Abstract
Aim: The availability of long-term (>2 years) safety outcomes of spinal cord stimulation (SCS) remains limited. We evaluated safety in a global SCS registry for chronic pain. Methods: Participants were prospectively enrolled globally at 79 implanting centers and followed out to 3 years after device implantation. Results: Of 1881 participants enrolled, 1289 received a permanent SCS implant (1776 completed trial). The annualized rate of device explant was 3.5% (all causes), and 1.1% due to inadequate pain relief. Total incidence of device explantation >3 years was 7.6% (n = 98). Of these, 32 subjects (2.5%) indicated inadequate pain relief as cause for removal. Implant site infection (11 events) was the most common device-related serious adverse event (<1%). Conclusion: This prospective, global, real-world study demonstrates a high-level of safety for SCS with low rate of explant/serious adverse events.
Clinical Trial Registration: NCT01719055 (ClinicalTrials.gov)
Background
Advances in medical device technology holds the promise of treating a variety of chronic disease states afflicting large segments of the human population, but the persistent push to improve the quality of healthcare must be balanced in kind with the fundamental obligation to strive for and ensure patient safety. Recently, calls have been made to overhaul the postmarket surveillance of commercially approved medical devices so as to more adequately recognize previously undetected treatment complications (e.g., adverse events, product design flaws) that may put the safety of patients at increased risk [Citation1,Citation2]. In particular, the collection and dissemination of real-world evidence (RWE) are thought to be critical components of this endeavor. Patient data collected as part of the standard delivery of care is typically the source from which RWE is derived, including but not limited to electronic health records, claims data and clinical registries based on retrospective chart review [Citation2]. These types of repositories of information, while valuable to inform real-world experience, provide for lower levels of evidence than registry or randomized trials. As such, large multicenter patient registries carried out using more rigorous observational study designs may represent a source of higher quality clinical data in support of obtaining RWE. Additionally, results obtained from randomized controlled trials designed to establish the efficacy of an investigational device may not be fully generalizable to the real-world clinical setting, particularly as it pertains to the analysis of safety, thus further signifying the value of RWE derived from large patient-outcome registries [Citation3,Citation4].
Spinal cord stimulation (SCS) has been used since the late 1960s to treat and manage chronic neuropathic pain. Recently, a personalized device-based therapy for chronic pain via the customized delivery of electrical signals to the spinal cord – and thereby enabling the precise optimization of treatment on the basis of the specific needs of each patient – has been in use [Citation5–7]. A variety of commercially available SCS devices are now equipped to provide for an array of neurostimulation methods, programming capabilities and/or available waveform modalities [Citation8–10]. Despite these advancements however, recent safety concerns have been raised, and as such, efforts have been undertaken to reaffirm the critical importance of patient selection and the need to conduct a trial period prior to permanent implant in certain patients deemed applicable [Citation11–14]. Moreover, critical scrutiny of SCS as a safe and effective therapeutic alternative to conventional opioid drug medications has also been documented in the popular press [Citation15].
Therefore, in an effort to support the recurring assessment of the safety performance of currently available SCS devices designed with enhanced waveform programming capabilities for use in the clinical management of chronic pain, we conducted a long-term analysis of complications and explantation rates as collected from a multicenter, global SCS registry that is comprised of a large, prospectively-enrolled cohort.
Materials & methods
Study design
RELIEF (Clinicaltrials.gov identifier: NCT01719055) is a global, multicenter, prospective, single-arm, observational registry designed to collect RWE for neurostimulation systems utilized for chronic pain indications by patients within routine clinical practice. Enrolled participants were established patients in a medical practice (e.g., pain management, surgery, physical medicine and rehabilitation) who were eligible to receive neurostimulation therapy to treat their pain condition utilizing a commercially approved SCS system (manufacturer: Boston Scientific, MA USA) per local directions for use (DFU). Participation in the study was completely voluntary and up to the patient and physician.
All consecutive patients were offered participation based on standard of care and no selection bias was introduced. All participants in the registry were required to provide a signed Institutional Review Board/Ethics Committee-approved consent form and be 18 years of age or older. Prospective patients were excluded if any contraindication in the SCS system (per local DFU) were met, or if diagnosed with a cognitive impairment or exhibiting any characteristic that would limit the registry candidate’s ability to assess pain relief or complete study assessments.
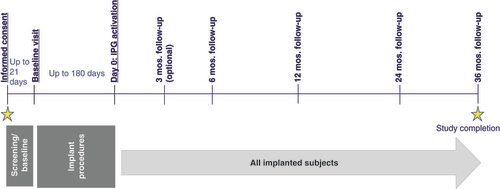
Enrolled patients underwent a trial procedure using lead(s), per local DFU and per standard of care. Patients with a successful trial continued participation in the study and received a permanent implant (i.e., stimulator), as applicable. A successful trial was determined per sites’ standard of care, typically, a minimum of 50% pain relief. However, since other clinical assessments may be assessed in conjunction with pain relief to determine stimulation trial success, final determination of trial success was at the discretion of the physician and patient. During the study, if needed, SCS system revision or replacement was allowed. Following device activation, patients returned for programming as needed over the course of the study, per standard of care. The study design of the RELIEF real-world outcomes study is displayed in .
Device description
This registry included commercially approved Boston Scientific-manufactured neurostimulation systems, per local DFU, including the following available implantable pulse generator (IPG) models: Precision, Precision Spectra, Montage, Novi and Spectra WaveWriter [Citation8,Citation16,Citation17]. All of these SCS systems are intended to treat chronic pain by electrically stimulating neurons to disrupt neuronally derived dysregulated pain signals using an IPG connected to leads that are implanted within the epidural space of the spinal canal. In addition, all of these systems are equipped with multiple independent current control technology, thereby allowing for flexible manipulation (and steering) of the current that can be delivered to each available lead contact for electro-neurostimulative therapy. As described previously, other SCS-programming technologies and/or algorithms accessible in at least some of the IPGs available to registry participants included anatomically guided neural targeting (Illumina 3D), subperception-based electric field targeting (Contour), multiple waveforms with waveform automation (sequential or simultaneous programming) and fast-acting subperception therapy [Citation8,Citation16–19]. Each of these SCS device enhancements represent features engineered to provide additional patient-specific control of therapeutic electro-neurostimulation allowing for greater customization of treatment according to the unique preferences and needs per individual patient. IPGs enabling full-body MRI (Montage) and nonrechargeable battery (Novi) features were also available to participants.
Data collection & analysis
Baseline demographic information was collected from each registry participant prior to SCS trial procedures. Study follow-up occurred at 6 months, 12 months, 24 months and 36 months to collect long-term data. Safety data including serious adverse events (SAEs), unanticipated adverse device effects/unanticipated serious adverse device effects, device-related adverse events and study procedure-related adverse events were collected. Adverse events were collected at specific study intervals or follow-up visits where any adverse events since last visit was reported. Subjects also reported adverse events (outside of scheduled visits) as needed to physicians. All device-related events were further subcategorized as hardware-related (i.e., those events that reasonably can be attributed to the mere presence of the device or a malfunction of the device or a deficiency of the device) or stimulation-related (i.e., those events that show a relationship to stimulation and undesired sensations), including those that are thought to be stimulation-related, but for which such a relationship cannot be demonstrated with stimulation on/off. Instances of device deficiencies, including device explantations, were also tracked throughout the course of the registry.
Results
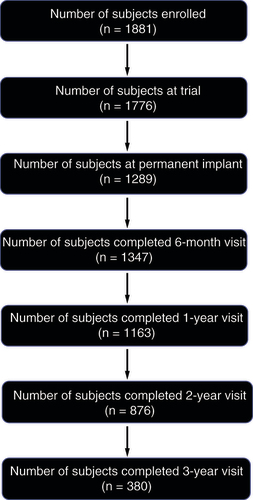
Of the 1881 registry participants enrolled between 21 January 2013 and 19 November 2021, 1776 completed the SCS trial procedure (). A total of 1289 subjects were permanently implanted with a commercially available SCS system at 79 global centers (). Mean (standard deviation) age at time of enrollment was 57.6 (13.7) years old (n = 1286), and 57% were female (). The duration of time in which permanently implanted participants reported suffering from chronic pain prior to enrolling in the registry was 11.6 ± 11.3 years (mean ± standard deviation; n = 1287) ().
Table 1. Demographics for implantedTable Footnote† subjects.
Among the 1289 permanently implanted registry participants, a total of 98 (7.6%) required an explant due to reasons shown in . Of these, 32 (2.5%) of all registry implanted participants later underwent device explantation due to inadequate pain relief. Among all device-related complications, high lead impedance (5.4%) and lead migration/movement (5.0%) were the most common events.
Table 2. Device-related complications including explants up to 3 years (n = 1289 implanted participants).
The annualized rate of explants (per year) was calculated based on number of explants per year among all registry participants over the course of 3 years. An overall annualized explant rate of 3.5% was noted, with an explant rate of 1.1% per year due to inadequate pain relief. A total of 109 subjects underwent revision including 43 for leads (3.3%) and 39 for IPG (2.9%) (note: the number of subjects is not mutually exclusive). 25 subjects (1.9%) underwent re-implant. Subjects reported their overall improvement or impression of change at follow-up compared with baseline, and 88.2% reported overall improvement (very much, much or minimally improved) at 3 year follow-up.
Among the reported adverse events, the following were reported in each of the following categories: 1) device- or procedure-related neurological deficits (three events) and 2) life-threatening local infections (two events: implant site infection, meningitis). No deaths were reported among implanted subjects. A total of 32 SAEs related to device and 51 events related to procedure (note: certain events overlapped) were reported among the 1881 consenting patients. Implant site infection (11 events) was the most common SAE related to the device. Among those related to the procedure, implant site infection (17 events) followed by implant site pain (five events) were among the common events (note: some events were reported as both related to device and procedure). provides a summary of SAEs including total number of events and those related to device, procedure or stimulation.
Table 3. Serious adverse events (related to either device, procedure or stimulation), totaling 32 serious adverse events related to device and 51 events related to procedure (overlapping events may be reported) reported among the 1881 consenting patients.
Discussion
This multicenter, prospective, registry-based assessment of SCS device-associated adverse events and complications represents one of the largest clinical evaluations of permanently implanted patients for long-term safety outcomes (i.e., out to 24- and 36-month follow-up), and therefore is an important new addition to the growing compendium of safety data relevant to the use of contemporary SCS devices for the management of chronic neuropathic pain. Multiple clinical studies published more than a decade ago reported that a substantial proportion (up to 30–40%) of SCS patients implanted with older-generation devices (following a successful trial) went on to experience at least one complication resulting in a revision and/or unanticipated explantation of device hardware, thereby greatly reducing the cost–effectiveness and quality of clinical outcomes associated with the use of SCS for treatment of chronic pain [Citation20–22]. Since then, however, published data from more recent studies and other patient registries have shown that adverse event and explantation rates in implanted SCS patients have steadily improved [Citation23–25]. In this regard, the registry data provided in this report offers key evidence for the substantial improvement in safety of SCS devices amidst the ongoing advances in hardware, software, neurostimulative waveforms, approaches to patient selection and procedural technique. For patients, providers and payers alike, these improved safety outcomes are particularly relevant given the recent drive to implement comprehensive integrative pain management on the basis of published recommendations aimed at supporting individualized patient care programs providing a range of pain care therapies such as cognitive therapies, restorative therapies, opioid and nonopioid pharmaceuticals, adjunctive therapies and neuromodulation [Citation26]. Moreover, recent CDC guidelines also have called for inclusion of all nonopioid pain therapies including neuromodulation, radiofrequency and epidural steroids, as an adjunct to decrease pain, suffering and the burden of opioids [Citation27]. We therefore assert that SCS undeniably has a key role within the comprehensive integrative pain management-based therapeutic armamentarium per its ongoing progression as a pertinent and safe therapeutic option when compared with conservative care alone in patients suffering from chronic, intractable trunk and/or limb pain.
Firstly, similar to this report, explantation data derived from a prospective, multicenter observational registry has been previously described but was carried out as part of an evaluation of 476 patients permanently implanted with St Jude Medical (since acquired by Abbott) devices who were followed out to 24 months [Citation23]. In that cohort, a total of 59 device hardware removal procedures occurred over the course of the follow-up period, corresponding to an overall explant rate of 9.6%. Of these, 13 (22.1%) were not due to an associated adverse event, while the remaining 46 (77.9%) were. Additionally, loss of analgesia was identified to be the most frequent complication (38% of all total adverse events) and occurred in a total of 70 subjects (11.4%). Secondly, a published report from Van Buyten et al. describes a multinational 5-year study using retrospective chart review to assess 822 patients at four European centers using conventional non-rechargeable, conventional rechargeable or high-frequency rechargeable SCS systems [Citation24]. Over the entire course of the study, the rate of explantation was 19%, while the annualized rate of explantation was determined to be 8% with over half of all total explants being due to inadequate pain relief. The total proportion of each type of device that was explanted because of inadequate pain relief was determined to be the following: 6.9% of nonchargeable SCS devices, 11.2% of conventional rechargeable SCS devices and 14.2% of high-frequency SCS devices. Interestingly, a higher probability of explant was associated with age (≥65 years) and females throughout most of the 5-year follow-up period after implant. Thirdly, a recent study from Al-Kaisy et al. describes a single-center retrospective-based assessment of 1177 patients implanted with different types of SCS devices over the course of an 11-year period [Citation25]. The cumulative explant rate at 3 years after permanent device implantation was determined to be 11.8%. In contrast to that reported by Van Buyten et al., no difference in explant rates according to age or sex was found in this study; however, a notable reduction in the potential likelihood of explantation appeared to be associated with the use of SCS devices capable of providing for multiple waveform options (e.g., tonic, 1–10 kHz, burst).
In comparison to these aforementioned studies, analysis of the explant data we describe in this report demonstrates a markedly lower incidence of overall device explantation (7.6%) versus Deer et al. (9.6%), Van Buyten et al. (19%) and Al-Kaisy et al. (11.8%) [Citation23–25]. This also corresponded to a lower annualized rate of device removal (3.5% per year), as well as a decreased yearly incidence of explant due to inadequate pain relief (1.41% per year), when compared with the abovementioned studies. As similarly suggested by Al-Kaisy et al., one probable explanation for the lower rates of device removal we observed (in comparison to these other studies) could be at least in part due to the various SCS-programming capabilities of the devices utilized by registry participants which are inherently designed to provide for individualized application of neurostimulation per the specific preferences and needs of each patient (e.g., multiple waveform programming and custom stimulation field targeting) [Citation25]. These device capabilities are thought to be important in helping to avoid ‘therapy habituation’, while also concurrently allowing for more efficient use of device energy. This in turn can foster a reduction in the amount of time needed for recharging (an established drawback of older and higher frequency SCS systems), an increasingly acknowledged risk factor for patient noncompliance and cessation of therapy and ultimately device explantation [Citation24,Citation28,Citation29]. Indeed, in support of this presumption, a recently published clinical study found there to be much higher than expected known cumulative explantation rates of 11.1% (1-year post-implantation) to 32.5% (3-years post-implantation) among patients implanted with a high frequency SCS system (n = 126) (vs those rates previously reported from earlier controlled studies of high-frequency SCS) [Citation30]. As such, the data described in this report provide an evidentiary basis on which to support future clinical investigation of the role that enhanced personalization of SCS therapy may have on safety and efficacy outcomes including explantation rate.
Studies comprising large-scale or ‘big data’ analytic-based evaluations of electronic health record and claims information represent an increasingly available and important resource from which to attain RWE associated with the post-market utilization of SCS devices for chronic pain. In one such longitudinal, retrospective study, 8727 individual patient datasets from the USA, for whom up to 3 years of data were available and who were permanently implanted between the years spanning 2007–2012, were identified from Commercial Claims and Encounters, Medicare Supplemental and Coordination of Benefits, and Medicaid data [Citation31]. In that study, 805 patients underwent an explant procedure, representing an overall device removal rate of 9.2%. This assessment, not surprisingly, reported that removal of an implanted SCS device was found to be significantly correlated with a total increase in healthcare resource utilization and costs [Citation31]. Notably, as described in this current report, the mean number of years since the initial onset of chronic pain (before SCS device implantation) among all patients assessed was approximately 11.5 years. Accordingly, one could speculate that not all of the patients included were ideal candidates for SCS therapy and some were perhaps challenging cases. Yet, despite this, we found an overall device explantation rate of 7.6% as measured over the course of a 3-year follow-up, thereby representing a modest but noteworthy improvement (i.e., lower incidence) in the total percentage of SCS device removal when compared with previous studies reporting similar data near or at this timepoint [Citation23–25,Citation32,Citation33].
Lead migration has long been documented as a common complication associated with the use of SCS [Citation34–38]. In particular, rates of lead migration have been previously reported to be as high as up to 24% [Citation2]. Accordingly, advances in lead design and device programming capability have been developed as an approach to cope with and mitigate this specific problem more effectively [Citation37]. This includes the development of percutaneous and/or paddle leads equipped with 32 contacts, tight-electrode spacing, multicolumn electrode configurations and a longer span capable of covering three vertebral levels (in comparison with other traditional lead designs) [Citation8,Citation39–41]. These various enhanced aspects of lead design, as well as algorithmic-based programming technologies (e.g., multiple independent current control, anatomically guided neural targeting, contoured field shaping, alternative waveforms) enable highly customized, patient-specific therapeutic neurostimulation by providing for the ability to adjust stimulation current steering and field shape (as well as other parameters) in response to slight or even severe changes in lead position or placement. In this registry-based evaluation, all analyzed patients were implanted with an SCS device equipped with at least some of these updated hardware designs in addition to other technological enhancements. Among those assessed in this registry, reports of lead migration (5%) are substantially lower than several previously published reports [Citation34–36,Citation42–44]. Whether or not this is at all or to some degree attributable to the benefits offered by the abovementioned advances in lead and/or upgraded SCS system designs cannot be definitively discerned from this analysis, but to our knowledge, this result does represent one of the lowest (if not the lowest) lead migration rates ever reported in a clinical study of this size.
Infection is an additional complication that must be closely monitored in SCS-device implanted patients given the significant association with higher healthcare costs and clinical morbidity [Citation45]. In various pertinent studies published over the last decade, reported rates of patient infection associated with SCS-device implantation have ranged from roughly 2% to 5% [Citation23,Citation24,Citation34,Citation46–48]. Encouragingly, the rate of device-related infection in this current registry evaluation was observed to be closest to the very low end of this range, as well as found to be slightly improved versus the SCS device-related infection rate previously reported in a comprehensive analysis of a large USA-based payer database (1% vs 3.11%) [Citation48]. This would therefore support the notion that risk of infection associated with the implantation of SCS devices continues to be improved in comparison to earlier reports. While it is not possible to definitively ascertain why device-related infection was observed to be decreased relative to known historical rates, it has been suggested by others that improved procedural adherence to standard operating protocols and technique among SCS device implanting physicians is likely a critical factor in lowering the risk of infection in patients treated with implanted SCS devices [Citation45,Citation48,Citation49]. It therefore is noteworthy that peer-reviewed publication of evidence-based guidelines aimed to help prevent infection in SCS-implanted patients occurred fairly close to the inception of this patient registry [Citation50].
Some limitations of observationally designed registries must be noted, including the inability to uncover specific reasons for outcomes obtained and the constraint that only observed, descriptive data can be collected. Future studies therefore will be required to determine the possible direct effects that new technologies or designs (or any other potential causal feature) may have on the rate of complications and/or device explantation associated with the use of SCS for management of chronic pain. Additionally, this presented analysis of safety data does not allow for rational predictions to be made regarding which types of patients may be more prone to complications or more likely to undergo a device explant. Finally, the aim of this registry was to only surveil outcomes associated with one specific manufacturer-based SCS devices, and thus the comprehensive tracking of the safety performance with regard to other similarly approved SCS devices that are also currently available for real-world use was not undertaken. We therefore recommend that future registries and/or meta-analyses amass or combine safety information from other large patient cohorts using different SCS systems designed and marketed by various device manufacturers, so as to accumulate data that is all-inclusive and more broadly reflective of the entirety of the patient population implanted with contemporary SCS devices for use in the treatment of chronic neuropathic pain.
Conclusion
This global, multicenter, real-world, prospective, SCS device registry constitutes one of the largest published studies to report long-term safety outcomes (n = 1289 permanently implanted patients; out to 36-months follow-up) and demonstrates low rates of device-associated complications and hardware removal procedures (explant) in comparison to other similar clinical studies and registries that previously reported safety data from implanted patients using SCS devices for chronic neuropathic pain. Additionally, the safety profile associated with the SCS devices utilized by patients in this registry was found to be good, including the rate of related SAEs. Furthermore, these data strongly suggest that the safety performance of SCS devices continues to progressively improve, thereby providing further evidence for SCS as a therapeutic tool of ever-increasing importance in the treatment of chronic pain.
Previously published clinical studies reported that a substantial proportion (up to 30–40%) of spinal cord stimulation (SCS) patients implanted with older-generation devices had a revision and/or explant, in turn limiting the cost–effectiveness of SCS.
Over the last decade, SCS device technologies have greatly advanced in capability for customized neurostimulative therapy allowing for improved patient-specific treatment strategies.
These medical device technology developments have occurred amidst an increased scrutiny for assessment of real-world patient safety and treatment complications.
The RELIEF registry collected real-world safety outcomes out to 3 years as derived from a large cohort of chronic pain patients (n = 1289) implanted with SCS systems at 79 sites located internationally.
The overall yearly incidence (rate) of device explant was 3.5%, and this decreased to 1.1% for explants occurring specifically as a result of inadequate pain relief.
The total percentage of device explant over the course of the registry (3 years) was 7.6% (n = 98), and this decreased to 2.5% for explants occurring specifically as a result of inadequate pain relief.
The most common noted serious adverse event was implant site infection (11 total events; <1%).
Results obtained from this prospective, real-world, international registry demonstrate an overall good safety profile with use of SCS systems for chronic pain and constitute one of the largest published studies to report long-term safety outcomes in patients using SCS for treatment of chronic pain.
Author contributions
Conception and design: RL Rauck, R Woon, K Lechleiter and R Jain. Patient enrollment and data collection: all authors. Data analysis: R Woon. Manuscript preparation: R Jain, N Patel, K Lechleiter, RL Rauck. All authors have critically reviewed and approved the published version of the submitted manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Acknowledgments
The authors wish to express their great appreciation to DS Halperin for providing substantial contribution to the writing and editing of this published manuscript.
Financial & competing interests disclosure
RL Rauck reports grants from SPR, Nalu and Nevro, personal fees from Presidio, and grants and personal fees from Boston Scientific and Saluda. E Loudermilk reports no conflicts. SJ Thomson reports consulting agreements with Boston Scientific, Mainstay Medical and Saluda Medical. JF Paz-Solis reports a consulting agreement with Boston Scientific. L Bojrab reports no conflicts. J Noles reports no conflicts. J Vesper reports consulting agreements with Medtronic, Abbott, Boston Scientific and Uniqure, and has received grants from the German Research Council and Medtronic and speaker honoraria from Abbott. J Atallah reports a consulting agreement with Boston Scientific. D Roth reports no conflicts. J Hegarty reports no conflicts. M Prud’Homme reports travel reimbursement from Boston Scientific, Medtronic and Abbott. GM Phillips reports a consulting agreement with Boston Scientific. SG Smith reports no conflicts. M Ibrahim reports no conflicts. CD Willoughby reports no conflicts. JB Obray reports no conflicts. M Gupta reports grants and personal fees from Nevro Corp., and consulting agreements from Averitas Pharma, US WorldMeds, Nalu Medical, Foundation Fusion Solutions, SPR Therapeutics, Inc., outside the submitted work. J Paez reports no conflicts. AP Berg reports a consulting agreement with Boston Scientific. NJ Harrison reports no conflicts. P Maino reports no conflicts. P Mambalam reports no conflicts. M McCarty reports no conflicts. G Towlerton reports no conflicts. S Love-Jones reports consulting agreements with Abbott, Boston Scientific, Medtronic, Nevro Corporation and Pfizer, and has received research support from Boston Scientific, Abbott, Nevro Corporation, Nalu Medical and Mainstay Medical. S Ahmed reports no conflicts. A Lee reports no conflicts. B Shah reports no conflicts. I Goor-Aryeh reports no conflicts. M Russo reports no conflicts. N Varela reports no conflicts. JB Phelps reports no conflicts. J Cid reports no conflicts. T Fernandez reports no conflicts. C Pérez-Hernández reports consultancy fees or honoraria from Grunenthal, Ferrer, Kyowa, Teva, Boston Scientific, Takeda, Prim, Pfizer and Medtronic. D Keehn reports no conflicts. JM Rosenow reports grants and personal fees from Boston Scientific, Monteris, Stryker and AIM Medical Robotics. N Haider reports no conflicts. AG Parrent reports advisory board membership for Medtronic. MM Lawrence reports no conflicts. P Georgius reports personal fees from Boston Scientific, Abbott and Spectrum. L Demartini reports personal fees from Boston Scientific and Abbott. A Mendiola reports no conflicts. V Mehta reports grants from Mainstay and Abbott, grants and personal fees from Boston Scientific and Medtronic. R Thoma reports no conflicts. AF Israel reports no conflicts. G De Carolis reports no conflicts. S Bhatia reports no conflicts. M Green reports no conflicts. A Villarreal reports no conflicts. MT Crooks reports no conflicts. RP Gwinn reports no conflicts. JG Pilitsis reports grant support from Medtronic, Boston Scientific, Abbott, NIH 2R01CA166379, NIH R01EB030324, NIH Blueprint 3U54EB015408 and NIH U44NS115111. She is the medical advisor for Aim Medical Robotics and has stock equity. H Sato reports no conflicts. S Maldonado Vega reports no conflicts. MG Hillegass reports no conflicts. P Carnes reports no conflicts. C Scherer reports travel and conference fees from Abbott, Boston Scientific and Medtronic. S Brill reports no conflicts. J Yu reports employment with Australian Medical Research, outside the submitted work. JJ Brennan reports no conflicts. K Gatzinsky reports consulting fees from Boston Scientific and Medtronic, honoraria from Abbott, Boston Scientific and Nevro and participated in advisory boards of Boston Scientific and Medtronic. A Navani reports no conflicts. LT Snook reports no conflicts. B Mugabure Bujedo reports no conflicts. J De Andrés Ares reports no conflicts. A Murillo reports no conflicts. AT Trobridge reports no conflicts. K Assil reports no conflicts. J Shah reports no conflicts. C McLeod reports no conflicts. J Buwembo reports no conflicts. O De Coster reports no conflicts. N Miller reports no conflicts. M Sanapati reports no conflicts. M Mikhael reports no conflicts. R Przkora reports no conflicts. N Sukenaga reports no conflicts. LJ Raso reports speakers Bureau for Boston Scientific, Aurora Spine, SurGen Tec, Vertiflex and FloSpine. He is a stockholder for Aurora Spine and receives royalties from Flospine and SurGen Tec. AK Calodney reports consultant fees or receives research support from Medtronic, Nevro, Stryker, Saluda, Nalu, Boston Scientific, Vertos, Painteq, Stimgenics, Spine BioPharma, Saol Therapeutics, Tissuetech, BioRestorative, FUSMobile and APEX Biologix. LE Cáceres Jerez reports no conflicts. T Uchiyama reports no conflicts. JW Kallewaard reports a consulting agreement with Boston Scientific and advisory board membership with Medtronic, Saluda, Abbott, Nevro and Boston Scientific. B Chandler reports no conflicts. F Piedimonte reports no conflicts. KD Candido reports no conflicts. TE Weaver reports consulting services to Medtronic and received research support from Medtronic, Boston Scientific, SPR Therapeutics and Heron Pharmaceutical. T Agari reports no conflicts. D Holthouse reports no conflicts. R Woon reports employment with Boston Scientific. N Patel reports employment with Boston Scientific. K Lechleiter reports employment with Boston Scientific. R Jain reports employment with Boston Scientific. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Data sharing statement
The data, analytic methods and study materials for this clinical study will be made available to other researchers in accordance with Boston Scientific Data Sharing Policy (https://www.bostonscientific.com).
Additional information
Funding
References
- Salazar JW , RedbergRF. Leading the call for reform of medical device safety surveillance. JAMA Intern. Med.180(2), 179–180 (2020).
- Resnic FS , MathenyME. Medical devices in the real world. N. Engl. J. Med.378(7), 595–597 (2018).
- Kim HS , LeeS , KimJH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J. Korean Med. Sci.33(34), e213 (2018).
- Booth CM , TannockIF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br. J. Cancer110(3), 551–555 (2014).
- Guzzi G , DellaTorre A , LaTorre Det al. Spinal cord stimulation in chronic low back pain syndrome: mechanisms of modulation, technical features and clinical application. Healthcare (Basel)10(10), 1953 (2022).
- Viswanath O , UritsI , BouleyE , PeckJM , ThompsonW , KayeAD. Evolving spinal cord stimulation technologies and clinical implications in chronic pain management. Curr. Pain Headache Rep.23(6), 39 (2019).
- Maheshwari A , PopeJE , DeerTR , FalowskiS. Advanced methods of spinal stimulation in the treatment of chronic pain: pulse trains, waveforms, frequencies, targets, and feedback loops. Expert Rev. Med. Devices16(2), 95–106 (2019).
- Veizi E , HayekSM , NorthJet al. Spinal cord stimulation (SCS) with anatomically guided (3D) neural targeting shows superior chronic axial low back pain relief compared to traditional SCS – LUMINA study. Pain Med.18(8), 1534–1548 (2017).
- Deer TR , LevyRM , KramerJet al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain158(4), 669–681 (2017).
- Russo M , BrookerC , CousinsMJet al. Sustained long-term outcomes with closed-loop spinal cord stimulation: 12-month results of the prospective, multicenter, open-label avalon study. Neurosurgery87(4), e485–e495 (2020).
- Sivanesan E , BicketMC , CohenSP. Retrospective analysis of complications associated with dorsal root ganglion stimulation for pain relief in the FDA MAUDE database. Reg. Anesth. Pain Med.44(1), 100–106 (2019).
- Pollard EM , LamerTJ , MoeschlerSMet al. The effect of spinal cord stimulation on pain medication reduction in intractable spine and limb pain: a systematic review of randomized controlled trials and meta-analysis. J. Pain Res.12, 1311–1324 (2019).
- Thomson S , HuygenF , PrangnellSet al. Applicability and validity of an e-health tool for the appropriate referral and selection of patients with chronic pain for spinal cord stimulation: results from a European retrospective study. Neuromodulation.26(1),164-171 (2022).
- US FDA .Conduct a Trial Stimulation Period before Implanting a Spinal Cord Stimulator (SCS) – Letter to Health Care Providers (2020). www.fda.gov/medical-devices/letters-health-care-providers/conduct-trial-stimulation-period-implanting-spinal-cord-stimulator-scs-letter-health-care-providers?utm_campaign=2020-09-03%20Conduct%20a%20Trial%20Stimulation&utm_medium=email&utm_source=Eloqua (Accessed 9September2022).
- Associated Press . Spinal-cord stimulators help some patients, injure others. NBC News, 28th November (2018). www.nbcnews.com/health/health-care/spinal-cord-stimulators-help-some-patients-injure-others-n940131
- Metzger CS , HammondMB , PylesSTet al. Pain relief outcomes using an SCS device capable of delivering combination therapy with advanced waveforms and field shapes. Expert Rev. Med. Devices17(9), 951–957 (2020).
- Clingan JA , PatelA , MaherDP. Survey of spinal cord stimulation hardware currently available for the treatment of chronic pain in the United States. Front. Pain Res. (Lausanne)1, 572907 (2020).
- Kallewaard JW , Paz-SolisJF , DeNegri Pet al. Real-world outcomes using a spinal cord stimulation device capable of combination therapy for chronic pain: a European, multicenter experience. J. Clin. Med.10(18), 4085 (2021).
- Metzger CS , HammondMB , Paz-SolisJFet al. A novel fast-acting sub-perception spinal cord stimulation therapy enables rapid onset of analgesia in patients with chronic pain. Expert Rev. Med. Devices18(3), 299–306 (2021).
- Kumar K , WilsonJR , TaylorRS , GuptaS. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J. Neurosurg. Spine5(3), 191–203 (2006).
- Rosenow JM , Stanton-HicksM , RezaiAR , HendersonJM. Failure modes of spinal cord stimulation hardware. J. Neurosurg. Spine5(3), 183–190 (2006).
- Simopoulos T , AnerM , SharmaS , GhoshP , GillJS. Explantation of percutaneous spinal cord stimulator devices: a retrospective descriptive analysis of a single-center 15-year experience. Pain Med.20(7), 1355–1361 (2019).
- Deer T , SkaribasI , McJunkinTet al. Results from the partnership for advancement in neuromodulation registry: a 24-month follow-up. Neuromodulation19(2), 179–187 (2016).
- Van Buyten JP , WilleF , SmetIet al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation20(7), 642–649 (2017).
- Al-Kaisy A , RoydsJ , Al-KaisyOet al. Explant rates of electrical neuromodulation devices in 1177 patients in a single center over an 11-year period. Reg. Anesth. Pain Med.45(11), 883–890 (2020).
- US Department of Health and Human Services, Alliance to Advance Comprehensive Integrative Pain Management . Pain Management Best Practices Inter-Agency Task Force Report (2019). https://painmanagementalliance.org/resources/hhs-report-2019/ (Accessed 28October2022).
- Dowell D , RaganKR , JonesCM , BaldwinGT , ChouR. CDC clinical practice guideline for prescribing opioids for pain – United States, 2022. MMWR Recomm. Rep.71(3), 1–95 (2022).
- Levy RM , MekhailN , KramerJet al. Therapy habituation at 12 months: spinal cord stimulation versus dorsal root ganglion stimulation for complex regional pain syndrome type I and II. J. Pain21(3–4), 399–408 (2020).
- Pope JE , DeerTR , FalowskiSet al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation20(6), 543–552 (2017).
- Wang VC , BounkousohnV , FieldsKet al. Explantation rates of high frequency spinal cord stimulation in two outpatient clinics. Neuromodulation24(3), 507–511 (2021).
- Han JL , MurphyKR , QasimHussaini SMet al. Explantation rates and healthcare resource utilization in spinal cord stimulation. Neuromodulation20(4), 331–339 (2017).
- Dombovy-Johnson ML , D’SouzaRS , HaCT , HagedornJM. Incidence and risk factors for spinal cord stimulator lead migration with or without loss of efficacy: a retrospective review of 91 consecutive thoracic lead implants. Neuromodulation25(5), 731–737 (2022).
- Murphy KR , HanJL , QasimHussaini SMet al. The volume–outcome effect: impact on trial-to-permanent conversion rates in spinal cord stimulation. Neuromodulation20(3), 256–262 (2017).
- Hayek SM , VeiziE , HanesM. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of 8 years of experience from an academic center database. Neuromodulation18(7), 603–8; discussion 608–609 (2015).
- Mironer YE , BrownC , SatterthwaiteJR , CohenM , TonderLM , GrummanS. A new technique of ‘midline anchoring’ in spinal cord stimulation dramatically reduces lead migration. Neuromodulation7(1), 32–37 (2004).
- Kumar K , BuchserE , LinderothB , MeglioM , Van BuytenJP. Avoiding complications from spinal cord stimulation: practical recommendations from an international panel of experts. Neuromodulation10(1), 24–33 (2007).
- Gatzinsky K , BaardsenR , BuschmanHP. Evaluation of the effectiveness of percutaneous octapolar leads in pain treatment with spinal cord stimulation of patients with failed back surgery syndrome during a 1-year follow-up: a prospective multicenter international study. Pain Pract.17(4), 428–437 (2017).
- Kim DD , VakharyiaR , KrollHR , ShusterA. Rates of lead migration and stimulation loss in spinal cord stimulation: a retrospective comparison of laminotomy versus percutaneous implantation. Pain Physician14(6), 513–524 (2011).
- Hegarty D . Spinal cord stimulation: the clinical application of new technology. Anesthesiol.Res. Pract.10.1155/2012/375691(2012).
- Rigoard P , JacquesL , DelmotteAet al. An algorithmic programming approach for back pain symptoms in failed back surgery syndrome using spinal cord stimulation with a multicolumn surgically implanted epidural lead: a multicenter international prospective study. Pain Pract.15(3), 195–207 (2015).
- Rigoard P , BasuS , DesaiMet al. Multicolumn spinal cord stimulation for predominant back pain in failed back surgery syndrome patients: a multicenter randomized controlled trial. Pain160(6), 1410–1420 (2019).
- Cameron T . Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J. Neurosurg.100(Suppl. 3 Spine), 254–267 (2004).
- Kumar K , TaylorRS , JacquesLet al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery63(4), 762–70; discussion 770 (2008).
- Shultz DM , CalodneyAK , MoglinerAYet al. Spinal cord stimulation (SCS) – the Implantable Systems Performance Registry (ISPR). Neuromodulation19(8), 857–863 (2016).
- Provenzano DA , DeerT , LuginbuhlPhelps Aet al. An international survey to understand infection control practices for spinal cord stimulation. Neuromodulation19(1), 71–84 (2016).
- Schultz DM , WebsterL , KosekP , DarU , TanY , SunM. Sensor-driven position-adaptive spinal cord stimulation for chronic pain. Pain Physician15(1), 1–12 (2012).
- Bendel MA , O’BrienT , HoelzerBCet al. Spinal cord stimulator related infections: findings from a multicenter retrospective analysis of 2737 implants. Neuromodulation20(6), 553–557 (2017).
- Falowski SM , ProvenzanoDA , XiaY , DothAH. Spinal cord stimulation infection rate and risk factors: results from a United States payer database. Neuromodulation22(2), 179–189 (2019).
- Thomson SJ , KruglovD , DuarteRV. A spinal cord stimulation service review from a single centre using a single manufacturer over a 7.5 year follow-up period. Neuromodulation20(6), 589–599 (2017).
- Deer TR , ProvenzanoDA , HanesMet al. The Neurostimulation Appropriateness Consensus Committee (NACC) recommendations for infection prevention and management. Neuromodulation20(1), 31–50 (2017).