desmethyl zolmitriptan metabolite for ADAM zolmitriptan compared with zolmitriptan tablets.
(A) Zolmitriptan groups A, B, C, D, F, G, zero to 24 h (B) Zolmitriptan groups A, B, C, D, F, G, zero to 2 h (C) Sumatriptan group E, zero to 24 h (D) Sumatriptan group E, zero to 2 h.
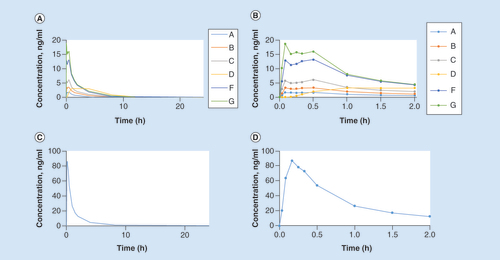
Table 3. Plasma pharmacokinetic parameters.
Table 4. Intergroup pairwise comparisons of AUC2h.
Overall, 95% (19/20) of participants experienced a total of 77 AEs during the study; 87% were considered mild, and none was severe. Among these, 87% (67/77) of AEs were considered possibly or probably related to treatment. No serious AEs occurred during the conduct of the study. details adverse events that occurred in more than one participant.
Table 5. Adverse events with incidence ≥1.
Evaluation of application site(s) 30 min after application showed mostly mild-to-moderate symptoms. Mild erythema remained 24 h after patch application for all groups. By 24 h after administration, edema and bleeding returned to near baseline levels for all groups. Superficial punctate bruising returned to baseline for all groups except group C (). One patient in group F reported pain at the application site.
Table 6. Application site assessments at 30 min and 24 h after removal of the Adhesive Dermally-Applied Microarray.
Conclusion & discussion
Optimal migraine treatment provides rapid pain relief with good tolerability. Triptans are the first-line acute migraine treatment in patients with disabling migraines and no vascular contraindications.
Orally administered triptans may be difficult to swallow or retain in patients with nausea. They can be slowly absorbed, which may be exacerbated by gastrointestinal dysmotility in migraine, resulting in inconsistent delivery. To circumvent these issues, intranasal and subcutaneously administered formulations have been developed. While administering the drugs via these routes of administrations may result in faster absorption, intranasal formulations suffer from poor palatability, and many patients are averse to the use of needles.
Here we have described an ADAM system for intracutaneous administration of zolmitriptan. ADAM is a needle-free device that is applied to the skin where microprojections penetrate the stratum corneum delivering drug proximal to the capillaries, with limited likelihood of stimulating sensory nerve endings.
In this Phase I trial, zolmitriptan delivery with ADAM provided rapid and reproducible zolmitriptan delivery. The tmax was less than 20 min and was comparable to subcutaneously administered sumatriptan. Absorption was substantially faster than for orally administered zolmitriptan, with less measurable N-desmethyl zolmitriptan metabolite, presumably due to a lack of first pass metabolism. PK parameters for subcutaneous sumatriptan and oral zolmitritpan in this study were similar to those previously reported [Citation14,Citation15].
Most AEs (88%) were consistent with those commonly seen in previous trials with triptans [Citation16] It should be noted that headache is the most frequently reported adverse event in clinical trials in healthy volunteers, and the incidence reported here is in keeping with these findings [Citation17–19]. ADAM was well tolerated, with only one participant in one treatment group reporting any pain at the application site. Where application site reactions were observed, they were generally mild and resolved after 24 h.
These initial data indicate that ADAM zolmitriptan is a promising approach for rapid and tolerable delivery of zolmitriptan. Results from a recently concluded Phase II/III trial will further elucidate safety and efficacy in migraine treatment. A generally painless, easy to use, skin delivery system for zolmitriptan, with PK comparable to injectable sumatriptan, is likely to be very useful clinically as a nonoral alternative for acute migraine treatment.
The flexibility of the ADAM system with respect to drug formulation makes it amenable to multiple additional indications, particularly when rapid needle-free delivery is warranted.
Migraine treatment
Despite the availability of multiple treatment options, considerable unmet need persists in the treatment of migraine.
Triptans are the first-line acute migraine treatment in patients with disabling migraines and no vascular contraindications.
Optimal migraine treatment provides rapid pain relief with good palatability; currently available formulations and routes of administration for migraine medications do not fully meet these criteria.
Adhesive dermally applied microarray
The Adhesive Dermally Applied-Microarray (ADAM) is a device for intracutaneous drug administration. It consists of a disposable adhesive backing, with an array of drug (zolmitriptan) coated titanium microprojections mounted on the skin-facing surface. The ADAM microprojection array measures 3 cm2 in diameter with 1987 titanium microprojections, each 340 μm in length.
It is applied to the skin using a low cost, reusable, handheld applicator. Following application, the microprojections penetrate the stratum corneum proximal to the microcapillaries in skin. The shallow depth of penetration and the minuteness of the size of the microprojections limit the likelihood of stimulating sensory nerve endings.
The ADAM is removed after 30 min and is disposed of.
Each ADAM is individually packaged and stored at room temperature.
Pharmacokinetic evaluation of zolmitriptan delivery using ADAM
The pharmacokinetics of zolmitriptan delivery using ADAM was evaluated in a Phase I, single center, open-label, ascending dose, tolerability Phase I study in healthy volunteers, aged 18–60 years (n = 20).
The time to maximum concentration was less than 20 min and was comparable to subcutaneously administered sumatriptan.
Absorption was substantially faster than for orally administered zolmitriptan with less N-desmethyl zolmitriptan metabolite, presumably due to a lack of first pass metabolism.
Tolerability of ADAM
In the Phase I study, most adverse events were consistent with those commonly seen in previous trials with triptans.
The most commonly reported adverse events (≥10% in any group) were paresthesia; headache; throat and jaw tightness, heaviness or ache; hot flushes; and upper respiratory tract infection.
ADAM was well tolerated, with only one participant in one of five ADAM treatment groups reporting any pain at the application site.
Where application site reactions were observed, they were generally mild and resolved after 24 h.
Informed consent disclosure
The study was reviewed and approved by The Alfred Hospital Ethics Committee and all participants provided informed consent.
Acknowledgements
The authors would like to thank Peter E Daddona and Thorsten Von Stein for their support during the initial stages of the project.
Financial & competing interests disclosure
DJ Kellerman and M Ameri are employees of Zosano, Inc. SJ Tepper serves as a consultant for Acorda Therapeutics, Alder Biopharmaceuticals, Allergan, Amgen, Autonomic Technologies, Inc., Avanir Pharmaceuticals, Biovision, electroCore, Eli Lilly, eNeura, Kimberly-Clark, Pernix Therapeutics, Pfizer, Teva Pharmaceutical Industries and Zosano Pharma. SJ Tepper serves on the advisor’s board for Alder Biopharmaceuticals, Allergan, Amgen, Autonomic Technologies, Inc., Avanir Pharmaceuticals, Charleston Laboratories, Dr Reddy’s, Kimberly-Clark, Pfizer, Scion Neurostim, Teva Pharmaceutical Industries and Zosano Pharma. SJ Tepper performs research (without personal compensation) for Alder Biopharmaceuticals, Allergan, Amgen, Autonomic Technologies, Inc., Avanir Pharmaceuticals, electroCore, eNeura, Scion NeuroStim, Teva Pharmaceutical Industries and Zosano Pharma. SJ Tepper has received stock options from Autonomic Technologies, Inc. SJ Tepper receives royalties from University of Mississippi Press and Springer. SJ Tepper receives salary compensation from Dartmouth-Hitchcock Medical Center and the American Headache Society. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support by Pamela Foreman, PhD and funded by Zosano Pharma. Statistical analyses provided by Pharmaceutical Solutions Ltd, and funded by Zosano Pharma.
References
- Lipton RB , BuseDC , SerranoD , HollandS , ReedML . Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study . Headache53 ( 8 ), 1300 – 1311 ( 2013 ).
- Gilmore B , MichaelM . Treatment of acute migraine headache . Am. Fam. Phys.83 ( 3 ), 271 – 280 ( 2011 ).
- Marmura MJ , SilbersteinSD , SchwedtTJ . The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies . Headache55 ( 1 ), 3 – 20 ( 2015 ).
- Evers S , AfraJ , FreseAet al. EFNS guideline on the drug treatment of migraine–revised report of an EFNS task force . Eur. J. Neurol.16 ( 9 ), 968 – 981 ( 2009 ).
- Dahlof CG . Non-oral formulations of triptans and their use in acute migraine . Curr. Pain Headache Rep.9 ( 3 ), 206 – 212 ( 2005 ).
- Houghton LA , FowlerP , KeeneON , ReadNW . Effect of sumatriptan, a new selective 5HT1-like agonist, on liquid gastric emptying in man . Aliment. Pharmacol. Ther.6 ( 6 ), 685 – 691 ( 1992 ).
- Cipolla G , SaccoS , CremaF , MoroE , De PontiF , FrigoG . Gastric motor effects of triptans: open questions and future perspectives . Pharmacol. Res.43 ( 3 ), 205 – 210 ( 2001 ).
- Tack J . The physiology and the pathophysiology of the gastric accommodation reflex in man . Verhandelingen62 ( 3 ), 183 – 207 ; discussion 207–110 ( 2000 ).
- Scott AK . Sumatriptan clinical pharmacokinetics . Clin. Pharmacokinet.27 ( 5 ), 337 – 344 ( 1994 ).
- ONZETRA Xsail, package Insert . Avanir Pharmaceuticals, Inc. , CA, USA ( 2016 ).
- Munjal S , GautamA , OffmanE , Brand-SchieberE , AllenbyK , FisherDM . A randomized trial comparing the pharmacokinetics, safety, and tolerability of DFN-02, an intranasal sumatriptan spray containing a permeation enhancer, with intranasal and subcutaneous sumatriptan in healthy adults . Headache56 ( 9 ), 1455 – 1465 ( 2016 ).
- Yates R , NairnK , DixonR , KempJV , DaneAL . Pharmacokinetics, dose proportionality, and tolerability of single and repeat doses of a nasal spray formulation of zolmitriptan in healthy volunteers . J. Clin. Pharmacol.42 ( 11 ), 1244 – 1250 ( 2002 ).
- Zomig, package insert . Impax Pharmaceuticals , CA, USA ( 2012 ).
- Lionetto L , CasollaB , MastropietriFet al. Pharmacokinetic evaluation of zolmitriptan for the treatment of migraines . Expert Opin. Drug Metab. Toxicol.8 ( 8 ), 1043 – 1050 ( 2012 ).
- Lionetto L , NegroA , CasollaB , SimmacoM , MartellettiP . Sumatriptan succinate: pharmacokinetics of different formulations in clinical practice . Expert Opin. Pharmacother.13 ( 16 ), 2369 – 2380 ( 2012 ).
- Ferrari MD , GoadsbyPJ , RoonKI , LiptonRB . Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials . Cephalalgia22 ( 8 ), 633 – 658 ( 2002 ).
- Rosenzweig P , BrohierS , ZipfelA . The placebo effect in healthy volunteers: influence of experimental conditions on the adverse events profile during phase I studies . Clin. Pharmacol. Ther.54 ( 5 ), 578 – 583 ( 1993 ).
- Lutfullin A , KuhlmannJ , WensingG . Adverse events in volunteers participating in Phase I clinical trials: a single-center five-year survey in 1,559 subjects . Int. J. Clin. Pharmacol. Ther.43 ( 5 ), 217 – 226 ( 2005 ).
- Sibille M , DeigatN , JaninA , KirkesseliS , DurandDV . Adverse events in phase-I studies: a report in 1015 healthy volunteers . Eur. J. Clin. Pharmacol.54 ( 1 ), 13 – 20 ( 1998 ).