Abstract
Aim: Ketamine is an anesthetic agent that at lower doses can be a potent analgesic. There has been an interest in the use of low dose ketamine in treatment of chronic pain syndromes. Patients & methods: We report the results of a retrospective observational study for patients diagnosed with a chronic noncancer pain syndrome receiving a 2-week continuous subanesthetic IV ketamine infusion. Results & conclusion: We conclude that a 10–14 days of subanesthetic ketamine infusion in chronic patients results in clinically significant lowering of patients’ numerical pain score. Further studies looking at subanesthetic ketamine infusion in a prospective trial of multi-day IV ketamine infusion in chronic refractory chronic neuropathic pain are needed to further assess the efficacy of ketamine.
Lay abstract
Ketamine is a pharmacological agent that was developed in the 1960s. There has been an increase in interest in the use of ketamine at low doses in the treatment of chronic pain syndromes. In this study, we report the results of a study that investigated patients diagnosed with a chronic noncancer pain syndrome that received a 2-week continuous ketamine infusion. We hypothesized that patients receiving IV ketamine infusion will experience acute and chronic lowering of pain intensity on the numerical rating pain level scale and reduce patient’s opioid requirements. We concluded that a 10–14 day of subanesthetic ketamine infusion in chronic patients results in clinically significant lowering of patients’ numerical pain score during the ketamine infusion.
Ketamine is an anesthetic agent developed as a safer alternative to phencyclidine [Citation1,Citation2]. At typical doses, it produces a dissociative anesthetic state, but at lower doses it is a potent analgesic [Citation1,Citation3]. In the central nervous system, ketamine is predominantly a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist. One postulated mechanism underlying these conditions is the reversal of magnesium ion blockade at the NMDA receptors allowing glutamate activation in response to chronic noxious input to the dorsal horn cells [Citation4].
Ketamine’s oral, sublingual and rectal bioavailability has been estimated at 20–30% due to a large first-pass effect [Citation5]. The plasma concentration of norketamine exceeds ketamine concentration following a ketamine infusion, possibly contributing to the analgesic profile given that it is also a non-competitive NMDA receptor antagonist [Citation1]. However, a small randomized placebo-controlled trial of 12 healthy male volunteers has reported a negative contribution of (S)-norketamine to the analgesic effects of (S)-ketamine on acute heat pain [Citation6].
A systematic review on perioperative ketamine conducted by Bell et al. in 2005 showed that subanesthetic intravenous ketamine ranging from 0.15 to 1.0 mg/kg reduced overall pain intensity, patient-controlled analgesia (PCA) morphine consumption and rescue analgesic requirement, with only mild adverse effects [Citation7]. Subanesthetic ketamine infusion has also been studied in different chronic pain conditions including complex regional pain syndrome (CRPS), phantom limb pain, postherpetic neuralgia, fibromyalgia and migraines [Citation8]. In 2010, Noppers et al. reviewed 36 RCTs published between 1992 and 2008 and found that while most studies showed acute analgesic effects, three later trials also showed evidence of pain relief lasting for months after multi-day ketamine infusions for CRPS and neuropathic pain [Citation1,Citation9–11]. Importantly, the ketamine infusion regimens were different in all of these studies, thus current evidence to guide ketamine infusion therapy in terms of dose and duration is poor [Citation8].
Evidence regarding ketamine’s specific effect on opioid requirements in chronic pain patients is still relatively limited as it has not been adequately explored in the literature. Three early prospective studies found that intrathecal, epidural or oral ketamine use did decrease opioid use in adults with cancer pain [Citation12–15]. A 2016 prospective study on continuous subcutaneous 3–7 day ketamine infusion with chronic nonmalignant pain found an overall opioid use reduction of 59% [Citation16]. In this study, we explored the effect of 10–14 days of continuous ketamine infusion on reducing opioid use in patients with refractory chronic pain. We also assessed the effects of ketamine infusion on reducing pain scores in patients with refractory chronic pain, either if they were taking opioids or not at baseline. We postulated a significant reduction of their pain ratings acutely, as well as reduction or discontinuation of their opioid use.
Methodology
We performed a retrospective chart review of all inpatients admitted to St. Paul’s Hospital from October 2015 to July 2019 for subanesthetic continuous IV ketamine infusion to manage chronic nonmalignant pain. All data presented in this study is available by request by the study authors. Although some patients had received outpatient IV ketamine in the past, no patients had been admitted for continuous IV ketamine prior to October 2015. The main inclusion criteria were patients with treatment-refractory chronic pain that had not benefited from standard treatments and had a high numerical pain rating scale (NRS) at baseline (>6). Exclusion criteria included inability to provide informed consent, pregnancy, past or present drug or alcohol use disorders, past or present psychosis, unstable current psychiatric functioning and abnormal ECG or documented coronary artery disease.
Patients had a planned admission of 10–14 days. The starting dose for all patients was 5 mg/h regardless of weight, titrating to a maximum of 30 mg/h (based on 0.5 mg/kg) as tolerated by 1–5 mg/h. Once an effective dose was reached (defined as at least 30% reduction in NRS score), opioids were reduced as clinically indicated. Many other studies have used midazolam to reduce the incidence of central nervous system–related adverse events secondary to ketamine, and even possible additional analgesic benefit [Citation8]. However, we opted not to use this agent in order to limit confounding variables.
Ketamine was delivered through a peripherally inserted central catheter, following investigations including renal function, liver enzymes, TSH, complete blood count and ECG. During infusion, nurses assessed for dysphoric symptoms (restlessness, anxiety), hallucinations, vivid dreams and delirium every 4 h. Clonidine was prescribed as needed for elevated blood pressure (over 140 mm systolic), headache, or withdrawal symptoms in patients using opioids. For headache, other options included adjustment of the ketamine dose, or a bolus of normal saline or D5NS. For nausea, patients received ondansetron and/or dimenhydrinate. All patients were assessed prior to admission by a pain specialist, the principal investigator of this study. After their admission, patients were typically scheduled for follow-up visits at 2, 6 and 12 weeks post-discharge. During these office visits, pain scores and medication changes were recorded.
The collected data included demographic parameters (age, gender), maximum ketamine dose, total number of infusion days, adverse events and pain scores and medications at admission, discharge and outpatient follow-up. Pain scores were assessed using a numerical rating scale (NRS) ranging from 0 (no pain) to 10 (unbearable pain). The admission and discharge pain scores were taken from the discharge summary. If a range of scores was given, then the average was taken (e.g., 3–4 would be 3.5).
Morphine milligram equivalents (MMED) were calculated for all opioid patients using the 2017 Canadian Guideline for Opioids for Chronic noncancer Pain [Citation17]. A conversion ratio of 0.15 was used for tramadol given that a range of 0.1-0.2 was provided in the guideline. The Center for Disease Control and Prevention guideline was used for fentanyl transdermal conversion ratio as it is not provided in the Canadian guideline [Citation18]. Buprenorphine was excluded from the comparison as there is currently no reliable conversion ratio and therefore no applicable guidelines. Methadone has complex pharmacokinetic, genetic polymorphism variability resulting in a wide range of inter-individual response complicating conversion schematics and potentially increasing patient risk with conversion [Citation19,Citation20]. Accordingly, methadone was not converted to MMED and instead analyzed separately. The project was approved by the University of British Columbia – Providence HealthCare Research Ethics Board (certificate number H17-02033).
Results
Patient characteristics & clinical information
Demographics and clinical information, including diagnoses and opioid use, for the patients are provided in . There were a total of 172 admissions between October 2015 and July 2019, with 44 excluded either due to scheduled admissions shorter than 10 days or treatment discontinuation due to intolerance or side effects. In total, we reviewed 128 admissions for a total of 87 patients. The mean age at time of first admission was 49 years. The majority of the admissions were female patients (107, 83.6%). The most common diagnosis for all admissions were central sensitization syndrome (fibromyalgia) and CRPS (each 37.5%). Most patients (79.3%) only had one admission between October 2015 and July 2019, while 10.3% had 2 admissions and 10.3% had 3 or more admissions. A total of nine admissions were longer than 2 weeks, for prolonged opioid withdrawal or other medical issues requiring further management. The majority of patients (92.2%) were seen by the primary investigator at the first follow-up visit, while 64.8% made it to the third visit. 53.1% of total admissions reached the maximum ketamine dose of 30 mg/h. There were a total of 52 admissions (40.6%) in which patients were taking opioids prior to the ketamine infusion. The most commonly used opioid at admission was tramadol (19.2%), followed by hydromorphone (13.5%).
Table 1. Summary table of baseline variables of all 128 admissions.
Pain scores
There were a total of 77 first admissions with clearly documented admission and discharge pain scores. The mean admission score for these encounters was 7.71 (std = 1.5) while the mean discharge score was 3.7 (std = 2.5). The majority of these visits had a clinically significant pain score decrease, defined as 30% or more [Citation21], by the time of discharge (72.7%). 34 patients had a documented admission and discharge score as well as a pain score during the first follow-up visit. Within this group, 88.2% had clinically significant reductions in pain scores at discharge, persisting for 73.5% at the first office visit. Beyond the initial follow-up, the number of patients returning for subsequent visits was very small but results are outlined in .
Table 2. Changes in pain ratings for all patients.
Within the opioid group specifically, 34 first admissions had documented admission and discharge pain scores. The mean admission score was 7.9 (std = 1.4) while the mean discharge score was 4.4 (std = 2.6). The majority of these visits had a clinically significant pain score decrease of 30% or more at discharge (61.8%). Again, the number of patients beyond the first visit were small but outlined in .
Using a Wilcoxon test, there was no significant difference between males and females in any of the outcomes evaluated, including pain ratings at baseline and change in pain ratings for opioids users, nonopioid users, or both combined.
Opioid usage
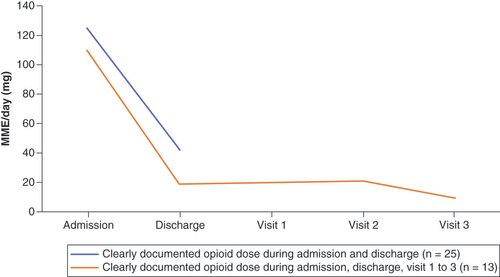
Based on 27 accurately documented opioid dosages, the MMED was 119.0 mg on admission. There were 29 accurately documented opioid dosages at discharge, with an MMED of 36.8 mg (reduction of 69%) (). In the methadone group, the mean dose on admission was 73.3 and 36.8 mg at discharge (reduction of 50%). Overall, 17 out of 33 opioid patients (52%) were off opiates completely at discharge while 11 had a dose reduction of 30% or more. Only 13 opioid patients at admission (excluding methadone) made it to the third follow-up, with nine of these off opioids at discharge and remaining off at all three visits. One additional patient stopped opioids subsequent to discharge, and subsequently remained off at the third follow-up. Therefore, 10/13 (77%) of patients were off opiates at the third follow-up interval (mean of 110 days).
Mixed effect model of pain rating scale
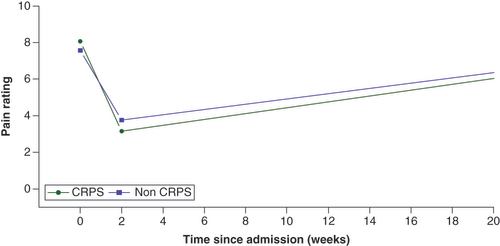
Using a mixed effect piecewise regression model of pain rating scale () adjusted for opioid use at admission, gender, and ketamine dose for all admissions and patients, there was no significant difference in the baseline pain rating scale between CRPS and non-CRPS patients (p = 0.28). However, the decrease in pain ratings during admission was significantly greater among CRPS patients compared with those without CRPS (p = 0.01). Following treatment, there was no significant difference in the clinical trajectory between the two groups (p = 0.71). At 20 weeks, neither group had yet reverted to their pre-ketamine baseline pain scores.
CRPS: Complex regional pain syndrome.
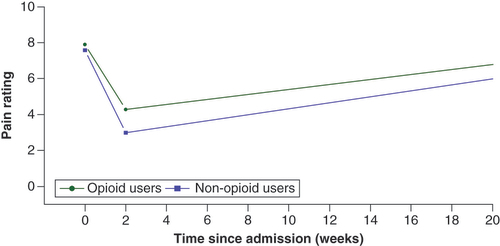
When using the mixed effect piecewise regression model adjusted for condition (CRPS vs non-CRPS), gender, and ketamine dose for all admissions and patients, there was no significant difference in baseline pain rating scale scores (p = 0.43) or opioid use status (). However, the decrease in pain ratings during infusion was significantly greater among nonopioid users compared with opioid users (p = 0.02).
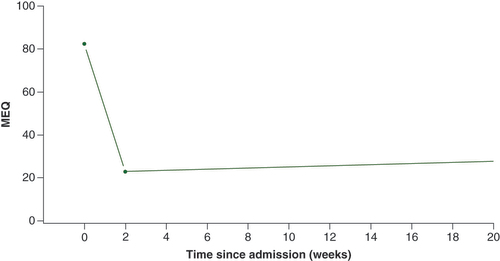
When using the mixed effect piecewise regression model adjusted for condition (CRPS vs non-CRPS), gender, and ketamine dose for all admissions and patients, there was a significant drop in the MMED for non-methadone opioid users during infusion (slope per 14 days = -59.3, p < 0.0001; ). After discharge, the MMED remained suppressed over time (slope per 14 days = 0.6, p = 0.34).
Side effects
Mild adverse effects were common, most frequently nausea (in 28 admissions), headache (25 admissions) and cognitive complaints (22 admissions). Other common side effects included dizziness, chest discomfort, urinary hesitancy and gastrointestinal complaints including constipation. Four patients experienced transaminitis that resolved after stopping the infusion.
Discussion
In this study, we found that a subanesthetic ketamine infusion for 10–14 days in patients with chronic noncancer related pain resulted in clinically significant decrease in baseline pain scores from 7.71 to 3.7 at discharge. Most of these patients maintained a clinically significant decrease in their pain score at the first visit (73.5%). Despite pain scores increasing after discharge, they did not reach the baseline scores at 20 weeks. Within the opioids users (excluding methadone), there was a statistically significant decrease of 69% in MMED after 10–14 days of ketamine infusion. 52% of opioids users were completely weaned off their opioids while on ketamine infusion. CRPS patients (compared with non-CRPS) and nonopioid patients had a more significant decrease in their pain scores during ketamine infusion. In addition, 10 out of 13 (77%) of patients were completely off opioids at the time of the third follow-up (110 days), with all others being lost to follow-up.
It is estimated that 15–25% of chronic pain patients have a predominantly neuropathic etiology [Citation22]. Neuropathic pain is defined as “pain caused by a lesion or disease of the somatosensory nervous system” by the International Association for the Study of Pain, which excludes any condition characterized by central sensitization in the absence of a frank somatosensory lesion [Citation23]. Although support for ketamine use has typically been strongest in neuropathic pain, a recent study showed pain type (neuropathic vs non-neuropathic; CRPS vs without) did not impact the magnitude of pain reduction [Citation24]. Our study therefore includes patients with neuropathic pain as well as with central sensitization in different conditions including headaches and chronic back pain.
It is believed that ketamine may be effective by ‘resetting’ the magnesium blockade of NMDA receptors, and other feedback mechanisms which become dysregulated with chronic noxious input. There is no current consensus on a standardized ketamine protocol, with our study targeting 0.5 mg/hg/h corresponding to up to 30 mg/h for a 70 kg adult [Citation22]. Maher et al. concluded in a 2017 review that the exact rate of infusion did not appear to be a factor in degree or duration of pain relief [Citation8]. This is consistent with a more recent meta-analysis of randomized controlled ketamine infusion trials finding that cumulative dose exceeding 400 mg was associated with greater and longer pain relief [Citation25]. Another recent review found that high doses during a shorter period of time (1–10 days) had better results than lower doses for longer [Citation26]. In our study, all completing patients received more 400 mg of ketamine over 10–14 days.
Michelet et al. found that ketamine infusion significantly decreased pain intensity at 4 weeks when they excluded high-risk-of-bias studies from their analysis. In contrast, a subgroup analysis from a recent Canadian Agency for Drugs and Technologies in Health (CADTH) review showed that IV ketamine infusion significantly reduced pain scores versus placebo 2 weeks post-treatment, but no longer [Citation24].
In our study, 73.5% of the patients with documented pain scores at 2-week follow-up still had clinically significant improvement (>30%) compared with their baseline admission scores. Unfortunately, only 34 patients had clearly documented pain scores at first visit, limiting the interpretation of results. Our patient sample with documented pain scores at admission and beyond one follow-up was too small to confidently assess the clinical significance of their decreased pain scores. When looking at our pain score trends on the mixed effect piecewise regression models, scores increase immediately following discharge from hospital, but still remain below baseline some 20 weeks later.
A meta-analysis of randomized controlled trials analyzed pain intensity scores recorded more than 48 h after ketamine infusion in a subgroup of CRPS versus non-CRPS patients. Although not statistically significant, the reduction in pain scores was higher in CRPS patients than for other pain etiologies [Citation25]. This is partially consistent with our findings of greater pain reduction during treatment for CRPS patients, but no significant difference in the subsequent rate of change following discharge. This may suggest that CRPS patients respond particularly well acutely, suggesting a need for larger and more robust trials to further explore this trend.
Although a 2015 meta-analysis demonstrated a small effect size in lowering opioid consumption in the peri-operative setting using ketamine or other NMDA-receptor antagonists [Citation27], a more recent study found a significant reduction in opioid use following 3–7 days of subanesthetic ketamine infusion. The authors reported an MME decrease from 216 mg/day to 89 mg/day post-infusion (p < 0.005) with an overall opioid use reduction of 59% [Citation16]. In our study, we found a similar trend with MMED decreasing from 118.98 mg at admission (n = 27) to 36.8 mg on discharge (n = 29), or 69%. In addition, 10 out of 13 patients at the third follow-up (110 days) were completely off opioids. We postulate that the reason why patients with high opioid use had seemingly less effective response to ketamine is due to simultaneously undergoing opioid withdrawal which increases their pain intensity at the same time. Using the mixed effect piecewise regression model of pain rating scales, our MMED decrease was statistically significant.
Our study showed that the decrease in pain rating scale during the ketamine infusion was significantly greater among nonopioid users compared with opioid users. In contrast, a 2015 retrospective study found that oral administration of ketamine showed less failure in opioid users, and that opioid de-escalation was an expected benefit of oral ketamine [Citation28]. Therefore, more robust studies are needed to explore if oral ketamine is more efficient than IV administration in opioid users with chronic refractory noncancer pain.
This study has several limitations. This was a retrospective chart review with no control group, therefore any changes to pain scores may have been influenced by factors other than ketamine i.e. neuropathic pain medications taken concurrently. The use of the numeric rating scale can cause variability in the results as its subjectivity is influenced by many factors including but not limited to cultural factors, mood, and education. In addition, some pain scores were not consistently charted in the discharge summaries and at the follow-up visits. Nevertheless, this study showed significant reduction of opiate usage in refractory chronic pain patients following 10–14 days of IV ketamine infusion.
Conclusion
In summary, in this retrospective observational study, we conclude that 10–14 days of subanesthetic ketamine infusion in chronic patients results in clinically significant lowering of patients’ numerical pain score during the ketamine infusion and at least at the first office visit approximately 34 days following discharge. The pain scores at 20 week following Ketamine infusion did not return to their previous baseline. In addition, ketamine infusion successfully reduced opioid usage in chronic neuropathic pain patients. Overall, the pain NRS was significantly reduced in all patients with chronic refractory neuropathic pain at discharge, with greater reductions for patients with CRPS compared with non CRPS. The overall pain NRS was reduced more in non opioid users compared with opioid users at discharge possibly due to effects of opiate withdrawal. There was an MMED reduction from 119 to 36.8 mg (69%) at discharge and methadone reduction from 73.3 to 36.8 mg (50%) at discharge. In addition, at discharge 52% of patients were discontinued off opiates completely. For patients at the third follow-up, 10/13 or 77% remained off of all opiates. We found that 73.5% of the patients that had a documented pain score during the first office visit after their first admission still had clinically significant decrease in their pain score (≥30%) when compared with their baseline admission pain score. Further studies looking at subanesthetic ketamine infusion in a placebo-controlled prospective trial are needed to further assess the efficacy of ketamine in treatment of refractory chronic pain.
Ketamine is an anesthetic agent that at lower doses can act as a potent analgesic. It acts as noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist in the central nervous system.
Many studies and systematic reviews have concluded that subanesthetic intravenous ketamine reduces overall pain intensity and patient opioid use.
In this study, we found that a subanesthetic ketamine infusion for 10–14 days in patients with chronic noncancer related pain resulted in clinically significant decrease in baseline pain scores.
Within the opioids users (excluding methadone), there was a statistically significant decrease in morphine milligram equivalents (MMED) after 10–14 days of ketamine infusion.
Our study revealed a decrease in pain rating scale during the ketamine infusion was significantly greater among nonopioid users compared with opioid users.
We postulate that the reason why patients with high opioid use had seemingly less effective response to ketamine is due to simultaneously undergoing opioid withdrawal which increases their pain intensity at the same time.
We conclude that 10–14 days of subanesthetic ketamine infusion in chronic patients results in clinically significant lowering of patients’ numerical pain score during the ketamine infusion and at least at the first office visit approximately 34 days following discharge.
Further studies looking at subanesthetic ketamine infusion in a placebo-controlled prospective trial are needed to further assess the efficacy of ketamine in treatment of refractory chronic pain.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
St. Paul’s Hospital Foundation provided the research grant (H17-02033). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Data availability statement
If requested, we can share all the data gathered and used in this report for research purposes. Please direct data requests to the corresponding author.
Additional information
Funding
References
- Noppers I , NiestersM , AartsL , SmithT. Ketamine for the treatment of chronic noncancer pain. Expert Opin. Pharmacother.11(14), 2417–2429 ( 10AD).
- Bell RF , KalsoEA. Ketamin in der Schmerztherapie. Der Schmerz.33(2), 156–164 (2019).
- Hilal-Dandan R , BruntonLL , GoodmanLS. Goodman and Gilman’s Manual of Pharmacology and Therapeutics.Hilal-DandanR, BruntonLL (Eds). 2nd ed. McGraw-Hill, NY, USA.
- Bennett GJ . Update on the neurophysiology of pain transmission and modulation: focus on the NMDA-receptor. J. Pain Symptom Manage.19(1), 2–6 (2000).
- Yanagihara Y , OhtaniM , KariyaSet al. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm. Drug Dispos.24(1), 37–43 (2003).
- Olofsen E , NoppersI , NiestersMet al. Estimation of the contribution of norketamine to ketamine-induced acute pain relief and neurocognitive impairment in healthy volunteers. Anesthesiology117(2), 353–364 (2012).
- Bell RF , DahlJB , MooreRA , KalsoE. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review). Acta Anaesthesiol. Scand.49(10), 1405–1428 (2005).
- Maher DP . Intravenous ketamine infusions for neuropathic pain management: a promising therapy in need of optimization. Anesth. Analg.124(2), 661–674 ( 2AD).
- Sigtermans MJ , van HiltenJJ , BauerMCRet al. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain145(3), 304–311 (2009).
- Amr YM . Multi-day low dose ketamine infusion as adjuvant to oral gabapentin in spinal cord injury related chronic pain: a prospective, randomized, double blind trial. Pain Physician13(3), 245–249 (2010).
- Schwartzman RJ , AlexanderGM , GrothusenJR , PaylorT , ReichenbergerE , PerreaultM. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain147(1–3), 107–115 (2009).
- Bredlau AL , ThakurR , KoronesDN , DworkinRH. Ketamine for pain in adults and children with cancer: a systematic review and synthesis of the literature. Pain Med.14(10), 1505–1517 (2013).
- Lauretti GR , LimaIC , ReisMP , PradoWA , PereiraNL. Oral ketamine and transdermal nitroglycerin as analgesic adjuvants to oral morphine therapy for cancer pain management. Anesthesiology90(6), 1528–1533 (1999).
- Yang C-Y , WongC-S , ChangJ-Y , S-THO , SevarinoFB. Intrathecal ketamine reduces morphine requirements in patients with terminal cancer pain. Surv. Anesthesiol.41(5), 305 (1997).
- Lauretti GR , GomesJMA , ReisMP , PereiraNL. Low doses of epidural ketamine or neostigmine, but not midazolam, improve morphine analgesia in epidural terminal cancer pain therapy. J. Clin. Anesth.11(8), 663–668 (1999).
- Zekry O , GibsonSB , AggarwalA. Subanesthetic, subcutaneous ketamine infusion therapy in the treatment of chronic nonmalignant pain. J. Pain Palliat. Care Pharmacother.30(2), 91–98 (2016).
- Busse JW , CraigieS , JuurlinkDNet al. Guideline for opioid therapy and chronic noncancer pain. Can. Med. Assoc. J.189(18), LP–E666 (2017).
- https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf
- Rennick A , AtkinsonT , CiminoNM , StrasselsSA , McPhersonML , FudinJ. Variability in opioid equivalence calculations: variability in opioid equivalence. Pain Med.n/a–n/a (2015).
- Li Y , KantelipJ-P , SchieveenPG , DavaniS. Interindividual variability of methadone response: impact of genetic polymorphism. Mol. Diagn. Ther.12(2), 109–124 (2008).
- Farrar JT , YoungJP , LaMoreauxL , WerthJL , PooleRM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain94(2), 149–158 (2001).
- Cohen SP , BhatiaA , BuvanendranAet al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg. Anesth. Pain Med.43(5), 521–546 (2018).
- Finnerup NB , HaroutounianS , KamermanPet al. Neuropathic pain: an updated grading system for research and clinical practice. Pain157(8), 1599–1606 (2016).
- Tran K , McCormackS. Ketamine for Chronic noncancer Pain: A Review of Clinical Effectiveness, Cost–Effectiveness, and Guidelines.Canadian Agency for Drugs and Technologies in Health, ON, Canada.
- Orhurhu V , OrhurhuMS , BhatiaA , CohenSP. Ketamine infusions for chronic pain: a systematic review and meta-analysis of randomized controlled trials. Anesth. Analg.129(1), 241–254 (2019).
- Michelet D , BrasherC , HorlinALet al. Ketamine for chronic noncancer pain: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur. J. Pain (United Kingdom).22(4), 632–646 (2018).
- Wu MD L , HuangMD X , SunMD L. The efficacy of N-methyl-D-aspartate receptor antagonists on improving the postoperative pain intensity and satisfaction after remifentanil-based anesthesia in adults: a meta-analysis. J. Clin. Anesth.27(4), 311–324 (2015).
- Marchetti F , CoutauxA , BellangerA , MagneuxC , BourgeoisP , MionG. Efficacy and safety of oral ketamine for the relief of intractable chronic pain: a retrospective 5-year study of 51 patients: Oral ketamine for intractable pain. Eur. J. Pain.19(7), 984–993 (2015).