Abstract
Aim: To explore fracture outcomes with tapentadol or oxycodone, two opioids with differing mechanisms of action. Materials & methods: Retrospective cohort pilot study, using MarketScan® Commercial and Medicare Supplemental claims databases, on patients with postoperative pain, back pain, or osteoarthritis and ≥1 claim for tapentadol (n = 16,457), oxycodone (n = 1,356,920), or both (n = 15,893) between June 2009 and December 2015. Results: During 266,826 and 9,007,889 days of tapentadol and oxycodone treatment, patients evidenced 1080 and 72,275 fractures, respectively. Fracture rates per treatment-year were 1.512 for tapentadol and 3.013 for oxycodone. Conclusion: Examination of administrative claims has inherent limitations, but this exploratory analysis indicates a lower fracture rate with tapentadol than oxycodone in the analyzed dataset, which needs confirmation by further clinical trials.
The use of strong opioids for the treatment of severe pain, while decreased in recent years, remains at high levels [Citation1]. Although opioids can be very effective as analgesics, they are not a panacea for every type of pain and major concerns have been raised with their long-term use. There is consensus that they must only be used appropriately in selected and supervised patients with pain as part of a comprehensive, multimodal, multidisciplinary approach [Citation2,Citation3]. Importantly, opioids can be associated with an array of adverse effects such as sedation, constipation, endocrinopathy and addiction. Additionally, there is evidence that chronic opioid use is associated with an increased risk of bone fracture. Yue et al., in a recent meta-analysis of 12 cohort studies and six case-control studies, reported an overall fracture risk of 1.78 (95% CI: 1.53–2.07) associated with opioid use [Citation4]. Fractures are, in turn, related to increased mortality rates, declines in both physical and mental health and substantial healthcare costs [Citation5–8].
The mechanism underlying the association between opioid use and fracture risk is unknown to date. Two potential mechanisms have been discussed in the literature. One is that opioids increase the risk of somnolence/dizziness, leading to falls and fractures, particularly in the elderly [Citation5–7]. The second postulated mechanism is that chronic opioid use reduces bone mass density, either directly through an effect on osteoblasts [Citation8–10] or by an indirect effect via opioid-induced hypogonadism [Citation11]. Higher doses of opioids are reported to confer a higher fracture risk [Citation12], but there is a lack of studies on fracture risk in relation to duration of opioid therapy, formulation, or the specific opioid agent used.
Oxycodone is an opioid prescribed for the relief of moderate-to-severe acute and chronic pain, including osteoarthritic [Citation13], neuropathic [Citation14], postoperative [Citation15] and cancer pain [Citation16]. The efficacy of oxycodone is attributed primarily to its mu-opioid receptor agonist activity [Citation17,Citation18]. Tapentadol is an atypical opioid [Citation19] with a dual mechanism of action via mu-opioid receptor agonism and noradrenaline reuptake inhibition [Citation20,Citation21]. Tapentadol has been shown to be effective for the relief of chronic [Citation22–27], neuropathic [Citation28–30], acute [Citation31–34] and cancer pain [Citation35], with analgesic effects comparable to oxycodone but with a more tolerable side effect profile, particularly for gastrointestinal effects [Citation36–39]. Pooled analyses and meta-analyses of published studies support these conclusions [Citation40–42].
Assessment of the fracture risk associated with individual opioids based on real-world data is challenging, for several reasons. Although not supported by clinical practice guidelines, the concomitant use of multiple opioids is common and the time-to-event pattern for fractures is unknown. Furthermore, opioids are widely used in elderly populations, in whom the risks of low bone density, falls and fractures are inherently elevated [Citation43,Citation44]. Moreover, details related to the circumstances leading to the fracture may not be known. Despite these challenges, we initiated an exploratory claims database study of tapentadol immediate release (IR) and prolonged release (PR) and oxycodone IR and PR formulations, to investigate in a descriptive way the population characteristics and fracture outcomes in patients treated with these opioids with differing mechanisms of action.
Materials & methods
This is a retrospective cohort pilot study investigating the population characteristics and fracture outcomes in patients treated with tapentadol IR and/or PR or oxycodone IR and/or PR. The data sources of this study were the MarketScan® Commercial and Medicare Supplemental claims databases in the USA, which contain inpatient, outpatient and prescription drug administrative healthcare claims for individuals insured through commercial or Medicare supplemental insurance plans. Data for this study spanned 1 June 2008 through 31 December 2016.
Study sample
The study sample was composed of patients with acute or chronic pain with evidence of ≥1 claim for tapentadol or oxycodone. Acute pain was defined as at least one surgery, while chronic pain was defined as a diagnosis of back pain or osteoarthritis; these pain indications were selected as part of a wider program of investigations of pain management that concentrated specifically on surgery, back pain and osteoarthritis. Patients with a claim for oxycodone or tapentadol between 1 June 2009 and 31 December 2015 were selected; the date of the first claim served as the index date. The period of initiation in 2009 was chosen based on the start of tapentadol availability in the USA and the period end was chosen based on the latest point of data availability in the database at the time of the analysis. This analysis was the first in a series of planned analyses in this pilot study. As this is an exploratory pilot study, sample size calculation was not performed a priori; all patients from the MarketScan database who met the inclusion criteria were included in the study.
To be eligible for the study, patients had to have at least 12 months of continuous medical and pharmacy eligibility prior to and following study index, be ≥18 years of age at index and could not have had a claim for tapentadol or oxycodone in the 12 months prior to index. Patients were also required to evidence a surgery or diagnosis for back pain or osteoarthritis during the 24-month period surrounding index (12 months pre-index through 12 months postindex). Surgery was defined as the presence of ≥1 claim for a surgical procedure, while diagnoses of back pain and osteoarthritis were defined as ≥2 nondiagnostic claims for back pain or osteoarthritis, respectively. Individuals with a claim for cancer or opioid dependence at any time during the study period were excluded; cancer patients were excluded as the pain management protocols for cancer generally differ from chronic noncancer pain, as well as between different types and stages of cancer, while patients with opioid dependence were excluded because of different usage patterns and possible misuse. No other exclusion criteria such as prior medications or comorbid conditions were applied.
Eligible patients were followed over a 12-month preperiod and variable postperiod (minimum 12 months in duration). Patients exited the cohort based on one of the three situations: inpatient death, stop of data availability, or reaching the study period end date (31 December 2015). Having a fracture was not a reason for stopping follow-up.
Outcomes
Fractures in the postperiod were identified via the presence of ICD-9 or ICD-10 diagnosis codes in the claim record. Patients were allowed to evidence multiple fractures over the follow-up period, thus overall fracture rates and not incidence rates are reported. After the first fracture, subsequent fracture claims meeting any of the following criteria were considered to be related to the first fracture; thus, these events did not count as a new fracture event: second claim occurred within 14 days of the first fracture claim; second claim occurred within 180 days of the first fracture claim and had the same first three digits of the diagnosis code (indicates fracture in same/similar location); or the second claim did not occur in the inpatient or emergency setting. Diagnostic and other code lists can be seen in the Supplementary material.
Fractures were classified as tapentadol fracture, oxycodone fracture, or other fracture, depending on the treatment during the occurrence of the fracture (see ). If the fracture occurred during a period of tapentadol treatment, it was assigned to tapentadol, while it was classified as an oxycodone fracture if the event occurred during a period of oxycodone. If the event occurred while the patient was taking tapentadol and oxycodone concomitantly, it was assigned to both. Fractures occurring during a period of neither tapentadol nor oxycodone treatment were classified as other fracture.
The exact person-exposure time to tapentadol and oxycodone was recorded to account for the total treatment duration with the respective treatments. A single patient could have contributed person-time to tapentadol, oxycodone, or both during the study period.
The analysis included patient- and fracture-level outcomes for overall fracture rates. Patient-level outcomes included the rate of overall tapentadol or oxycodone fractures per-patient-treatment-year, defined as the total number of tapentadol or oxycodone fractures divided by the total duration (in years) of tapentadol or oxycodone treatment during the postperiod for the entire sample (patients with or without fractures). The total duration of tapentadol or oxycodone treatment was calculated by summing the number of days in all tapentadol or oxycodone treatment periods during follow-up. A treatment period was defined as the duration of tapentadol or oxycodone treatment, based on days’ supply, without discontinuation (>30 days with no drug on hand). Under this scheme, gap days were attributed to days of tapentadol or oxycodone treatment as long as the patient did not show evidence of discontinuation. This approach was utilized, as tapentadol and oxycodone are frequently used on an as-needed basis, thus a prescription may last longer than the associated days’ supply if a patient is not taking the medication on a daily basis. Patients were permitted to evidence multiple tapentadol and/or oxycodone treatment periods over the variable follow-up.
Fracture-level outcomes were assessed for each fracture and included the patient age at time of fracture, duration of continuous tapentadol or oxycodone use prior to fracture, opioid days’ supply (noncontinuous) prior to fracture, location of fracture, use of concomitant medications during follow-up and presence of comorbidities during follow-up. The duration of continuous tapentadol or oxycodone utilization was defined as the number of days in the tapentadol or oxycodone treatment period prior to the fracture. The opioid days’ supply prior to fracture was the total days’ supply of any opioid (including tapentadol and oxycodone) on record at any time (start of preperiod on) prior to the fracture; treatment in this case was not required to be continuous. Other opioids assessed included: codeine, dihydrocodeine, tramadol, buprenorphine, fentanyl, hydrocodone, hydromorphone, meperidine, methadone and morphine.
Fracture location was based on the first three digits of ICD-9 and ICD-10 codes and classified as skull, spine and trunk, upper limb, lower limb or multiple locations. Concomitant medication use and comorbidities that have been associated with fracture risk were reported as the number and percentage of patients with evidence of use of the following medications or conditions prior to the fracture: medications included androgen deprivation therapy and gonadotrophin-releasing hormones, antidepressants, antiepileptics/anticonvulsants, aromatase inhibitors, bisphosphonates, calcineurin inhibitors, chemotherapy, glucocorticoids, heparin, medroxyprogesterone acetate, muscle relaxants, proton pump inhibitors, selective serotonin reuptake inhibitors and thiazolidinediones [Citation45–51]; comorbidities included celiac disease, Crohn’s disease, ulcerative colitis, depression, diabetes, osteopenia and osteoporosis [Citation52–56].
Patient and fracture outcomes were stratified based on duration of continuous tapentadol or oxycodone days’ supply prior to fracture into the following subgroups: <30, 31–90 and >90 days.
Results
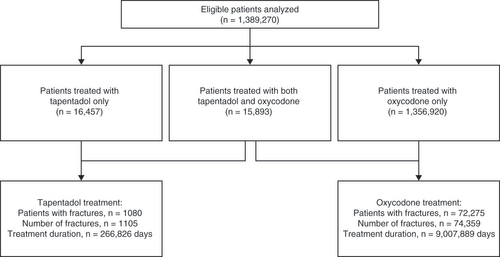
A total of 1,389,270 patients who met the eligibility criteria, including 16,457 who received tapentadol only, 1,356,920 who received oxycodone only and 15,893 who received both tapentadol and oxycodone, were included in the analysis ().
Of the 1,389,270 patients analyzed, 185,369 (13.3%) patients had at least one fracture over a mean length of enrollment of 1107.2 (standard deviation [SD] 599.8) days. The total number of fractures among all qualifying patients was 204,522; of these, 1105 fractures were assigned as tapentadol fractures, 74,359 as oxycodone fractures and 129,058 as other fractures. The mean age of patients at first fracture was 55.2 (SD 16.3) years in the overall population. The mean age of patients at the time of fracture was similar during tapentadol and oxycodone treatment at 56.7 (SD 15.6) and 54.3 (SD 54.3) years, respectively. The mean duration of continuous tapentadol or oxycodone treatment prior to fracture was greater for tapentadol than oxycodone fractures at 95.5 (SD 247.4) and 34.9 (SD 151.7) days, respectively. The mean total number days’ supply of any opioid at any time prior to fracture was also higher for tapentadol than oxycodone fractures at 518.7 (SD 629.1) mg versus 285.5 (SD 500.3) mg, respectively.
Subanalyses of tapentadol and oxycodone fractures based on the mean duration of continuous tapentadol or oxycodone prior to fracture (i.e., <30, 31–90 or >90 days) showed that for both tapentadol and oxycodone fractures, the majority of fractures occurred in patients with <30 days’ supply of tapentadol or oxycodone at the time of fracture but the proportion was higher in the oxycodone-treated patients (75.4 and 91.0%, for tapentadol and oxycodone, respectively).
During treatment with tapentadol, 1080 patients had 1105 fractures over a total duration of treatment of 266,826 days, providing a fracture rate per tapentadol treatment-year of 1.512. During treatment with oxycodone, in other words, in a population with different patient characteristics and different treatment patterns, 72,275 patients had 74,359 fractures over a total duration of treatment of 9,007,889 days, giving a fracture rate per oxycodone treatment-year of 3.013.
Analyses of data at the fracture level are shown in . The majority of fractures occurred in the lower limbs (43.8%, tapentadol fractures; 40.3%, oxycodone fractures), followed by the spine and trunk, including hips (27.6, 22.5%) and the upper limbs (23.1, 30.3%). The most common concomitant medications prescribed prior to fracture were glucocorticoids (62.4%, tapentadol; 46.1%, oxycodone), antidepressants (50.4, 38.6%), antiepileptics/anticonvulsants (36.2, 22.0%), muscle relaxants (33.9, 23.6%), proton pump inhibitors (33.8, 26.0%), selective serotonin reuptake inhibitors (27.1, 24.9%) and bisphosphonates (9.3, 6.8%). Common comorbidities prior to fracture included depression (20.7%, tapentadol; 18.5%, oxycodone), diabetes (17.9, 16.5%) and osteoporosis (11.1, 6.9%).
Table 1. Fracture-level characteristics among fractures in tapentadol- and oxycodone-treated patients, during variable length study period.
Discussion
This exploratory post hoc analysis of a US claims database was aimed primarily at comparing bone fracture rates in patients using tapentadol or oxycodone, while highlighting the challenges in assessing fracture risks for individual opioids based on real-world data. In this analysis, a lower fracture rate was observed with tapentadol than oxycodone. Because of the study design limitations, including the lack of information on potential confounding factors such as smoking, diet, exercise, etc., these results should be interpreted with great care.
Review of patient characteristics in the tapentadol and oxycodone groups showed that approximately three-fourths were aged <65 years, most fractures occurred in the lower limbs, comorbidities including depression, diabetes and osteoporosis were common and concomitant medication use – notably glucocorticoids and antidepressants was frequent. Because of this study design, there were differences between tapentadol and oxycodone groups in the frequencies of these characteristics.
Despite the exploratory nature of the study, the data may offer insights into the association underlying opioid-related fractures. In particular, the observation that the majority of fractures during both tapentadol and oxycodone treatment occurred within less than 30 days of continuous drug use at the time of fracture indicates that acute effects of oxycodone or tapentadol treatment, such as somnolence/dizziness, may have contributed more to fracture rate than a chronic effect such as reduced bone density.
Limitations
Because of the origin of these data (i.e., real world, claims database), there are several limitations to the study, including lack of information on the specific indications for opioid treatment and physicians’ reasons for prescribing tapentadol versus oxycodone (including whether first- vs second or third-line therapy). Further, as this study included patients with prior opioid use, the individuals’ full history of opioid use and potential cumulative effects of switching opioids were not fully assessed. The duration of continuous tapentadol or oxycodone use was examined prior to the fracture and over the course of the study. However, patients may have been using other opioids at the time or had a history of opioid use which could have affected fracture outcomes. Additionally, as dose was not assessed as part of this study, no conclusions can be drawn on dose–effect relationships for the two opioids. Potential confounders such as prior medication, concomitant intake of other opioids, comorbidities, information on compliance with treatment, diet, exercise and other risk factors such as gender and smoking were not taken into account in the analyses; these modifiers could have significantly influenced the risk of fracture. Finally, the claims included in the analysis were limited to those from patients eligible for commercial or Medicare supplemental insurance in the USA.
Conclusion
This exploratory analysis of real-world data originating from a claims database revealed a lower rate of fracture with tapentadol versus oxycodone treatment. There are, however, inherent limitations in this study design that hinder a reliable assessment of associations of opioid use with outcomes such as fractures. It cannot be determined if the estimated fracture risk is due to the treatment alone or due to confounding factors in the populations treated. In order to confirm fracture risks for individual opioids such as those reported here, additional studies are warranted that would include a comparator opioid-naive population, an assessment of patients who used a single opioid, the potential for gender differences and the influence of risk factors. Such studies can address the key questions and unmet needs in populations at risk of fracture who are using or need to use opioids.
Tapentadol and oxycodone are opioids with differing mechanisms of action.
This exploratory post hoc analysis of a US claims database compared bone fracture rates in patients with postoperative pain, back pain, or osteoarthritis treated with tapentadol or oxycodone.
During treatment with tapentadol, 1080 patients had 1105 fractures over a total duration of 266,826 days.
During treatment with oxycodone, 72,275 patients had 74,359 fractures over a total duration of 9,007,889 days.
Fracture rates per treatment-year were 1.512 for tapentadol and 3.013 for oxycodone.
This exploratory analysis indicates a lower fracture rate with tapentadol than oxycodone in the analyzed dataset.
Study limitations include the investigation of patient groups with different characteristics and treatment patterns and lack of information on potential confounding factors.
Because of the study design limitations, these results should be interpreted with great care.
Author contributions
All authors contributed to the analysis and interpretation of data for the work, revised it critically for important intellectual content; and provided final approval of the version to be published.
Acknowledgments
Analyses of data were provided by BL Brady at IBM Watson Health.
Financial and competing interests disclosure
This study was supported by Grünenthal GmbH. Bart J Morlion Over the last 5 years, grants and/or honoraria received for: clinical research: Novartis, Pfizer, Janssen, Shionogi; speaker’s and/or consultancy activities: consultancy activities: Astellas, Boehringer Ingelheim, Grünenthal, Janssen, Mundipharma, TEVA, GSK, Kyowa-Kirin, Pfizer, Lilly, Boston Scientific, P&G. I Wild, R Karra, H Liedgens and M Sohns are employees of Grünenthal GmbH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support was provided by B Wolvey, Parexel and was funded by Grünenthal GmbH.
Additional information
Funding
References
- Centers for Disease Control and Prevention . U.S. opioid prescribing rate maps. (2020). www.cdc.gov/drugoverdose/maps/rxrate-maps.html
- O’Brien T , ChristrupLL , DrewesAMet.al European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur. J. Pain21(1), 3–19 (2017).
- Dowell D , HaegerichTM , ChouR. CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA315(15), 1624–1645 (2016).
- Yue Q , MaY , TengYet.al An updated analysis of opioids increasing the risk of fractures. PLoS ONE15(4), e0220216 (2020).
- Buckeridge D , HuangA , HanleyJet.al Risk of injury associated with opioid use in older adults. J. Am. Geriatr. Soc.58(9), 1664–1670 (2010).
- Ensrud KE , BlackwellT , MangioneCMet.al Central nervous system active medications and risk for fractures in older women. Arch. Intern. Med.163(8), 949–957 (2003).
- Vestergaard P , RejnmarkL , MosekildeL. Fracture risk associated with the use of morphine and opiates. J. Intern. Med.260(1), 76–87 (2006).
- Perez-Castrillon JL , OlmosJM , GomezJJet.al Expression of opioid receptors in osteoblast-like MG-63 cells and effects of different opioid agonists on alkaline phosphatase and osteocalcin secretion by these cells. Neuroendocrinology72(3), 187–194 (2000).
- Rosen H , Bar-ShavitZ. Dual role of osteoblastic proenkephalin derived peptides in skeletal tissues. J. Cell. Biochem.55(3), 334–339 (1994).
- Rico H , CostalesC , CabranesJA , EscuderoM. Lower serum osteocalcin levels in pregnant drug users and their newborns at the time of delivery. Obstet. Gynecol.75(6), 998–1000 (1990).
- Coluzzi F , PergolizziJ , RaffaRB , MattiaC. The unsolved case of “bone-impairing analgesics”: the endocrine effects of opioids on bone metabolism. Ther. Clin. Risk Manag.11, 515–525 (2015).
- Saunders KW , DunnKM , MerrillJOet.al Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J. Gen. Intern. Med.25(4), 310–315 (2010).
- Friedmann N , KlutzaritzV , WebsterL. Long-term safety of Remoxy® (extended-release oxycodone) in patients with moderate to severe chronic osteoarthritis or low back pain. Pain Med.12(5), 755–760 (2011).
- Gaskell H , DerryS , StannardC , MooreRA. Oxycodone for neuropathic pain in adults. Cochrane Database Syst. Rev.7, Cd010692 (2016).
- Cheung CW , ChingWong SS , QiuQ , WangX. Oral oxycodone for acute postoperative pain: a review of clinical trials. Pain Physician20(2s), Se33–se52 (2017).
- Schmidt-Hansen M , BennettMI , ArnoldS , BromhamN , HilgartJS. Efficacy, tolerability and acceptability of oxycodone for cancer-related pain in adults: an updated Cochrane systematic review. BMJ Support Palliat. Care8(2), 117–128 (2018).
- Moradi M , EsmaeiliS , ShoarS , SafariS. Use of oxycodone in pain management. Anesth. Pain Med.1(4), 262–264 (2012).
- Yang PP , YehGC , YehTKet.al Activation of delta-opioid receptor contributes to the antinociceptive effect of oxycodone in mice. Pharmacol. Res.111, 867–876 (2016).
- Schug SA . The atypical opioids: buprenorphine, tramadol and tapentadol. Medicine Today.19(Suppl. 9), 5–11 (2018).
- Tzschentke TM , JahnelU , KogelBet.al Tapentadol hydrochloride: a next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today (Barc.)45(7), 483–496 (2009).
- Wade WE , SpruillWJ. Tapentadol hydrochloride: a centrally acting oral analgesic. Clin. Ther.31(12), 2804–2818 (2009).
- Afilalo M , EtropolskiMS , KuperwasserBet.al Efficacy and safety of Tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled Phase III study. Clin. Drug Investig.30(8), 489–505 (2010).
- Hale M , UpmalisD , OkamotoA , LangeC , RauschkolbC. Tolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, double-blind study. Curr. Med. Res. Opin.25(5), 1095–1104 (2009).
- Vorsanger G , XiangJ , OkamotoA , UpmalisD , MoskovitzB. Evaluation of study discontinuations with tapentadol inmmediate release and oxycodone immediate release in patients with low back or osteoarthritis pain. J. Opioid. Manag.6(3), 169–179 (2010).
- Buynak R , RappaportSA , RodKet.al Long-term safety and efficacy of tapentadol extended release following up to 2 years of treatment in patients with moderate to severe, chronic pain: results of an open-label extension trial. Clin. Ther.37(11), 2420–2438 (2015).
- Kavanagh S , KwongWJ , HammondGC , NelsonW , UpmalisD , YangM. Pain relief and tolerability balance of immediate release tapentadol or oxycodone treatment for patients with moderate to severe osteoarthritis or low back pain. Pain Med.13(9), 1110–1120 (2012).
- Wild JE , GrondS , KuperwasserBet.al Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract.10(5), 416–427 (2010).
- Baron R , JansenJP , BinderAet.al Tolerability, safety and quality of life with tapentadol prolonged release (PR) compared with oxycodone/naloxone PR in patients with severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, Phase IIIb/IV trial. Pain Pract.16(5), 600–619 (2016).
- Baron R , LikarR , Martin-MolaEet.al Effectiveness of tapentadol prolonged release (PR) compared with oxycodone/naloxone PR for the management of severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, Phase IIIb/IV study. Pain Pract.16(5), 580–599 (2016).
- Schwartz S , EtropolskiM , ShapiroDYet.al Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr. Med. Res. Opin.27(1), 151–162 (2011).
- Haeseler G , SchaefersD , PrisonN , AhrensJ , LiuX , KarchA. Combatting pain after orthopedic/trauma surgery- perioperative oral extended-release tapentadol vs. extended-release oxycodone/naloxone. BMC Anesthesiol.17(1), 91 (2017).
- Vorsanger GJ , KlopferAM , XiangJ , BensonCJ , MoskovitzBL , RosenthalNR. Immediate-release tapentadol or oxycodone for treatment of acute postoperative pain after elective arthroscopic shoulder surgery: a randomized, Phase IIIb study. J Opioid Manag.9(4), 281–290 (2013).
- Daniels SE , UpmalisD , OkamotoA , LangeC , HaeusslerJ. A randomized, double-blind, Phase III study comparing multiple doses of tapentadol IR, oxycodone IR and placebo for postoperative (bunionectomy) pain. Curr. Med. Res. Opin.25(3), 765–776 (2009).
- Daniels S , CassonE , StegmannJUet.al A randomized, double-blind, placebo-controlled Phase III study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute pain. Curr. Med. Res. Opin.25(6), 1551–1561 (2009).
- Imanaka K , TominagaY , EtropolskiMet.al Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Curr. Med. Res. Opin.29(10), 1399–1409 (2013).
- Etropolski M , KellyK , OkamotoA , RauschkolbC. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Adv. Ther.28(5), 401–417 (2011).
- Merchant S , ProvenzanoD , ModyS , HoKF , EtropolskiM. Composite measure to assess efficacy/gastrointestinal tolerability of tapentadol ER versus oxycodone CR for chronic pain: pooled analysis of randomized studies. J. Opioid Manag.9(1), 51–61 (2013).
- Hartrick C , Van HoveI , StegmannJU , OhC , UpmalisD. Efficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end-stage joint disease: a 10-day, Phase III, randomized, double-blind, active- and placebo-controlled study. Clin. Ther.31(2), 260–271 (2009).
- Biondi D , XiangJ , BensonC , EtropolskiM , MoskovitzB , RauschkolbC. Tapentadol immediate release versus oxycodone immediate release for treatment of acute low back pain. Pain Physician16(3), E237–246 (2013).
- Etropolski M , LangeB , GoldbergJ , SteupA , RauschkolbC. A pooled analysis of patient-specific factors and efficacy and tolerability of tapentadol extended release treatment for moderate to severe chronic pain. J. Opioid Manag.9(5), 343–356 (2013).
- Lange B , KuperwasserB , OkamotoAet.al Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain. Adv. Ther.27(6), 381–399 (2010).
- Riemsma R , ForbesC , HarkerJet.al Systematic review of tapentadol in chronic severe pain. Curr. Med. Res. Opin.27(10), 1907–1930 (2011).
- Krebs EE , PaudelM , TaylorBCet.al Association of opioids with falls, fractures and physical performance among older men with persistent musculoskeletal pain. J. Gen. Intern. Med.31(5), 463–469 (2016).
- Miller M , SturmerT , AzraelD , LevinR , SolomonDH. Opioid analgesics and the risk of fractures in older adults with arthritis. J. Am. Geriatr. Soc.59(3), 430–438 (2011).
- Moura C , BernatskyS , AbrahamowiczMet.al Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos. Int.25(5), 1473–1481 (2014).
- Shen C , ChenF , ZhangY , GuoY , DingM. Association between use of antiepileptic drugs and fracture risk: a systematic review and meta-analysis. Bone64, 246–253 (2014).
- Thong BKS , Ima-NirwanaS , ChinKY. Proton pump inhibitors and fracture risk: a review of current evidence and mechanisms involved. Int. J. Environ. Res. Public Health.16(9), 1571 (2019).
- Tseng OL , SpinelliJJ , GotayCC , HoWY , McBrideML , DawesMG. Aromatase inhibitors are associated with a higher fracture risk than tamoxifen: a systematic review and meta-analysis. Ther. Adv. Musculoskelet. Dis.10(4), 71–90 (2018).
- van Staa TP , LeufkensHG , AbenhaimL , ZhangB , CooperC. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford.)39(12), 1383–1389 (2000).
- Wallander M , AxelssonKF , LundhD , LorentzonM. Patients with prostate cancer and androgen deprivation therapy have increased risk of fractures-a study from the fractures and fall injuries in the elderly cohort (FRAILCO). Osteoporos. Int.30(1), 115–125 (2019).
- Zhu ZN , JiangYF , DingT. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone68, 115–123 (2014).
- Heikkila K , PearceJ , MakiM , KaukinenK. Celiac disease and bone fractures: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab.100(1), 25–34 (2015).
- Poiana C , CapatinaC. Fracture risk assessment in patients with diabetes mellitus. J. Clin. Densitom.20(3), 432–443 (2017).
- Schuit SC , vander Klift M , WeelAEet.al Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone34(1), 195–202 (2004).
- Shirazi KM , SomiMH , RezaeifarP , FattahiI , KhoshbatenM , AhmadzadehM. Bone density and bone metabolism in patients with inflammatory bowel disease. Saudi J. Gastroenterol.18(4), 241–247 (2012).
- Wu Q , LiuJ , Gallegos-OrozcoJF , HentzJG. Depression, fracture risk and bone loss: a meta-analysis of cohort studies. Osteoporos. Int.21(10), 1627–1635 (2010).