Abstract
Introduction & aim: Temporary (60-day) percutaneous peripheral nerve stimulation (PNS) has demonstrated effectiveness for the treatment of chronic post-amputation pain, and this pilot study aims to evaluate the feasibility of temporary percutaneous PNS for the treatment of acute post-amputation pain. Patients & methods: Sixteen veterans undergoing lower extremity amputation received PNS and standard medical therapy or standard medical therapy alone. Results: The PNS group reported greater reductions in average phantom limb pain, residual limb pain and daily opioid consumption, and there were fewer participants taking opioids through 3 months post-amputation. Conclusion: This pilot study suggests that PNS is feasible in the acute postoperative period following lower limb amputation and may provide a non-pharmacologic analgesic therapy that lowers pain scores and reduces opioid consumption, and thus warrants further investigation.
Lay abstract
A small study was done to evaluate placing a wire lead near a nerve to electrically stimulate it for 60 days after a leg amputation surgery to see if it helps reduce pain. The study looked at 16 veterans who had an amputation to their leg. These patients were divided into either a group that received nerve stimulation plus normal pain control medications or a group that just received pain medications. The group that received nerve stimulation had less pain in the remaining leg and less phantom pain (pain in the missing leg). They also required fewer narcotic medications. The study suggested that nerve stimulation may provide an effective way to manage pain after amputation and reduce the use of pain medications.
Clinical Trial Registration Number: NCT03484429
Major limb amputation commonly causes severe acute and chronic pain. Acute perioperative pain is a significant predictor of the development of chronic residual limb pain (RLP) and phantom limb pain (PLP) [Citation1], and early intervention is critical for several important reasons. Optimal control of acute post-amputation pain can decrease the postsurgical inflammatory and sympathetic responses allowing for reduced rates of chronic pain, decreased opioid usage, increased mobility and rehabilitation, increased patient satisfaction, decreased lengths of stay, and possibly decreased readmission rates [Citation2,Citation3].
Current standard medical therapy (SMT) focuses on pharmacologic agents, which include oral and parenteral narcotics, as well as perineural catheter (PNC) infusions of local anesthetics, all of which pose risk of toxicity and are limited by significant side effects [Citation4]. For example, the harmful effects of opioids are well known, and efforts to minimize their use throughout the surgical period are ongoing. The use of PNCs infusing local anesthetics in the perioperative period has gained in popularity and has been shown to reduce pain scores and opioid consumption, but their efficacy can be limited due to several reasons: the short (<7-day) duration of therapy due to the risk of infection and local anesthetic toxicity, worsening periods of pain after removal of the catheter and motor and sensory deficits from local anesthetic blockade that can increase the risk of falling [Citation4–6].
Peripheral nerve stimulation (PNS) was traditionally considered a treatment of last resort for chronic pain. The development of percutaneous leads that can be temporarily implanted for up to 60 days has enabled the use of PNS in the acute to subacute postoperative period without permanent implants and without increased risk of infection [Citation7,Citation8]. PNS has been reported to decrease pain and opioid requirements following total knee arthroplasty and ambulatory foot and shoulder surgeries [Citation9–11], and a recent randomized, double-blind, placebo-controlled trial demonstrated significant relief of chronic post-amputation pain [Citation12,Citation13]. No studies to date have evaluated the postoperative use of percutaneous PNS for the treatment of acute or subacute pain following major lower limb amputation. This prospective, randomized, controlled pilot study is the first to evaluate the feasibility of a 60-day percutaneous PNS treatment for potential evaluation in a future randomized controlled trial (RCT), including the potential for reducing pain scores, opioid usage, and multiple other secondary outcomes in the postoperative period following major lower limb amputation surgery.
Methods
Study design & population
A single center, prospective, randomized, controlled pilot study was designed to evaluate the feasibility of using percutaneous PNS to control pain in the postoperative period after lower extremity amputation. The study was prospectively approved by the McGuire Institutional Review Board (no. 2343; Richmond, Virginia), submitted for registration with ClinicalTrials.gov in November 2017 (NCT03484429) and was conducted in accordance with relevant sections of the US Code of Federal Regulations, the Declaration of Helsinki, and the International Conference on Harmonization guidelines for Good Clinical Practice.
After obtaining written informed consent, veterans undergoing major lower limb amputation at the Hunter Holmes McGuire VA Medical Center were screened for eligibility. Key inclusion criteria included >18 years of age, scheduled to undergo non-traumatic transtibial (TT) or transfemoral (TF) amputation and reporting postoperative RLP or PLP ≥4/10 on a 0–10 numerical rating scale using the Brief Pain Inventory–Short Form (BPI-SF) Question #5 [Citation14]. Key exclusion criteria included Beck Depression Inventory score >20, systemic infection, immunosuppressive disorder, other implanted electronic devices, previous allergy to skin contact materials and/or anesthetic agent, altered mental status at time of evaluation or inability to provide consent, greater than 180 mg oral morphine equivalent(s) (OME) per day preoperatively and pregnancy.
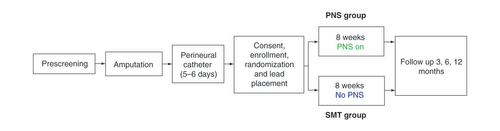
Eligible participants were randomized 1:1 via a sealed envelope by the study investigators to receive either a 60-day percutaneous PNS treatment and SMT (PNS ‘treatment’ group) or SMT alone (control group) (). All participants in both groups had femoral and sciatic PNCs infusing a low dose local anesthetic (ropivacaine 0.2% at 10 ml/h) placed on the day prior to or day of surgery, which were routinely removed post-amputation after 5–7 days of therapy. Of note, the peripheral nerve catheter is part of the standard perioperative care plan for patients undergoing amputation surgeries at the authors’ institution, and the catheter was administered to all patients regardless of their group randomization as part of the SMT. The duration and dosing were consistent with standard protocols, which were applied consistently for patients across groups. After PNCs were discontinued and once the effects of the local anesthetic had dissipated (~24–48 h after catheter removal), the PLP and RLP baseline scores were assessed, and participants who reported scores ≥4/10 were consented and enrolled by the research team. Psychometric scale questionnaires and functional independence measure (FIM) scores were assessed upon enrollment. Participants randomized to the treatment group had PNS placed immediately after study enrollment. All participants were followed daily until hospital discharge, then weekly through 8 weeks during the treatment phase, and at 3, 6 and 12 months for follow-up (data analysis for 6 and 12 months is ongoing).
Interventions
Standard medical therapy
SMT was defined as the routine medical therapy following amputation surgery, which may include opioid and non-opioid pain medications, injections, physical rehabilitative therapies or complementary and alternative therapies. Subjects were permitted to continue the use of all analgesic medications throughout the study. Subjects in both groups received SMT throughout the study.
PNS treatment
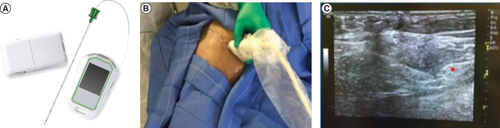
In addition to receiving SMT, participants in the PNS group were implanted with commercially available percutaneous PNS leads (SPRINT® PNS System, SPR Therapeutics, OH, USA) under ultrasound guidance approximately 1–3 cm distant from the femoral and sciatic nerves for up to 60 days of treatment (). A test stimulation needle was used to determine the optimal location prior to lead placement, confirmed by stimulation-evoked sensations overlapping the regions of RLP and/or PLP. A fine-wire (100 μm diameter) coiled percutaneous lead (MicroLead; SPR Therapeutics) was then deployed via an introducer needle and the proximal end of the lead was connected to an external, wearable pulse generator (i.e., the ‘stimulator’). Stimulation parameters were chosen to achieve the desired end point of full coverage of the region of pain with comfortable, stimulation-evoked sensations. The PNS system used in the present study delivers stimulation (asymmetric charge-balanced biphasic pulse train) at a fixed frequency of 100 Hz. The other parameters (amplitude and pulse width) are adjustable as a single intensity variable that range from 0 to 100, which corresponds to a range of amplitudes (0–30 mA) and pulse widths (10–200 us) determined to be safe for human use. During stimulation testing, the intensity of stimulation was increased and coverage of comfortable paresthesia sensations was assessed based on patient report to identify the optimal stimulation range for patients, and patients were given the ability to adjust stimulation independently using a remote control in the 0–100 intensity range, to their own comfort level at any time. Wound closure adhesive (2-octyl 2-cyanoacrylate) was applied to the skin at the lead exit site, then covered with a sterile dressing. The external pulse generator was secured to the skin using an adhesive hydrogel pad that also served as the return electrode.
Fine-wire coiled percutaneous leads with an external stimulator and remote (A) were implanted under ultrasound guidance. This example shows a lead placement targeting the femoral nerve (B). After identifying the optimal location using a test stimulation needle, the leads were placed approximately 1–3 cm distant from the femoral and sciatic nerves (C).
The PNS group received active stimulation based on parameters programmed to deliver comfortable sensations covering the entire region affected by RLP and/or PLP. Subjects were instructed to use stimulation continuously during the 8-week treatment period and could adjust stimulation intensity to maintain comfortable coverage of their region of pain. In the event of lead migration or dislodgement, indicated by loss of stimulation-evoked paresthesias, participants were given the option to undergo lead replacement and complete the remainder of the 60-day treatment or withdraw from the study. At the end of PNS treatment, participants returned to the clinic for lead removal by an investigator.
Outcome measures
Average, best, and worst RLP and PLP were assessed using the BPI-SF [Citation14]. Mean scores standard deviation ( SD) as well as percent reductions from baseline over time were calculated for each group. Responders were defined as participants with ≥50% reductions in average RLP and PLP over time, and the proportion of responders was determined for each group. Greater than 50% pain score reduction was considered substantial improvement based on previous consensus definitions [Citation15].
Additional outcome measures included opioid consumption as determined by average OMEs, FIM scores [Citation16], pain Interference (PI) scores measured by BPI-SF question 9 [Citation14], Patient Global Impression of Change (PGIC) [Citation17], Pain Catastrophizing Scale (PCS) scores [Citation18], pain disability measured by the Pain Disability Index (PDI) [Citation19], length of hospital stay postoperatively and 30-day readmission rates.
Statistical analysis
No hypothesis testing was performed due to the nature of the trial as a feasibility study. Instead, the study was designed to generate data to help power a subsequent clinical trial. A convenience sample of 16 participants was enrolled and statistics were not applied to the data due to the small sample size. Demographics, baseline characteristics and outcome means SD are reported in tables by group.
Results
Patient characteristics
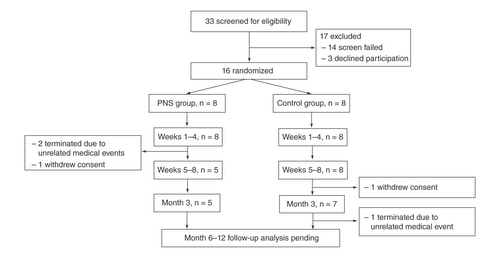
Subjects were enrolled from December 2017 to March 2019. A total of 33 participants were screened for eligibility, of whom 14 did not meet screening criteria and 3 declined to participate. The remaining 16 participants were consented, enrolled and randomized to either the PNS group (n = 8) or control group (n = 8) (). Recruitment was stopped when the target enrollment was reached to evaluate study feasibility. Three participants in the PNS group did not complete the 60-day treatment period: 1 participant required a revision operation and withdrew consent, and 2 others withdrew due to complications from unrelated medical issues. Two additional participants in the control group required revisions to their amputations and elected to restart the study visits at the time of reoperation. Two participants in the PNS group required lead re-implantation due to accidental dislodgement (e.g., unintentional tension on the externalized portion of the lead that caused the lead to dislodge fully, such as during a dressing change).
Subject flow diagram showing the progression of participants from screening through all study visits. Long-term follow-up is ongoing, and participants still in follow-up are noted as in progress.
A majority of participants were males (94%) undergoing TF amputations (56%). The most common cause of amputation was peripheral vascular disease (44%) (). PNCs were placed and maintained by the primary teams for an average of 5.5 ± 2.5 days prior to placement of PNS leads. In the PNS group, 38% of participants required opioids preoperatively compared with 25% in the control group.
Table 1. Patient demographics.
Primary outcome measures
Pain scores
Both groups showed reductions in overall pain scores over time from baseline through 3 months (A & C). By week 8, the PNS treatment group showed the greatest percent reduction in average phantom (76 vs 29%) and residual (86 vs 52%) pain scores compared with the control group (B & D). Despite starting at higher baseline average, worst and best pain scores, the PNS group ended the 8-week treatment period with lower scores in all measures compared with the control group ().
(A) Group means and (B) percent reduction from baseline for average PLP scores. (C) Group means and (D) percent reduction from baseline for average RLP scores. Data are shown at postoperative baseline and mean of weeks 1–4, weeks 5–8 and at the 3-month follow-up visit. Stimulation was active in the PNS group for 8 weeks beginning after baseline.
PLP: Phantom limb pain; PNS: Peripheral nerve stimulation; RLP: Residual limb pain.
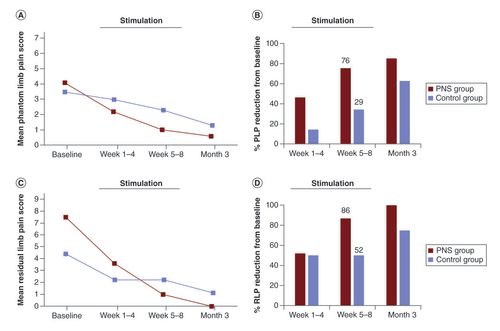
Table 2. Summary of pain scores and percent reduction from baseline over time.
Based on average RLP and PLP pain scores, the treatment responder rate (the proportion of participants reporting ≥50% pain relief compared with baseline in at least one of RLP and/or PLP) during PNS treatment at 8 weeks was 100% (5/5) compared with 50% (4/8) in the control group at 8 weeks of SMT. At 3 months, the PNS group responder rate remained 100% (n = 5/5), and the control group rate increased to 86% (n = 6/7).
Secondary outcome measures
Opioid consumption
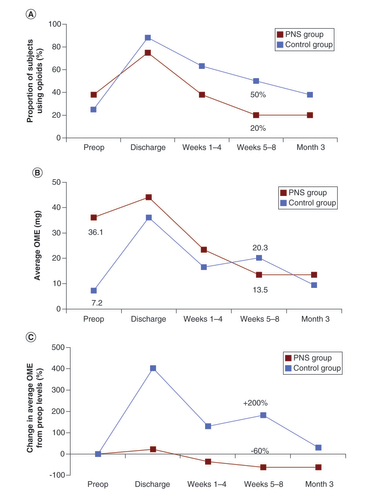
Though preoperatively the treatment group had a higher percentage of subjects using opioids and a higher average OME usage compared with the control group (36.1 mg vs 7.2 mg), fewer subjects in the PNS group were using opioids compared with SMT alone at each postoperative time point ().
Table 3. Summary of average oral morphine equivalents and % participants taking opioids over time.
By the end of PNS treatment at week 8, 20% of subjects (1/5) in the PNS group were still using opioids compared with 50% (4/8) in the SMT group (A). Opioid usage increased relative to preoperative levels in the SMT group to 20.3 mg by the end of Week 8 (>200% increase), while usage decreased relative to preoperative levels to 13.5 mg (>60% decrease) in the PNS group (B & C). Only in the PNS group did average OME fall below preoperative levels within the first 3 months post-amputation (C).
(A) The proportion of participants in the PNS group and control group that were taking opioids is shown for the preoperative period, at the time of hospital discharge, during Weeks 1–4, during Weeks 5–8 and at Week 12. The percent reduction in oral morphine equivalents (OME) consumption in the PNS group fell to 69% by the end of the 8-week treatment period, while the control group did not fall that same amount (70%) until Week 12. (B) Average OMEs and (C) percent reduction in average OMEs in the PNS group steadily decreased from the date of hospital discharge to 3 months and fell below preoperative levels by 1–4 weeks. The control group remained higher than preoperative levels throughout the entire 12 weeks. (C) The PNS group saw an earlier rate of decline in the proportion of participants taking opioids and a larger percent reduction of OMEs from baseline over time.
OME: Oral morphine equivalent; PNS: Peripheral nerve stimulation.
30-day readmission rates & length of stay
Thirty-day readmission rates were reviewed for both groups. The PNS group did not have any readmissions (0%), whereas the control group readmission rate was 25%. Average historical 30-day readmission rates according to the Veterans Affairs Surgical Quality Improvement Program (VASQIP) database (July 2015–2016) for TTs and TFs are 14.8 and 14.3%, respectively [Citation20].
Hospital length of stay (LOS) was calculated from day of surgery to day of discharge from inpatient hospital ward to rehab facility. In this study, the PNS group mean LOS was 7.1 ± 1.6 days and that of the control group was 7.9 ± 5.5 days. Historical VASQIP data from year prior to study start date (July 2015 to June 2016) showed the average LOS for TTs to be 13.4 days and TFs to be 14 days [Citation20].
FIM scores
FIM scores in all categories for both groups showed reductions over time (). Average FIM scores for the selected domains were similar between the PNS and control groups at baseline, 4 weeks and 8 weeks.
Table 4. Functional independence measure scores over time.
Other secondary outcomes
PI scores over time in both groups were similar for all of the studied variables (e.g., mood, sleep, enjoyment, relationships or general activities) (). PGIC scores were similar in both groups at 4 and 8 weeks but worsened at 12 weeks, indicating both groups perceived disease deterioration. This perceived deterioration was less dramatic in the PNS group (4.1 to 3.8, 7% reduction) than in the control group (5.1 to 2.4, 53% reduction), indicating there may be some prolonged benefit of PNS even after the treatment has ended (). PCS scores were also similar in both groups at 4 and 8 weeks but worsened at 12 weeks. Noteworthy, both groups reported increases in pain interference and disability at 12 weeks, suggesting natural disease progression rather than the lack of a treatment effect.
Table 5. Other secondary outcomes over time.
PDI scores in the PNS treatment group were twofold to sixfold higher than those in the control group throughout the entire 12 weeks, though this may be explained by the small sample size and missing secondary outcomes data for some participants ().
Study-related adverse events
There were no unanticipated serious study-related adverse events. No infections occurred during the 8-week lead implantation period. Two patients required lead re-implantation due to lead dislodgement. No suspected lead fractures were observed during treatment or lead removal.
Discussion
The principles of neuromodulation and its applications have been well described for the management of chronic somatic and neuropathic pain [Citation21,Citation22], but few studies have evaluated the effectiveness of PNS in the postoperative period, and none have evaluated its use for controlling acute to subacute post-amputation pain [Citation12,Citation13,Citation23]. Neurostimulation has historically involved the permanent implantation of leads and pulse generators, making it unsuitable for temporary use in the postoperative period. A novel PNS system is available that avoids permanent implantation by utilizing temporary removable leads connected to an external pulse generator [Citation7,Citation8]. The goal of this prospective, randomized, controlled feasibility study was to determine if 60-day PNS treatment could be implemented in the acute to subacute postoperative period as a novel non-pharmacologic treatment for mitigating pain in patients undergoing major lower limb amputation.
Early and effective acute pain management that reduces chronic pain incidence has long been a goal in peri- and postoperative anesthesia [Citation24,Citation25]. Conventional management paradigms have focused on pharmacologic agents, which pose risk of toxicity and are limited by significant side effects [Citation3]. Borghi et al. reported the potential benefits of prolonged PNC infusions, suggesting that effective postoperative pain management for 30 days may help reduce the incidence of chronic pain [Citation26]. Unfortunately, PNCs are more commonly removed after a few days due to infection risk while postoperative pain may still be significant. The novel percutaneous PNS device used in this study is approved for up to 60 days and carries a lower risk of infection compared with PNCs [Citation4,Citation8]. The risk of falling due to decreased sensory and motor blockade from PNC infusions is also mitigated by the use of PNS over local anesthetics [Citation6]. Initial findings of this study support that temporary percutaneous PNS may be a safe and reliable method of treating acute to subacute post-amputation pain that addresses some of the key limitations of conventional acute pain management strategies.
In addition to PNS, new surgical techniques have also been developed to address nerve management during amputation surgeries with potential benefits for post-operative pain and alleviation of subacute and chronic post-amputation pain. For example, targeted muscle reinnervation (TMR) involves placing a severed nerve into a new, reinnervated muscle target either during the initial amputation surgery (primary TMR) or months to years following amputation (secondary TMR) for the treatment of neuroma pain and PLP. Studies have shown promising results in pain reduction in lower limb amputations [Citation27–29]. Regenerative peripheral nerve interfaces (RPNIs) similarly use muscle grafts as the physiologic target and have shown promise in reducing neuroma pain and PLP [Citation30,Citation31]. Although conventional PNS leads are placed surgically in some instances, the present approach and PNS system involve a minimally invasive percutaneous placement proximal to the site of amputation with patient feedback during stimulation testing, making intraoperative placement impractical. Surgical techniques are often performed by a peripheral nerve surgeon, which may limit their applicability at institutions that lack the necessary specialists, but nonetheless they have shown promise and may offer possibilities for multimodal pain management alongside peripheral nerve stimulation for pain in future studies [Citation32].
Inadequate perioperative pain control and postoperative phantom pain have been reported as a chief unmet need among lower extremity amputees, especially following hospital discharge [Citation33]. Phantom and residual limb pain have also been linked to poor rehabilitation outcomes, quality of life and prosthetic limb usage [Citation34]. In this study, participants in the PNS group achieved a greater reduction in residual and phantom limb pain scores at an earlier time point compared with those in the control group. Improvements in phantom pain were notable with PNS compared with SMT and largely occurred during the 8-week PNS treatment through the 3-month follow-up, suggesting that PNS may extend effective pain relief well beyond discharge. Several studies support that post-amputation pain is a key limitation in the rehabilitation process and prosthesis use [Citation35,Citation36]. Achieving lower pain scores at earlier time points may help improve rehabilitation outcomes for amputees, as suggested by reduced readmission rates and shorter lengths of stay.
Preoperative opioid use has been associated with greater postoperative opioid usage and worse pain outcomes following many surgeries, including amputation [Citation37–39]. In this study, however, the PNS group achieved greater and faster pain relief than the control group despite higher average preoperative opioid usage (). Furthermore, by the end of the treatment period (Week 8), only 20% of the PNS group were still using opioids compared with 50% in the control group. These findings are consistent with previous studies that used percutaneous PNS following total knee arthroplasty and foot and shoulder surgeries, in which lower opioid usage and faster opioid cessation were recorded compared with historic data for the same surgeries with SMT [Citation9–11]. The present study suggests that the relationship between preoperative opioid use and post-amputation outcomes could be redefined with percutaneous PNS included as part of the postoperative pain management algorithm to more effectively treat pain for a longer period following discharge, and this relationship warrants further study.
Two patients in the PNS group underwent amputation surgery for the primary diagnosis of severe preoperative pain in the affected limb after failing other pain treatment modalities. Their preoperative opioid requirements and pain scores were the highest among all study participants. Despite this challenge, their outcomes were among the best in the PNS group, with 1 participant reporting no PLP or RLP and 50% reduction in preoperative opioid usage by the 3-month follow-up, and the other participant reporting no PLP, 50% reduction in baseline RLP, and no opioid usage by the end of the 8-week treatment period (after which the participant withdrew from the study due to an unrelated medical complication). Further investigation evaluating differences in surgical indications and the potential role that PNS may play in pain management for limb salvage prior to amputation should be considered in future studies.
PNS for acute postoperative pain has been demonstrated in the literature with lead placement at various times perioperatively. In the present study, PNS leads were placed in the acute postoperative period, after removal of PNCs, an average of 5.5 days post-amputation. Previous studies placing percutaneous PNS leads preoperatively have found that initiating stimulation immediately after surgery may not provide sufficient pain relief and additional analgesia may be required [Citation9–11,Citation23], but by postop day 1 and beyond, stimulation produced pain relief almost immediately upon initiation, consistent with the present study. Based on these findings, the best timing for lead placement in lower extremity amputation is still unknown, and it may be that earlier placement, even preoperatively, could enable earlier pain relief and opioid cessation following amputation.
This feasibility study highlights several challenges to be addressed in the design of future studies. First, lower limb amputations are associated with increased morbidity and mortality, likely related to the serious comorbidities and reduced functional status of the patients requiring this operation [Citation40]. Unfortunately, this patient population is at higher risk of adverse health events, including the need for reoperations, due to their complex comorbidities. The need for a reoperation in the middle of the treatment or follow-up periods can contribute to worsening pain and need for increased opioids, which may significantly confound the results. Design of future larger studies should be powered to account for a higher likelihood of participant withdrawals due to unrelated adverse health events or reoperations. Second, the effects of PNS treatment may be impacted by the number of hours the device is utilized by the patient. Though participants were instructed to use the device continuously, the authors did not collect usage data. Future studies may opt to collect usage data to better understand their impact on outcomes. Finally, variations in subject compliance with device usage may impact outcomes, and strategies to thoroughly educate patients on device operability and compliance with treatment plans would be beneficial.
Conclusion
Overall, the potential advantage of a temporary percutaneous PNS system over SMT alone for the treatment of acute to subacute postsurgical pain following lower extremity amputation may provide improved pain relief and less opioid consumption through 3 months postoperatively, as demonstrated in this randomized, controlled feasibility study. Long-term analysis through 12 months is ongoing. Though larger studies are needed to confirm these findings, initial results for PNS treatment in lower extremity amputation are promising as an alternative non-opioid, temporary, non-local anesthetic pain treatment modality in the acute and subacute postoperative setting.
Major limb amputation commonly causes severe acute and chronic pain, and current treatment regimens have largely been ineffective.
Minimally invasive percutaneous peripheral nerve stimulation (PNS) therapy is a feasible nonpharmacologic modality of managing both neuropathic and nociceptive pain in acute and chronic phantom limb pain.
PNS therapy may be used in the perioperative setting to help reduce acute postoperative pain.
The duration of pain relief from temporary, percutaneous PNS therapy may extend beyond the duration of the treatment period.
PNS therapy reduces daily opioid consumption and reduces the number of patients still taking opioids for postsurgical pain at 3 months after amputation.
Percutaneous PNS therapy can be utilized in a clinic, preoperative, or postoperative holding area, which makes this treatment modality widely accessible at many institutions.
Larger studies are necessary to reproduce the results found in this feasibility study, determine the optimal timing of PNS therapy, determine surgical indications, and guide patient selection.
Author contributions
B Albright-Trainer, T Phan, R Trainer, N Crosby, D Murphy, M Amendola, and D Lester contributed to study design and protocol development. B Albright-Trainer, T Phan, R Trainer and D Lester helped with data collection and analytics. B Albright-Trainer, T Phan, R Trainer, N Crosby, D Murphy and D Lester contributed to drafting and revision of the manuscript. B Albright-Trainer: This author helped with protocol development, grant proposal, screening, recruitment, enrollment and consent of study subjects; assisted in intervention, placement of devices, data collection, follow-up of patients and summary statistics; and is primary writer of the manuscript draft. T Phan: This author was primary author of protocol development and grant proposal and helped with screening, recruitment and enrollment of study subjects; assisted with data collection and follow-up of patients; and is main contributing author of this manuscript. R Trainer: This author contributed to protocol development and grant proposal and the screening and enrollment of study subjects as well as assisted in intervention, placement of devices and follow-up of patients. N Crosby: This author provided guidance during protocol development and assisted with edits of this manuscript. D Murphy: This author contributed to protocol development and grant proposal, helped recruit study subjects, assisted with follow-up of patients, reviewed data for accuracy and contributed to edits of this manuscript. M Amendola: This author contributed to protocol development and grant proposal, helped recruit study subjects and contributed to revisions of this manuscript. D Lester: This author is the primary investigator of the study and contributed to protocol development, development and submission of grant proposal, delivery of interventions, placement of devices, data collection, follow-up of patients, review of data for accuracy and analysis, and contributed to revisions of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Acknowledgments
The authors wish to thank A Gorgey for his help with statistical guidance. Thank you to E Baker, C Johnson, K Stutz and C Kem-Bumbala for their help as our research personnel, and thank you to S Ianchulev and D Drake for departmental support. Thanks to A Barden, S Kastury and TG Scott for their assistance in conducting functional independence measure assessments on participants. And thanks to S Henderson for her work in data collection as our hired study coordinator.
Financial & competing interests disclosure
N Crosby is an employee of SPR Therapeutics. The study was funded by a Hunter Holmes McGuire Research Pilot Grant and half of the devices were provided by SPR Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
The authors certify that this manuscript reports original clinical trial data, NCT03484429. All study data are currently available from ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT03484429) in accordance with the requirements of the repository.
Additional information
Funding
References
- Hanley MA , JensenMP , SmithDG , EhdeDM , EdwardsWT , RobinsonLR. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J. Pain8, 102–109 (2007).
- Karanikolas M , ArethaD , TsolakisI , MonanteraG , KiekkasP , PapadoulasSet al. Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency, a prospective, randomized, clinical trial. Anesthesiology114, 1144–1154 (2011).
- Kent ML , HsiaHJ , Vande Ven TJ , BuchheitTE. Perioperative pain management strategies for amputation: a topical review. Pain Med.18, 504–519 (2017).
- Capdevila X , BringuierS , BorgeatA. Infectious risk of continuous peripheral nerve blocks. Anesthesiology110, 182–188 (2009).
- Jeng CL , TorrilloTM , RosenblattMA. Complications of peripheral nerve blocks. BJA105(1), 97–107 (2010).
- Ilfeld BM , DukeKB , DonohueMC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth. Analg.111, 1552–1554 (2010).
- Ilfeld BM , GrantSA. Ultrasound-guided percutaneous peripheral nerve stimulation for postoperative analgesia: could neurostimulation replace continuous peripheral nerve blocks?Reg. Anesth. Pain Med.41, 720–722 (2016).
- Ilfeld BM , GabrielRA , SaulinoMF , ChaeJ , PeckhamPH , GrantSAet al. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract.17, 753–762 (2017).
- Ilfeld BM , BallST , GabrielRA , SztainJF , MonahanAM , AbramsonWBet al. A feasibility study of percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty. Neuromodulation22, 653–660 (2019).
- Ilfeld BM , GabrielRA , SaidET , MonahanAM , SztainFJ , AbramsonWBet al. Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the sciatic nerve for postoperative analgesia following ambulatory foot surgery, a proof-of-concept study. Reg. Anesth. Pain Med.43, 580–589 (2018).
- Ilfeld BM , FinneranJJ , GabrielRA , SaidET , NguyenPL , AbramsonWBet al. Ultrasound-guided percutaneous peripheral nerve stimulation: neuromodulation of the suprascapular nerve and brachial plexus for postoperative analgesia following ambulatory rotator cuff repair. A proof-of-concept study. Reg. Anesth. Pain Med.44, 310–318 (2019).
- Gilmore C , IlfeldB , RosenowJ , LiS , DesaiM , HunterCet al. Percutaneous peripheral nerve stimulation for the treatment of chronic neuropathic postamputation pain: a multicenter, randomized, placebo-controlled trial. Reg. Anesth. Pain Med.44, 637–645 (2019).
- Gilmore C , IlfeldB , RosenowJ , LiS , DesaiM , HunterCet al. Percutaneous 60-day peripheral nerve stimulation implant provides sustained relief of chronic pain following amputation: 12-month follow-up of a randomized, double-blind, placebo-controlled trial. Reg. Anesth. Pain Med.45, 44–51 (2020).
- Cleeland CS , RyanKM. Pain assessment: global use of the Brief Pain Inventory. Ann. Acad. Med. Singapore23, 129–138 (1994).
- Dworkin RH , TurkDC , WyrwichKW , BeatonD , CleelandCS , FarrarJTet al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain9, 105–121 (2008).
- Linacre JM , HeinemannAW , WrightBD , GrangerCV , HamiltonBB. The structure and stability of the functional independence measure. Arch. Phys. Med. Rehabil.75, 127–132 (1994).
- Ferguson LJ , SchemanJ. Patient global impression of change scores within the context of a chronic pain rehabilitation program. J. Pain10(4), S73 (2009).
- Sullivan MJ , BishopSR , PivikJ. The Pain Catastrophizing Scale: development and validation. Psychol. Assess.7(4), 524 (1995).
- Anagnostis C , GatchelRJ , MayerTG. The pain disability questionnaire: a new psychometrically sound measure for chronic musculoskeletal disorders. Spine29(20), 2290–2302 (2004).
- Massarweh NN , KajiAH , ItaniKM. Practical guide to surgical data sets: Veterans Affairs Surgical Quality Improvement Program (VASQIP). JAMA Surgery153, 768–769 (2018).
- Deer TR , MekhailN , ProvenzanoD , PopeJ , KramesE , LeongMet al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation17, 515–550 (2014).
- Deer TR , NaiduR , StrandN , SparksD , Abd-ElsayedA , KaliaHet al. A review of the bioelectronic implications of stimulation of the peripheral nervous system for chronic pain conditions. Bioelectron. Med.6(9), 1–13 (2020).
- Rauck RL , CohenSP , GilmoreCA , NorthJM , KapuralL , ZangRHet al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation17, 188–197 (2014).
- Bruce J , QuinlanJ. Chronic post-surgical pain. Rev. Pain5, 23–29 (2011).
- Imani F . Postoperative pain management. Anesth. Pain Med.1(1), 6–7 (2011).
- Borghi B , D’AddabboM , WhitePF , GalleraniP , ToccaceliL , RaffaeliWet al. The use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndrome. Anesth. Analg.111(5), 1308–1315 (2010).
- Dumanian GA , PotterBK , MiotonLM , KoJH , CheesboroughJE , SouzaJMet al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann. Surg.270(2), 238–246 (2019).
- Valerio IL , DumanianGA , JordanSW , MiotonLM , BowenJB , WestJMet al. Preemptive treatment of phantom and residual limb pain with targeted muscle reinnervation at the time of major limb amputation. J. Am. Coll. Surg.228(3), 217–226 (2019).
- McNamara CT , IorioML. Targeted muscle reinnervation: outcomes in treating chronic pain secondary to extremity amputation and phantom limb syndrome. J. Reconstr. Microsurg.36(4), 235–240 (2020).
- Woo SL , KungTA , BrownDL , LeonardJA , KellyBM , CedernaPS. Regenerative peripheral nerve interfaces for the treatment of postamputation neuroma pain: a pilot study. Plast. Reconstr. Surg. Glob. Open4(12), 1038 (2016).
- Kubiak CA , KempSW , CedernaPS. Regenerative peripheral nerve interface for management of postamputation neuroma. JAMA Surg.153(7), 681–682 (2018).
- Agrawal NA , GfrererL , HengM , ValerioIL , EberlinKR. The use of peripheral nerve stimulation in conjunction with TMR for neuropathic pain. Plast. Reconstr. Surg. Glob. Open9(6), 3655 (2021).
- Columbo JA , DaviesL , KangR , BarnesJA , LeinweberKA , SuckowBDet al. Patient experience of recovery after major leg amputation for arterial disease. Vasc. Endovascular. Surg.52(4), 262–268 (2018).
- Houghton A , NichollsG , HoughtonA , SaadahE , McCollL. Phantom pain: natural history and association with rehabilitation. Ann. R. Coll. Surg. Engl.76(1), 22–25 (1994).
- Gallagher P , AllenD , MacLachlanM. Phantom limb pain and residual limb pain following lower limb amputation: a descriptive analysis. Disabil. Rehabil.23(12), 522–530 (2001).
- Raichle KA , HanleyMA , MoltonI , KadelNJ , CampbellK , PhelpsEet al. Prosthesis use in persons with lower- and upper-limb amputation. J. Rehabil. Res. Dev.45(7), 961–972 (2008).
- Armaghani SJ , LeeDS , BibleJE , ArcherKR , ShauDN , KayHet al. Preoperative opioid use and its association with perioperative opioid demand and postoperative opioid independence in patients undergoing spine surgery. Spine (Phila PA 1976)39(25), e1524–e1530 (2014).
- Roullet S , Nouette-GaulainK , BiaisM , BernardN , BenardA , RevelPet al. Preoperative opioid consumption increases morphine requirement after leg amputation. Can. J. Anesth.56(12), 908–913 (2009).
- Smith SR , BidoJ , CollinsJE , YangH , KatzJN , LosinaE. Impact of preoperative opioid use on total knee arthroplasty outcomes. J. Bone Joint Surg. Am.99(10), 803–808 (2017).
- Rosen N , GigiR , HaimA , SalaiM , ChechikO. Mortality and reoperations following lower limb amputations. Isr. Med. Assoc. J.16(2), 83–87 (2014).