Abstract
Background: A modified algorithm for the treatment of lumbar spinal stenosis with hypertrophic ligamentum flavum using minimally-invasive lumbar decompression (mild®)was assessed, with a focus on earlier intervention. Patients & methods: Records of 145 patients treated with mild after receiving 0–1 epidural steroid injections (ESIs) or 2+ ESIs were retrospectively reviewed. Pain assessments as measured by visual analog scale (VAS) scores were recorded at baseline and 1-week and 3-month follow-ups. Results: Improvements in VAS scores at follow-ups compared with baseline were significant in both groups. No statistically significant differences were found between the two groups. Conclusion: Multiple ESIs prior to mild showed no benefit. A modified algorithm to perform mild immediately upon diagnosis or after the failure of the first ESI is recommended.
Lay abstract
Physicians use a structured decision-making process (an algorithm) to decide how best to treat lumbar spinal stenosis (LSS) that results from abnormal thickening of the spinal ligaments that run the length of the spinal cord. Early treatments can include one or more epidural steroid injections (ESIs). This study evaluated a change to the algorithm that involves earlier intervention with a minimally invasive, short outpatient procedure that removes a major root cause of the abnormal thickening (lumbar decompression) and leaves no implants behind. Records of patients treated with minimally-invasive lumbar decompression (mild®) after receiving either a single ESI procedure or none at all, were compared with the records of patients who underwent the mild procedure after receiving two or more ESIs (145 total patients). The patients’ pain scores before surgery, at 1 week postsurgery and at 3 months postsurgery were reviewed. The improvements in pain scores following the mild procedure were compared within each group and between the two groups. The improvements in pain scores at both the 1-week and 3-month follow-up visits indicated that the mild procedure had a positive effect for both groups. Further, there were no significant differences in how much pain scores improved when the two groups were compared. Since neither group experienced significantly more pain relief than the other, there appears to be no benefit to having multiple ESI procedures before undergoing the mild procedure. The authors recommend that the algorithm be modified to perform the mild procedure either as soon as LSS is diagnosed or after the failure of the first ESI procedure.
Lumbar spinal stenosis (LSS) is a decrease in the effective diameter of the spinal canal, which often leads to compression of the neural elements and subsequent neurogenic claudication [Citation1,Citation2]. LSS is generally the result of degenerative spinal conditions including intervertebral disc bulging and herniation, facet arthropathy and hypertrophic ligamentum flavum (HLF) [Citation3,Citation4]. The treatment algorithm for LSS begins with conservative management, which may include home exercise programs, physical therapy regimens, back braces and pain medications [Citation5]. For patients refractory to noninvasive conservative care, epidural steroid injections (ESIs) are commonly considered as the next step in the treatment continuum. While common, with an estimated 65% of patients with LSS receiving at least one ESI [Citation6], ESIs for neurogenic claudication have significant drawbacks. The steroid component in ESI treatments may address intraspinal inflammation related to radicular pain [Citation7], but the compression of neural structures causing neurogenic claudication is not related to inflammation and ESI treatment generally results in limited and short-term symptom relief [Citation8]. While studies have shown pain relief for up to 6 months in some LSS patients receiving ESI treatments, other studies have demonstrated limited effectiveness [Citation6,Citation9]. To achieve effectiveness over two to three years, five or more ESI treatments per year are often required [Citation10,Citation11]. The North American Spine Society (NASS) recommendations for treatment of degenerative spinal stenosis conclude that multiple ESIs for symptomatic relief of LSS with neurogenic claudication may be considered an optional treatment based on minimal evidence (recommendation grade C) [Citation12], with insufficient evidence to make a recommendation of transforaminal ESI in the treatment of lumbar radicular pain in the setting of central stenosis [Citation13]. The immunosuppressive properties of the steroids commonly used in ESI treatments are well-known and have been shown to increase susceptibility to opportunistic infections, leading to recommendations for patients with existing risk factors for infection to consider avoiding or limiting steroid therapy [Citation14,Citation15]. Even the targeted use of steroids in ESIs has been shown to result in suppression of the hypothalamic-pituitary-adrenal (HPA) axis as measured by cortisol levels, potentially reducing a host defense against infection [Citation16–18]. Studies also suggest that ESIs may cause bone loss, with larger doses conferring greater risk [Citation19].
A percutaneous image-guided lumbar decompression (PILD) treatment (mild®; Vertos Medical, CA, USA) offers a minimally invasive, steroid-free alternative to repeated ESI treatments for patients with moderate-to-severe LSS. This percutaneous direct decompression procedure, which increases space in the spinal canal by debulking thickened ligamentum flavum after gaining access through the lamina, leaves no implants behind and patients typically resume normal activity within 24 h with no restrictions. The mild procedure has been previously described in detail [Citation20]. This treatment has been shown to provide statistically superior efficacy compared with ESIs at follow-up intervals ranging from 6 months to 1 year [Citation21], and strong durability for the relief of LSS symptoms over 5 years [Citation22].
As a result of the superior efficacy of mild over ESI treatments and the ability to provide long-term symptom relief as safely as with ESIs, many physicians have established their standard of care to perform mild after failure of the first ESI or to eliminate the use of ESIs altogether. This multicenter study was performed to evaluate the impact of prior ESI therapy on pain relief for mild patients during follow-up and provide objective evidence supporting a change to the treatment algorithm for LSS patients suffering from neurogenic claudication secondary to HLF.
Materials & methods
Six centers participated in a retrospective study of LSS patients presenting with symptoms of neurogenic claudication primarily caused by HLF and treated with the mild procedure. All patients at each site that met these criteria and were treated from January 2020 through October 2020 were included in the study with no limitations on the number of patients included. Due to maintenance of patient anonymity, IRB approval and patient consent was not required for this study per US regulations regarding human clinical studies. Demographics, spinal comorbidities and procedure data were collected, and visual analog scale (VAS) assessments at baseline, 1-week and 3-month follow-up were recorded. Patients were divided into two groups: those receiving either one or no ESIs prior to treatment with mild, and those receiving two or more ESIs prior to treatment with mild. Improvement in VAS at each follow-up was compared between groups. Statistical significance was set at p < 0.05.
Outcome measures
All patients at the six centers who were treated with mild and had documented VAS scores recorded at baseline, 1-week and 3-month follow-ups were included in the study. Patient demographics, spinal comorbidities, treatment details and pain assessment as measured by VAS were collected. VAS measures pain level using a numeric scale, with zero representing no pain and ten indicating the worst pain. Patients included in the study were divided into two groups; those receiving zero or one ESI procedure prior to treatment with mild, and those receiving two or more ESI procedures before subsequent mild treatment. VAS scores at 1-week and 3-month follow-up were compared with baseline scores within each group to determine the magnitude of improvement and its statistical significance following the mild procedure. VAS scores were also compared between the two groups at all time points to identify any influence that the number of prior ESI treatments might have on mild outcomes.
Statistical methods
Data from all sites were pooled prior to analysis. Descriptive analysis was performed using mean ± standard deviation (SD) for continuous variables and count (percent) for categorical variables. Statistical analysis included a paired t-test for patient age and a test of proportions for all other patient demographic and clinical characteristics when comparing the cohort receiving ≤1 ESI treatment to those receiving two or more treatments. The significance of procedural information was assessed using a test of proportions for comparing the levels treated between the two cohorts and a Pearson’s chi-square test for the number of levels treated between cohorts. A paired t-test was used to determine if improvements in VAS scores between baseline and 1 week as well as between baseline and 3 months for each cohort were significant. The differences in VAS scores between the two cohorts at baseline, 1 week and 3 months were also compared using a t-test. The significance level for all statistical tests was set at 5% (p < 0.05). Analyses were performed using Stata 14 statistical software (StataCorp LLC, TX, USA).
Results
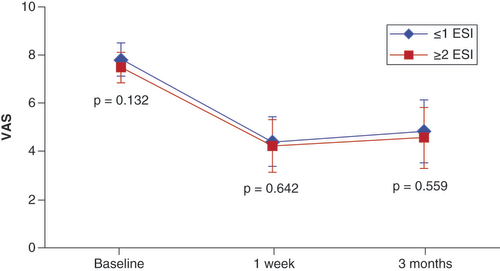
A total of 145 patients were included in the study, with the number of patients treated at each of the six sites ranging from 15 (10.3%) to 34 (23.4%). The average age was 72.7 years (range 40–92) and 53.8% (n = 78) were female. All patients presented with symptoms of neurogenic claudication and HLF. All but six patients (95.9%) presented with two or more comorbidities. There were no significant differences between the two ESI cohorts for any of the patient demographic or clinical characteristics measured (). The lack of any significant differences supports the homogeneity of the two cohorts.
Table 1. Baseline demographics and clinical characteristics.
Most patients received the mild treatment at one or two lumbar disc levels (n = 125, 86.2%). The most frequent level treated was L4–L5 (n = 112, 77.2%). There were no significant differences between the two cohorts for specific levels treated or number of levels treated (). There were no significant differences in absolute VAS scores between the two groups at any time point (). Accordingly, there were no statistically significant differences between the two groups in the degree of improvement in VAS scores at 1-week and 3-month follow-up compared with respective baseline values. Comparison of VAS scores is shown in .
Table 2. mild procedure data.
Table 3. VAS scores.
Discussion
Historically, ESIs have been an accepted intervention on the LSS care continuum for patients who are refractory to noninvasive treatments for painful neurogenic claudication symptoms. Commonly consisting of local anesthetic and steroid components, ESIs are intended to suppress pain and reduce inflammation [Citation23]. The likely mechanism for corticosteroids in affecting the neuropathic pain cascade remains unclear, although it may be related to reduced swelling of neural tissue [Citation24,Citation25].
The need for steroids in epidural injections in the treatment of LSS is also unsettled; while the inclusion of steroids has been shown to be more effective than injection of anesthetic alone in a study of 36 patients [Citation26], three separate randomized, controlled trials with a total of 620 patients found no significant difference in outcomes between groups of patients treated with an anesthetic plus steroid and those treated with anesthetic alone [Citation10,Citation11,Citation27]. The questionable benefit of the use of steroids in treating patients with LSS is further compounded by the increased risk of opportunistic infections. Research on suppression of the HPA axis following ESI is conclusive and shows that even a targeted delivery of steroids to the site of inflammation, using a route that does not rely on blood flow and should therefore minimize delivery to high-blood-flow organs, results in cortisol reductions and likely impairment of the immune system response to infections [Citation7,Citation16,Citation18]. Further, a relationship between ESI and fracture risk has been suggested and may be related to bone loss caused by ESI exposure [Citation19]. Given this, the benefits of epidural injections with steroids should be carefully considered so as not to subject patients to undue risks. Regardless of whether steroids are included, it is clear that epidural injections treat only the symptoms of LSS, and there is no indication that such injections reduce the physical compression of neural tissue.
As with conservative care, ESI represents a step in the LSS care continuum that may not be effective for all patients. Investigators have reported between 52% and 76% of LSS patients with average ages ranging 66.4–78.7 years were nonresponsive to ESI treatment when measuring pain with the Numeric Rating Scale (NRS), with responders typically defined as a score improvement of at least 50% or 2 points [Citation16,Citation21,Citation28–31]. While two studies of LSS patients with average ages ranging 52.3–56.3 demonstrated better NRS results, between 13% and 26% of LSS patients still did not respond to ESI. For patients who are responsive to ESI treatment, multiple subsequent treatments are often necessary to provide long-term relief, with an average of 2–3 injections needed per year [Citation10,Citation16,Citation21,Citation27,Citation31]. The number of injections needed to maintain pain relief for an individual patient has been measured as high as seven over one year [Citation11] and sixteen over three years [Citation31]. This high injection rate indicates a lack of efficacy and increases the risk of immunosuppression and bone loss.
The mild treatment serves as an effective, less invasive step on the care continuum for LSS patients suffering from neurogenic claudication and HLF before considering open surgery. Unlike conservative therapies and ESI, which focus on suppressing the symptoms of LSS, the mild treatment physically removes a major root cause of the symptoms by preferentially resecting small pieces of lamina and debulking the thickened ligamentum flavum to increase space in the spinal canal. mild leaves no implants behind and patients typically resume normal activity within 24 h with no restrictions. 6 months following treatment in a randomized controlled trial, 55.9% of 143 patients receiving the mild treatment experienced an NRS improvement of 2 or more points compared with 33.3% of the 129 patients receiving ESI; the 22.6% difference was statistically significant [Citation21]. These outcomes are consistent with those reported by other investigators [Citation4,Citation8,Citation30,Citation32–36].
At 12-month follow-up, the difference in responder rates of patients receiving mild versus those receiving ESIs increased to 30.2%, even with patients in the ESI group receiving an average of one additional injection procedure. The superiority of results at both 6- and 12-month follow-up extended to the reduction of disability and improvement of claudication symptoms as measured with the Oswestry Disability Index (ODI) and Zurich Claudication Questionnaire (ZCQ), respectively, as well as patient satisfaction [Citation21]. Device- or procedure-related adverse events occurred at a similar rate (1.3%) for both the ESI and mild cohorts in this study, with no serious device- or procedure-related adverse events reported in either group. By the end of one year, 2 patients in the mild group (1.4%) went on to surgery for unrelieved symptoms compared with 12 of the patients receiving ESI (9.3%). By the 2-year follow-up, the average NRS score showed continued improvement, although an additional 6 patients in the mild group underwent a subsequent surgical procedure at the index level, for a total of only 5.6% [Citation37]. This rate is consistent with the 5.2% rate reported for an earlier study of the mild treatment at 2-year follow-up [Citation35]. At 5-year follow-up, a reported 12% rate of subsequent surgical decompression indicates a low 2.4% per year rate of surgery after treatment with mild [Citation22]. Conversely, systematic reviews of multiple randomized trials comparing ESIs with placebo injections have concluded that the efficacy of spinal injections is limited and that ESIs do not reduce the rate of subsequent surgery [Citation38].
ESI and mild therapies have been shown to possess similar safety profiles. While complications such as infection, intravascular injection, nerve damage and others are recognized as general risks of ESI procedures [Citation39], serious adverse events rarely occur in the treatment of LSS [Citation40] although a rate of 2.3% has been reported in a study of 400 patients receiving transforaminal or interlaminar ESI procedures for LSS symptoms [Citation16]. The authors of 14 published studies involving a total of 708 patients treated with the mild procedure reported that no serious adverse events occurred [Citation4,Citation8,Citation21,Citation30,Citation33–37,Citation41–46].
The superiority of mild therapy over ESI procedures extends beyond clinical performance to cost-effectiveness. An analysis of cost over two years following initial procedures of mild, ESI and open surgery (laminectomy) for treatment of LSS with moderate to severe symptoms was performed, with effects measured as change in quality-adjusted life years (QALY) [Citation47]. The analysis, which included calculation of cost-effectiveness ratios and sensitivity analysis, revealed that mild was the most cost-effective at $43,760/QALY, with ESI costing 86% more (an additional $37,758/QALY) and laminectomy surgery totaling $125,985/QALY.
Given the superior performance of mild over ESI in the treatment of neurogenic claudication, together with a comparable safety profile without the need for steroid administration and substantially lower QALY than ESI, the historic utility of ESI as a bridge between conservative care and mild becomes less clear. The purpose of this cohort study was to determine if multiple ESI procedures imparted a clinical benefit to the patient undergoing a mild procedure that would justify the cost of the additional injections and the delay in providing long-term pain relief. While this study was limited by the retrospective, nonrandomized nature of the data collection and use of a single patient-reported outcome measure, the results were similar to previous studies of the mild procedure. No significant differences in VAS score changes were found between patients having 0–1 prior ESI treatments and those receiving 2 or more ESI treatments in this study. Accordingly, there appears to be no clinical benefit to the patient in performing multiple ESI procedures before receiving the longer lasting and more clinically and cost-effective mild treatment. The results of this study suggest a need to modify the treatment algorithm for LSS patients with neurogenic claudication to provide improved patient outcomes and reduced overall cost.
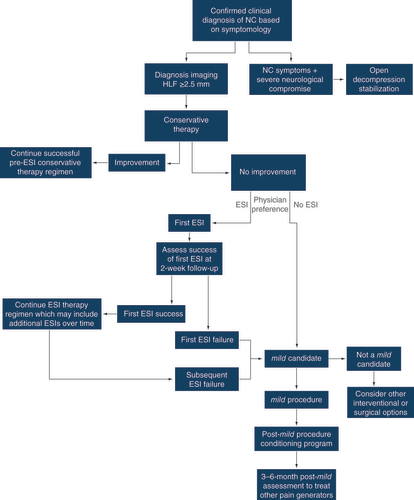
An updated algorithm is shown in . This algorithm spans patient care from initial neurogenic claudication diagnosis through follow-up at 3–6 months following a mild procedure. Patients nonresponsive to conservative care and having confirmation of HLF from diagnostic imaging (≥2.5 mm thickness) are immediately considered as candidates for mild. The modified algorithm recognizes the continued use of ESI as a treatment option based on the healthcare professional’s assessment of individual patient needs while positioning mild as the next step after the first ESI fails or directly after symptoms of neurogenic claudication are identified and confirmed with imaging.
Conclusion
mild has been shown to provide superior clinical performance to ESIs, a similar safety profile and substantially better cost–effectiveness. This study demonstrated that there is no clinical benefit in performing multiple ESI procedures and delaying long-lasting treatment with mild image-guided direct lumbar decompression for patients otherwise indicated for this percutaneous procedure. Elimination of multiple ESI procedures and utilizing the mild treatment immediately upon diagnosis of neurogenic claudication with HLF, or after failure of the first ESI procedure is recommended as part of a modified algorithm.
The efficacy of direct minimally invasive lumbar decompression (mild®) has been shown to be superior to epidural steroid injections (ESIs) for the treatment of symptomatic lumbar spinal stenosis (LSS) resulting primarily from hypertrophic ligamentum flavum.
With a safety profile similar to ESI, mild offers the potential for long-term symptom relief without first subjecting LSS patients to multiple ESI treatments.
The mild procedure leaves no implants behind and patients typically resume normal activity within 24 h with no restrictions.
The purpose of this cohort study was to determine if multiple ESI procedures imparted a clinical benefit to patients undergoing a mild procedure that would justify the cost of the additional injections and the delay in providing long-term pain relief.
Patients receiving zero or one ESI prior to treatment with mild were compared with patients receiving two or more ESI procedures prior to mild.
There were no significant differences in absolute VAS scores between the two groups at baseline or at any follow-up time point.
There appears to be no clinical benefit in performing multiple ESI procedures before receiving the longer-lasting and more clinically effective mild treatment.
The superiority of mild therapy over ESI procedures extends beyond clinical performance to cost-effectiveness.
Elimination of multiple ESI procedures and utilizing the mild treatment immediately upon diagnosis of neurogenic claudication with HLF, or after the failure of the first ESI procedure, is recommended as part of a modified algorithm.
Author contributions
All authors contributed to the analysis and interpretation of data, drafted or revised the manuscript critically for important intellectual content, provided final approval of the version to be published and are accountable for all aspects of the work.
Ethical conduct of research
Due to maintenance of patient anonymity, IRB approval and patient consent were not required for this study per US regulations regarding human clinical studies. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors thank Mark Hensley, PhD and Britt Norton of Medavise LLC for statistical analysis and manuscript assistance.
Financial & competing interests disclosure
P Pryzbylkowski: Consultant for Vertos, Nevro and Camber Spine. A Bux: Consultant for Boston Scientific, Vertos, AIS Healthcare and Flowonix. V Khemlani: Consultant for Vertos and Biotronik. S Puri: Consultant for Omnia Medical. H Sukumaran: Consultant for Vertos, Boston Scientific/Vertiflex, Nevro and SPR Therapeutics. J Rosenberg: Consultant for Vertos, Abbott and Boston Scientific/Vertiflex. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Statistial analysis was provided by Mark Hensley, PhD, and writing assistance was provided by Britt Norton, both from Medavise LLC (8725 Columbine Road #44952, Eden Prairie, MN 55344, USA). Funding was provided by Vertos Medical.
Additional information
Funding
References
- Haig AJ , TomkinsCC. Diagnosis and management of lumbar spinal stenosis. JAMA303(1), 71–72 (2010).
- Kalichman L , ColeR , KimDHet al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J.9(7), 545–550 (2009).
- Levy RM , DeerTR. Systematic safety review and meta-analysis of procedural experience using percutaneous access to treat symptomatic lumbar spinal stenosis. Pain Med.13(12), 1554–1561 (2012).
- Deer TR , KimCK , BowmanRG2nd , RansonMT , YeeBS. Study of percutaneous lumbar decompression and treatment algorithm for patients suffering from neurogenic claudication. Pain Physician15(6), 451–460 (2012).
- Deer TR , GriderJS , PopeJEet al. The MIST guidelines: the lumbar spinal stenosis consensus group guidelines for minimally invasive spine treatment. Pain Pract.19(3), 250–274 (2019).
- Diwan S , SayedD , DeerTR , SalomonsA , LiangK. An algorithmic approach to treating lumbar spinal stenosis: an evidenced-based approach. Pain Med.20(Suppl. 2), S23–S31 (2019).
- Abdi S , DattaS , TrescotAMet al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician10(1), 185–212 (2007).
- Mekhail N , CostandiS , AbrahamB , SamuelSW. Functional and patient-reported outcomes in symptomatic lumbar spinal stenosis following percutaneous decompression. Pain Pract.12(6), 417–425 (2012).
- Fukusaki M , KobayashiI , HaraT , SumikawaK. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin. J. Pain14(2), 148–151 (1998).
- Manchikanti L , CashKA , McmanusCD , DamronKS , PampatiV , FalcoFJ. A randomized, double-blind controlled trial of lumbar interlaminar epidural injections in central spinal stenosis: 2-year follow-up. Pain Physician18(1), 79–92 (2015).
- Friedly JL , ComstockBA , TurnerJAet al. Long-term effects of repeated injections of local anesthetic with or without corticosteroid for lumbar spinal stenosis: a randomized trial. Arch. Phys. Med. Rehabil.98(8), 1499–1507.e1492 (2017).
- Baisden JL , GilbertTJ , HwangSHet al. Evidence-based clinical guidelines for multidisciplinary spine care (NASS). Diagnosis and Treatment of Degenerative Lumbar Spinal Stenosis (2011). https://www.spine.org/Research-Clinical-Care/Quality-Improvement/Clinical-Guidelines
- Akuthota V , BogdukN , EasaJEet al. Lumbar transforaminal epidural steroid injections review & recommendation statement (NASS). (2013). https://www.spine.org/Portals/0/assets/downloads/ResearchClinicalCare/LTFESIReviewRecStatement.pdf.
- Klein NC , GoCH , CunhaBA. Infections associated with steroid use. Infect. Dis. Clin. North Am.15(2), 423–432viii (2001).
- Youssef J , NovosadSA , WinthropKL. Infection risk and safety of corticosteroid use. Rheum. Dis. Clin. North Am.42(1), 157–176ix–x (2016).
- Friedly JL , ComstockBA , TurnerJAet al. A randomized trial of epidural glucocorticoid injections for spinal stenosis. N. Engl. J. Med.371(1), 11–21 (2014).
- Sim SE , HongHJ , RohK , SeoJ , MoonHS. Relationship between epidural steroid dose and suppression of hypothalamus-pituitary-adrenal axis. Pain Physician23(4s), S283–S294 (2020).
- Rensen N , GemkeRJ , Van DalenEC , RotteveelJ , KaspersGJ. Hypothalamic-pituitary-adrenal (HPA) axis suppression after treatment with glucocorticoid therapy for childhood acute lymphoblastic leukaemia. Cochrane Database Syst. Rev.11(11), Cd008727 (2017).
- Krez A , LiuY , KanbourS , ClareS , WaldmanS , SteinEM. The skeletal consequences of epidural steroid injections: a literature review. Osteoporos. Int. doi:10.1007/s00198-021-05986-4 (2021).
- Jain S , DeerT , SayedDet al. Minimally invasive lumbar decompression: a review of indications, techniques, efficacy and safety. Pain Manag.10(5), 331–348 (2020).
- Benyamin RM , StaatsPS , MiDASEncore I. MILD® is an effective treatment for lumbar spinal stenosis with neurogenic claudication: MiDAS ENCORE randomized controlled trial. Pain Physician19(4), 229–242 (2016).
- Mekhail N , CostandiS , NageebG , EkladiosC , SaiedO. The durability of minimally invasive lumbar decompression procedure in patients with symptomatic lumbar spinal stenosis: long-term follow-up. Pain Pract. doi:10.1111/papr.13020 (2021).
- Manchikanti L , KayeAD , ManchikantiK , BoswellM , PampatiV , HirschJ. Efficacy of epidural injections in the treatment of lumbar central spinal stenosis: a systematic review. Anesth. Pain Med.5(1), e23139 (2015).
- Rijsdijk M , Van WijckAJ , KalkmanCJ , YakshTL. The effects of glucocorticoids on neuropathic pain: a review with emphasis on intrathecal methylprednisolone acetate delivery. Anesth. Analg.118(5), 1097–1112 (2014).
- Manchikanti L , AbdiS , AtluriSet al. An update of comprehensive evidence-based guidelines for interventional techniques in chronic spinal pain. Part II: guidance and recommendations. Pain Physician16(Suppl. 2), S49–S283 (2013).
- Nam HS , ParkYB. Effects of transforaminal injection for degenerative lumbar scoliosis combined with spinal stenosis. Ann. Rehabil. Med.35(4), 514–523 (2011).
- Manchikanti L , CashKA , McmanusCD , PampatiV , FellowsB. Results of 2-year follow-up of a randomized, double-blind, controlled trial of fluoroscopic caudal epidural injections in central spinal stenosis. Pain Physician15(5), 371–384 (2012).
- Do KH , KimTH , ChangMC. Effects of interlaminar epidural steroid injection in patients with moderate to severe lumbar central spinal stenosis: a prospective study. Ann. Palliat. Med.9(2), 163–168 (2020).
- Farooque M , SalzmanMM , YeZ. Effectiveness of bilateral transforaminal epidural steroid injections in degenerative lumbar spinal stenosis patients with neurogenic claudication: a case series. PM R9(1), 26–31 (2017).
- Brown LL . A double-blind, randomized, prospective study of epidural steroid injection vs. the mild® procedure in patients with symptomatic lumbar spinal stenosis. Pain Pract.12(5), 333–341 (2012).
- Lee JW , MyungJS , ParkKWet al. Fluoroscopically guided caudal epidural steroid injection for management of degenerative lumbar spinal stenosis: short-term and long-term results. Skeletal Radiol.39(7), 691–699 (2010).
- Mekhail N , VallejoR , ColemanMH , BenyaminRM. Long-term results of percutaneous lumbar decompression mild® for spinal stenosis. Pain Pract.12(3), 184–193 (2012).
- Basu S . Mild procedure: single-site prospective IRB study. Clin. J. Pain28(3), 254–258 (2012).
- Wong WH . mild Interlaminar decompression for the treatment of lumbar spinal stenosis: procedure description and case series with 1-year follow-up. Clin. J. Pain28(6), 534–538 (2012).
- Chopko BW . Long-term results of percutaneous lumbar decompression for LSS: two-year outcomes. Clin. J. Pain29(11), 939–943 (2013).
- Lingreen R , GriderJS. Retrospective review of patient self-reported improvement and post-procedure findings for mild (minimally invasive lumbar decompression). Pain Physician13(6), 555–560 (2010).
- Staats PS , ChafinTB , GolovacSet al. Long-term safety and efficacy of minimally invasive lumbar decompression procedure for the treatment of lumbar spinal stenosis with neurogenic claudication: 2-year results of MiDAS ENCORE. Reg. Anesth. Pain Med.43(7), 789–794 (2018).
- Deyo RA , MirzaSK , TurnerJA , MartinBI. Overtreating chronic back pain: time to back off?J. Am. Board Fam. Med.22(1), 62–68 (2009).
- Epstein NE . The risks of epidural and transforaminal steroid injections in the spine: commentary and a comprehensive review of the literature. Surg. Neurol. Int.4(Suppl. 2), S74–S93 (2013).
- Liu K , LiuP , LiuR , WuX , CaiM. Steroid for epidural injection in spinal stenosis: a systematic review and meta-analysis. Drug Des. Devel. Ther.9, 707–716 (2015).
- Chopko BW . A novel method for treatment of lumbar spinal stenosis in high-risk surgical candidates: pilot study experience with percutaneous remodeling of ligamentum flavum and lamina. J. Neurosurg. Spine14(1), 46–50 (2011).
- Deer TR , KapuralL. New image-guided ultra-minimally invasive lumbar decompression method: the mild procedure. Pain Physician13(1), 35–41 (2010).
- Durkin B , RomeiserJ , ShroyerALet al. Report from a quality assurance program on patients undergoing the MILD procedure. Pain Med.14(5), 650–656 (2013).
- Jassal NS . Single center experience of MILD procedure evaluating opioid and pain reduction at follow-up. Pain PhysicianJuly/August(22), E370 (2019).
- Mekhail NA , CostandiSJ , ArmanyousSet al. The impact of age on the outcomes of minimally invasive lumbar decompression for lumbar spinal stenosis. Med. Devices (Auckl.)13, 151–161 (2020).
- Wang JJ , BowdenK , PangG , CiptaA. Decrease in health care resource utilization with MILD. Pain Med.14(5), 657–661 (2013).
- Udeh BL , CostandiS , DaltonJE , GhoshR , YousefH , MekhailN. The 2-year cost-effectiveness of 3 options to treat lumbar spinal stenosis patients. Pain Pract.15(2), 107–116 (2015).