Abstract
The past decades witnessed the slow evolution of Europe’s heterogeneous stem cell (SC) policy and substantial scientific advances in the field. Parallel to these developments, professional organizations have grown in influence. With the recently revised International Society for Stem Cell Research’s Guidelines as a backdrop, we address the evolution of SC policies in 46 European countries and discuss how they fare against evolving ethical standards, societal views, and scientific advances. We identify areas of convergence, divergence, and the suitability of extant governance mechanisms to meet their stewardship roles. Europe represents a rich case study as it encompasses a wide range of policy approaches present worldwide. Comparative studies provide an opportunity to promote insight into national frameworks and to foster international harmonization.
summary
European countries have adopted different types of rules or policies, including laws and professional standards, to regulate stem cell research. These differences are because each country has different history and cultures. Also, individuals and institutions (e.g., religious leaders, politicians and advocacy organizations) have different degrees of power to influence the type of policies that are adopted in each country. Over the past decades, stem cell policies have evolved slowly even with significant scientific advances. Yet, during this time, professional organizations have grown in influence, for example, the prominent International Society for Stem Cell Research, whose guidelines (or rules) are considered ‘best practices’ in the field. In this article, we identify and analyze stem cell policies in 46 European countries, comparing them against the International Society for Stem Cell Research’s new Guidelines. In addition, we show the similarities and differences amongst these policies. Europe presents an interesting case study because the region includes a wide typology of policies like those adopted in the rest of the world, making this comparison useful for other countries as they consider the suitability of their own policies.
In Europe, the stem cell research–clinical translation continuum is characterized by a heterogeneity of governance and policy frameworks reflecting the continent’s diverse socio-cultural, economic and historical contexts. In the region, national and international sets of ‘hard’ (e.g., legislation, treaties) laws are supplemented by ‘soft’ ones (e.g., professional guidelines, funding policies, codes of conduct) offering different tools for enforceability and governance. Importantly, regardless of the approach, ethical considerations have acquired substantial importance as a normative tool for European policy making [Citation1]. Similarly, the public and the patient advocacy community have also gained prominence as influential policy actors, while professional organizations have maintained their persuasive role.
Globally, scientific advances in the stem cell (SC) field have occurred in parallel with the growth of professional organizations operating at the national and international stage. With different degrees of success, these entities have exerted influence in shaping the contours of responsible innovation, and thereby, fostering adaptive policy and governance. A notable example of this is the International Society for Stem Cell Research (ISSCR), whose Guidelines on stem cell research and clinical translation (hereafter: Guidelines) [Citation2] provide contemporary scientific, ethical and policy standards. Indeed, globally, ISSCR guidelines have been deemed more than metaphorically ‘customary law’ ever since their inception in 2006, as they have continued to gain legitimacy by raising awareness of ethical challenges, guiding scientific practice, and aiding in the interpretation and implementation of policy.
Recently, ISSCR updated its Guidelines, adopting strict recommendations for the regulation of clinical research and translation of SC-based interventions and substantially reconfiguring guidance for oversight of in vitro SC projects. Despite criticisms based on the absence of enforcement mechanisms and internal consistency [Citation3,Citation4], among other reasons, the revised Guidelines have the potential to exert influence in policy developments as they are designed to reflect community standards and, in that way, they aim to complement national frameworks for research governance.
Moreover, although both hard and soft laws affect the actual practice of SC research, it is unclear how European policy frameworks fare against evolving international norms represented, for instance, in the ISSCR guidelines. In addition, it is unclear how and why policies within European countries diverge and converge. Insights into these practices could lead to understanding possible gaps, contradictions and difficulties in governance and policy. Moreover, it could offer insights into how governance systems could equip researchers to act responsibly during the innovation process. In pursuit of these insights, this article addresses the evolution of SC policies in 46 European countries. With the Guidelines as a backdrop, we discuss central ethical and policy issues regarding contentious applications, including criteria for permissibility, oversight, and enforcement mechanisms. Comparative studies provide an opportunity to promote insight into national frameworks and to foster international harmonization.
The European region represents a rich case-study as it encompasses a wide range of policy approaches present across the globe. Thus, evaluating areas of convergence, divergence and progression in this region can contribute to policy debates and development worldwide.
Legislative building blocks past & present
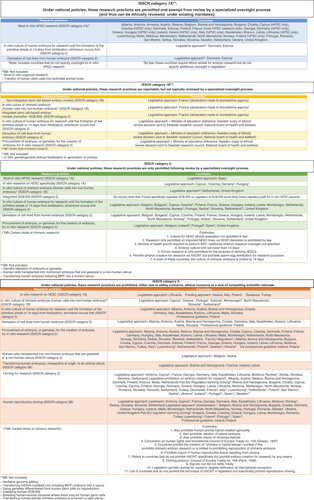
With no single policy comprehensively addressing SC across the entire research cycle, European SC policy frameworks are constituted by (i) a broad cluster of laws, directives, principles and norms governing assisted reproductive technologies (ART), biobanking, biomedical and genetics research which (ii) traverse the permissive to restrictive continuum (Appendix A Governance Approaches). Underpinning these models are foundational principles reflecting a society’s common vision, moral values, and beliefs. Moreover, a ‘common Ethics’ in the SC field is apparent from the ongoing adoption of research funding frameworks [Citation5], which include provisions for the inescapably controversial human embryonic SC (hESC) research as well as with the 1997 passing of the European Convention on Human Rights and Biomedicine [Citation6].
Across Europe, as in other regions, the regulation of SC research has generally followed a linear path, with discussions surrounding the human embryo’s biological, moral and legal status as an early central figure in the framing of socio-ethical debates and policy outcomes (e.g. Germany’s Embryo Protection Act; Belgium’s Law relating to research on embryo in vitro). This approach stems from the fact that early policy was embedded within normative frameworks governing ART (e.g. in Estonia [Citation7] and Greece [Citation8]). As such, primary goals have centered on preserving human dignity (e.g., preventing commodification, protecting the interests of unborn children) as well as integrity (e.g., cloning [Citation9] and genetic engineering bans). During these early stages, policy reforms reacted to changes in societal perceptions (e.g., toward biological parenthood) and increased technological uptake which required the promotion of the right to benefit from scientific advances and to protect welfare interests (e.g., facilitating ART research or expanding access to related services) [Citation10]. Similarly, striving to reach political compromises, European policy moved to regulate hESC research with blanket bans (e.g., Lithuania [Citation11]), moratoria (e.g., The Netherlands [Citation12]) or strict laws guided by the principles of proportionality and subsidiarity as pillars (e.g. UK, Czechia, France) [Citation13,Citation14]. From European funding policies to national laws, the passage of time has not altered this decisive criterion: the use of embryos (and gametes) is justified if it is the only means to achieve important scientific goals for societal benefits. Furthermore, early socio-ethical debates on genetic engineering, reproductive and research cloning were also influential in the framing of policy responses [Citation15] as reflected in the adoption of the Oviedo Convention [Citation16]. Importantly, the debates that took place during these times have now been revisited with the advent of gene editing tools and the emergence of SC-based embryo modeling as developments unforeseen by policymakers.
Stem cell-based embryo modeling & beyond
Contemporaneous developments in SC-based embryo modeling [Citation17], organoid [Citation18] and human–animal chimera research [Citation19] have triggered calls for policy review [Citation20] based on fears of potential loopholes inadvertently allowing controversial research to proceed without appropriate governance controls or societal support. For instance, advances in embryogenesis allow for hESCs and human induced pluripotent stem cells (hiPSCs) to be coaxed to organize into structures that mimic aspects of human embryonic development. These SC-based embryo models (SCB-EMs) have been used to model post-implantation stages of human development from the formation of the amniotic sac [Citation21] to neurulation [Citation22]. Additionally, improvements in human-animal chimera research using SC may enable investigators to study human development and organogenesis in an unprecedented manner. Furthermore, gastruloid [Citation23] research, which models events following the formation of the primitive streak, provides another interesting case study, as their scientific potential for disease modeling has driven a shift in well-established policy.
The possibility of yielding entities that might faithfully replicate embryonic developmental processes [Citation24] has prompted conceptual reexaminations, as SCB-EM do not fit neatly in pre-existing regulatory categories defining human embryos, gametes, or human research subjects. Because generally European national policies were initially created to govern ART, they often contain statutory decades-old definitions of what constitutes a human embryo (e.g., Bulgaria [Citation25], Iceland [Citation26] and Slovenia [Citation27]) (see statutory definitions of the human embryo). These definitions effectively establish distinct regulatory pathways depending on the embryo’s methods of creation, which is illustrated in the (often arbitrary) demarcation between embryos created by sperm-egg fertilization or by other means. An example is presented in Spain [Citation28] where an entity is deemed an ‘embryo’ or ’pre-embryo’ by reference to a human fertilized oocyte. Spanish law prohibits the creation of embryos for research purposes but allows pre-embryo creation via human somatic cell nuclear transfer to harvest stem cells. Other European laws categorize an embryo in terms of its potential to develop into a human individual or to reach a significant point on the developmental timeline (e.g., Belgium [Citation29], The Netherlands [Citation30] and Malta [Citation31]). In the UK, following legislative review [Citation32], the term embryo was considerably expanded, from the product of complete fertilization [Citation33] to “an egg that is in the process of fertilization or is undergoing any other process capable of resulting in an embryo” [Citation34] using human cells; or an ad-mixed entity that is the product of hybrid and chimeric processes. Importantly, here, expansive concepts and research have been accompanied by modernized, robust licensing and oversight systems [Citation35].
Largely, European countries have committed to ban embryo creation for research. This commitment arises by the ratification of the Oviedo Convention (albeit stipulated reservations). Furthermore, the Additional Protocol prohibiting human cloning [Citation36] or germline genetic engineering reflects European agreement that restrained compromises are necessary to protect human dignity and identity while advancing scientific progress (Appendix A lists countries that have ratified the Additional Protocol). Even countries adopting liberal approaches by permitting the creation of embryos for research purposes via different methods (e.g., UK [Citation37], Sweden [Citation38] and Belgium [Citation39]) align with this restrained approach by committing to provide “adequate protection of the embryo” where embryo research is allowed [Citation40]. In contrast, the ISSCR Guidelines diverge from common European policy by permitting the creation of embryos for research if projects stop at “well-defined timepoints” and undergo appropriate degrees of ethical review. Furthermore, to allow validation of increasingly advanced SCB-EM research, the Guidelines have issued a controversial [Citation41] call for governments and oversight bodies to reappraise the ‘14-day rule’ – a gold standard preventing human embryos cultured in vitro from developing for longer than 14 days [Citation42]. In the EU, regardless of where a country sits in the policy permissibility continuum, this rule continues to be ubiquitous (see for countries that have adopted the 14-day rule).
Similarly, the Guidelines sit in stark contrast with European policy regulating research on human–animal chimeric embryos. They recommend permitting human-animal chimera research and outline criteria for ethical review, while exempting in vitro culture from specialized review requirements. In contrast, almost half of European countries adopt a range of prohibitions on hybrid and/or chimera creation (e.g., Cyprus [Citation43], Portugal [Citation44], Switzerland [Citation45]) (). Only the UK regulates the practice extensively, while do not explicitly prohibit human–animal chimera research but ban different types of activities (e.g., implantation). Indeed, in most policies the term ’chimera’ is either not defined, or prohibitions refer to the combination of human and animal reproductive material, with unclear implications for human–animal chimera research using hPSCs [Citation46]. Direction from the Guidelines alone might prove to be insufficient to clarify the scope of extant legal prohibitions for research areas not explicitly described in law.
The gradual liberalization of EU national policy
A discrete trend toward policy liberalization continues to slowly emerge in Europe, which in turn, may have transformational effects in the entire region. Indeed, policy transfer might occur through joint problem solving in challenging issues such as with the curtail of unproven stem cell interventions or the regulation of interventions in early human development (e.g., embryo or germline). Alternatively, the promotion of more liberal policy models could happen by the active role of countries or international actors promoting their own models to foster their interests or seeking policy harmonization (ISSCR, European Commission). Finally, it can ensue as result of pressure exerted in the form of international scrutiny directing countries to legitimate their policy approaches.
Policy liberalization is particularly revealed in the progression of French law, which lifted its restrictive approach of the ‘90s toward hESC and chimeric research’ [Citation47,Citation48]. In 2021, France became the first country to specifically regulate the use of hPSC for gametes and SCB-EM creation, subject to the robust governance of the Biomedicine Agency and of local institutional ethics review bodies [Citation49]. Unlike many of its contemporaries, France did not place a legal temporal limit on embryo cultivation until 2021. Following a previous National Consultative Ethics Committee [Citation50] recommendation that embryo culture and research must stop after 7 days of development and, contemporaneous calls from researchers to not impose strict time limits, the revised law implements the 14-day rule. In addition, the law refines its prohibition on chimeric embryo research, forbidding the modification of human embryos by adding cells from other species but permitting the insertion of hPSC into animal embryos “for the purpose of its transfer to the female”, “subject to declaration to the Biomedicine Agency” [Citation51].
Similarly, several other countries have relaxed restrictions on hESC research over the past 15 years, while attempting to de-exceptionalize the SC field. For example, in the mid-2000s, moratoria were dropped and prohibitions on embryo research in countries such as Denmark [Citation52], Norway [Citation53] and Iceland [Citation54] were rescinded in favor of regulations allowing research on supernumerary embryos. Other countries shifted to allow cloning research (e.g., in Spain [Citation55], Iceland [Citation56] and the U.K. [Citation57]). The evolving power of stakeholders influenced some of these developments, albeit with different degrees of success. For instance, in Germany, the influence of the National Ethics Council and the German Research Foundation [Citation58] was effective for the relinquishment of blank prohibitions and for relaxing cut-off dates for the import and use of hESC lines. Also, in Ireland, the role of stakeholders (e.g., Catholic Church [Citation59]) was influential in maintaining a status quo [Citation60], despite calls from their National Bioethics Committee and other actors to adopt a more permissive approach [Citation61]. As a result, Ireland remains without specific stem cell legislation, while embryo research is considered a permissible activity since a ruling from its Supreme Court [Citation62] provided legal certainty regarding the in vitro embryo’s legal status. Across European countries (as in the rest of the world), religious [Citation63] and other cultural authorities continue to wield varying degrees of influence as stakeholders shaping policies related to embryo and stem cell research. Notable has been the uncompromising position of the Catholic Church, yet its influence has varied. For instance, in Italy [Citation64,Citation65] it continues to be a prevalent stakeholder hindering progressive stem cell policy, while in Spain, religious opposition was unable to stop policy liberalization [Citation66,Citation67]. In secular moral frameworks, permitting (under regulation) new and contentious uses of human pluripotent stem cells may be justified according to their potential for providing therapeutic benefit. Research on somatic stem cells has historically been favored as an alternative to research on the human embryo. However, it remains to be seen how SCB-EM and chimeras combining hiPSC with animal embryos fit within religious conceptions of the nature of human life.
Following decade-old attempts to shift an ‘embryo’-centric approach to one that focuses on governance, the Guidelines [Citation68] encourage nations and professional bodies to move toward a dynamic model of policy, where blanket bans are replaced by proportional and robust governance. It is thus timely to probe Europe’s current situation.
Challenging the status quo: the role of regulation & oversight
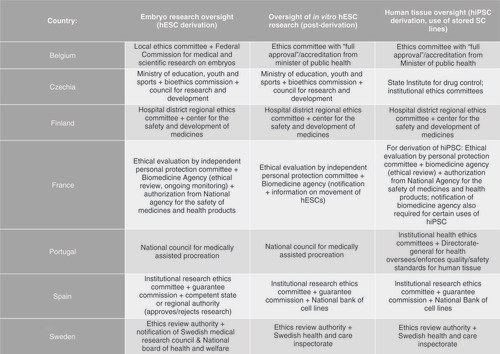
The dual mechanisms of licensing and ethics oversight play a pivotal role in fostering scientific integrity by influencing collective behavior. These deliberative bodies seek to rely on precedent, referencing previous dilemmas to judge similar proposals by consistent standards. Across Europe, governance is achieved through a plurality of actors and mechanisms, where a collection of laws and best practices establish the normative framework in which safety and ethical standards are implemented [Citation69–72]. Stewardship over SC research practices is often exercised through statutorily established licensing and oversight mechanisms. Ensuing competency and reporting requirements that guide oversight bodies are based on research stages such as tissue/embryo procurement, embryo research, derivation, and uses of hPSCs (see Governance mechanisms in selected countries).
As highlighted by ISSCR, prospective review and ongoing monitoring for compliance with ethical standards must be conducted and vetted by an independent committee. European approaches converge in this regard. They all establish local or national specialized oversight bodies of multidisciplinary composition (e.g., biology, law, ethics and in some instances community representatives) to conduct such activities. Moreover, most countries generally follow an ISSCR-style tiered approach to oversight, at least with respect to embryo and hESC related research, which are subject to specialized or centralized ethics review depending on pre-determined criteria. In Europe, mostly due to historical and political contexts, some entities charged with specialized oversight have as core focus licensing and oversight of ART interventions or embryo research activities (e.g., Belgium [Citation73], Greece [Citation74], Hungary [Citation75], Montenegro [Citation76], North Macedonia [Citation77], Portugal [Citation78], Slovenia [Citation79], UK [Citation80]), while others have a broader remit by evaluating biomedical research in general (e.g., Cyprus [Citation81], Czechia [Citation82], France [Citation83], Spain [Citation84], Switzerland [Citation85]) at the local level (Appendix B governance bodies tasked with review of embryo research).
The ISSCR Guidelines, revised to reflect evolving consensus within the field, are meant to complement existing systems of oversight, aligning new research with pre-existing societal goals. Their overarching goal is to guide researchers to seek appropriate levels of ethical review for in vitro research involving entities that could be deemed ethically or socially controversial. Among the substantial changes introduced is the categorization of permissible types of research and their corresponding oversight models. For instance, they recommend that the derivation of hESC and research on “integrated” SCB-EM be subject to the same oversight process. Notably, its previous version called for heightened scrutiny for projects involving embryo-like structures that might manifest “human organismal potential” [Citation86]. To this end, they abandon the latter nebulous and unmeasurable criterion and replace it with a proxy for researchers ‘intent and models’ capacity to “undergo further integrated development when cultured for additional time in vitro” [Citation87]. Thus, reflecting that the inherent potential of human and human-like organisms can only be realized through sustained and deliberate external influences. But while this justification has evolved, the ISSCR has remained consistent with recommendations to subject embryo modeling using hPSC to a higher level of scrutiny than what might be required by national laws. The Guidelines extend a premise fundamental to European research governance, that research on entities with questionable moral status should proceed with caution. However, SCB-EM in Europe appear to be outside the purview of national systems of oversight designed to apply and enforce ethical norms that govern embryo research. It remains to be seen whether these changes will effectively impact European policy.
Governance & the role of criminal law
Governance mechanisms, such as those related to licensing and oversight, rest on legitimacy and accountability. Governance approaches should endow researchers to act responsibly through the research cycle while providing the necessary tools for all stakeholders to seriously commit to their moral responsibilities. In Europe, the publicly documented cases of violations of SC-based laws were mostly misconduct cases of fraud [Citation88,Citation89] and failure to obtain ethics approvals. As with the rest of the world, punitive sanctions for violating research integrity adopt different modalities depending on the policy’s binding nature and the stringency of governance mechanisms, amongst other factors. The trend to uphold criminal law in biomedical research, while exceptional to the regulation of science itself, has been widely adopted in European SC-related policy. Indeed, a favored approach is the imposition of fines and harsh prison terms for crossing statutory boundaries. Pecuniary administrative or civil sanctions (e.g., malpractice, liability) are seldom adopted ( Sanctions for embryo and SC research-related misconduct).
Pink: Unspecified penalty; Purple: Administrative penalty; Yellow: Fine, no prison; Green: Permitted following specified oversight; Blue: Maximum penalty = prison <= 3 years; Red: Maximum penalty = prison >= 3 years; Purple + yellow = Combination of fine + administrative penalty; Other combinations of colors: Different penalties for noncompliance with regulations for hESC research versus regulations governing embryo research.
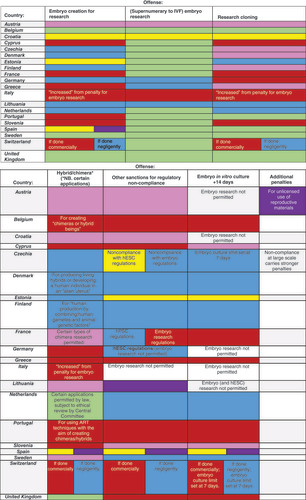
The widespread association of criminal sanctions with misconduct related to embryo and hESC research is quite notable across European policies. Criminal law constitutes an old and powerful tool which societies around the world use to send the strongest condemnatory message. At the same time, it achieves retribution, denunciation and/or deterrence. However, the use of criminal law in the biomedical research context should be used sparingly and limited to morally reprehensible behavior. Instead, other types of penalties, such as moral and professional sanctions embedded in soft law (e.g., codes of conduct, professional guidelines) could be equally powerful than criminal ones and should actively be pursued. Professional organizations have a central role in making effective the latter aided by governmental and societal support.
Legislative review in action
Overall, European countries have several embedded mechanisms to evaluate the strength of their policy frameworks and prompt change if required. While uptake of the Guidelines is voluntary, the ISSCR aims to provoke countries to evaluate whether the results of decades-old compromises, which project pluralistic beliefs into research governance, regulate emerging technologies as intended. Reflecting the importance of iterative, flexible regulation that keeps pace with “ill-defined and often moving targets” [Citation90], some European countries have adopted statutorily mandated periodic reviews to assess the implementation and effects of their laws (e.g., France [Citation91], Germany [Citation92] and The Netherlands [Citation93]). An illustrative example of this is found in France, where parallel to ISSCR’s policy revision process, the government conducted the mandated periodic review of its Bioethics Law [Citation94]. As with the Guidelines, this process also concluded with significant modifications. For instance, prior approval from the Biomedicine Agency is no longer required for hESC research. Additionally, research to form gametes or embryo models from hPSC, along with certain types of chimera research, can proceed as planned under a presumption of approval, unless the Biomedicine Agency opposes the proposed project within a set period. As outlined in an impact report that accompanied scheduled Parliamentary review [Citation95], the relaxation of requirements was due to the need to resolve regulation-induced delays which hamstrung French development in the field relative to countries like the UK, Spain, Belgium and the USA. The report shared consensus that hESCs were not “potential persons” and thus, hESC research was ethically distinct from research on the embryo. However, the National Consultative Ethics Committee remarked that both hESC and hiPSC can be used to produce “ethically sensitive cells” [Citation96]. Thus, the law was modified to avoid litigation that would inevitably ensue if decisions on the acceptability of contentious hPSC research were left entirely to the discretion of the Biomedicine Agency.
France’s legal foundations for the governance of biomedical research may facilitate adaptation as the bioethics law is entirely contained within the public health code, a feature which enable policy change when it can be justified as serving the broad and malleable goals of public health. But France pairs this feature with a tool available to legislators across EU: scheduled general parliamentary review of legislation every 5–7 years (the exact interval has varied over time) which must be preceded with public debate for “any reform project on ethical problems and social issues raised by advances in knowledge in the fields of biology, medicine and health” [Citation97]. Another example is found in The Netherlands, where the Embryo Law also incorporates scheduled review, ordered by the Ministry of Health, Welfare and Sport and conducted by academic researchers, every 5 years [Citation98]. In the most recent review, the Minister announced an intent to regulate the insertion of hiPSC into animal embryos under this law [Citation99]. In previous years, a moratorium on the creation of embryos for research purposes morphed into a general ban, subject to societal debate. The Dutch Minister’s review also stressed a need for “broad discussion” about the moral dilemmas arising from embryo-like structures cultured from iPSCs. French and Dutch experiences facilitating renewed public engagement with ethical dilemmas may prove instructive for other countries seeking to update legislation while balancing pluralistic moral concerns. Policy decisions informed by a broad range of stakeholders and enacted by officials who can be held democratically accountable may align scientific progress with diverse societal priorities.
While French and Dutch legislative review provisions seem to have catalyzed alignment with international norms, mechanisms for legislative evaluation do not guarantee change. However, they are still useful: for example, German legislative dormancy in the face of criticism may be interpreted as an affirmation of existing provisions. Review of German legislation by federally appointed experts has taken place continuously, and in recent years, consensus has built around a need for substantial revisions. The Stem Cell Act requires biannual evaluation by the Bundestag [Citation100], and an associated ordinance calls for an annual activity report from the Central Commission for Stem Cell Research, a federally appointed committee which issues a non-binding opinion on every German proposal for research involving hESCs [Citation101]. For years, the Commission’s reports have called for further relaxation of the statutory cut-off date for the derivation of hESC lines allowed to be imported for research uses [Citation102]. In addition, reports have called for other changes to keep with the spirit of the law. For instance, the Act requires that scientific research questions must have been clarified as far as possible with animal cells or experiments. However, as pointed out by the Commission, “in the future, cells derived from hESCs can contribute to significantly reducing the number of animals currently used for medical or pharmaceutical purposes”. Proposals with this aim in view would not be eligible for approval under the act in its current iteration. In its most recent report, the Bundestag also endorsed review of the ban on the use of hESC outside of a “research context” and stated that the “discourse about the ethical-legal classification” of “embryoids” should be “actively guided by science” [Citation103]. Finally, in 2014, the Conference of Health Ministers assigned the German Ethics Council, an independent council of experts [Citation104] with evaluating current developments in the field. A critical recommendation of the Council was to clarify and standardize the statutory definitions surrounding the human embryo across Germany’s Stem Cell Act and Embryo Protection Act [Citation105] to ensure entities like SCB-EM do not escape the reach of the law. To date, however, none of these concerns have been addressed in legislation.
The UK presents a different situation, where persistent court challenges did not impede the flexibilization of policy. For instance, in 2008, the Christian Legal Centre and Comment on Reproductive Ethics asked for a judicial review of the HFEA’s decision to approve research using human–animal cytoplasmic hybrid embryos, contesting the HFEA’s licensing powers and the rationality of their decisions, as they believed that such powers only covered fully human embryos, not human hybrid embryos [Citation106]. In contrast, the HFEA argued that the term ’human embryo’ was not defined by the Act and their interpretation relied on scientific expertise. The court ruled that the claimant’s case to be unarguable, that the HFEA pursued appropriate scientific guidance, and that the Act allowed for revision and reinterpretation following scientific advancements. Following this, revisions to the Act continued to follow a permissive path.
Conclusion
By eschewing bright-line prohibitions and eliminating ethical distinctions based on hPSCs provenance, the ISSCR Guidelines might have moved the forum for policy debates back to the halls of national Parliaments. They have further attempted to direct them to existing systems of ethical review in which research is “widely considered ethically acceptable” [Citation107]. The flexibility conferred by professional guidance (soft law) informed by discipline-specific expertise suggests this form of governance might be better suited to adapt to new ethical dilemmas posed by scientific developments. Yet, public deliberation is essential if a policy is to reflect societal values and priorities and thereby gain legitimacy.
Over the past decade, scientific organizations have repeatedly called for broad stakeholder engagement to reflect on and adapt governance strategies to address new ethical dilemmas that accompany scientific progress. True public deliberation on policy questions pertaining to stem cell research requires models for public engagement which have not yet materialized, perhaps due to lack of incentives [Citation108]. Legislative review processes such as those adopted in France, the UK and Denmark, adapted to solicit stakeholder perspectives on the governance of related emerging technologies, may provide useful models to make technocratic governance process more accessible and reflective of public interests. Because of the absence of true commitment to inclusiveness in policy debates, we do not anticipate a change in the status quo.
Future perspective
In Europe, existing pathways to assess the suitability of policy and governance frameworks have not been widely used. As stagnant norms continue to govern a dynamic field, it is difficult to assess whether these frameworks are still fulfilling their stewardship roles and how they might evolve in the next decades. Responsible innovation is predicated on the capacity to adapt in response to changing environments. As science and society co-evolve, are extant policy frameworks meeting stakeholders’ expectations as well as safeguarding important human interests? Promoting and delivering social value? Do they continue to satisfactorily reflect the socio-ethical principles that originally underpinned them? What is the weight of political, economic and other contextual factors in the adoption and implementation of policy? These are central questions to elucidate the social processes leading to, or required for, policy reform. The answers to these questions could also assist in evaluating whether governance structures are or will remain legitimate and sustainable in the future. These are all fundamental factors to support responsible research clinical translation, and we anticipate (or at least hope) that addressing these will occupy a central role in future policy and governance debates as well as their outcomes.
Introduction
In Europe (EU), the stem cell research-clinical translation continuum is characterized by a heterogeneity of governance and policy frameworks reflecting the continent’s diverse socio-cultural, economic and historical contexts.
Legislative building blocks past & present
Policy frameworks include a combination of national and international ‘hard’ (e.g., legislation, treaties) laws supplemented by ‘soft’ ones (e.g., professional guidelines, funding policies, codes of conduct) offering different tools for enforceability and governance.
Stem cell-based embryo modeling & beyond
Recently, the International Society for Stem Cell Research (ISSCR) updated its Guidelines, adopting strict recommendations for the regulation of clinical research and translation of stem cell (SC)-based interventions and substantially reconfiguring guidance for oversight of in vitro SC projects.
The possibility of yielding entities that might faithfully replicate embryonic developmental processes has prompted conceptual re-examinations, as stem cell embryo models do not fit neatly in pre-existing regulatory categories defining human embryos, gametes, or human research subjects. Because generally European national policies were initially created to govern Assisted Reproductive Technology (ART), they often contain statutory decades-old definitions of what constitutes a human embryo.
The ISSCR Guidelines diverge from common European policy by permitting the creation of embryos for research if projects stop at “well-defined timepoints” and undergo appropriate degrees of ethical review.
The Guidelines call for governments and oversight bodies to reappraise the ‘14-day rule’ – a gold standard preventing human embryos cultured in vitro from developing for longer than 14 days. In Europe, this rule continues to be ubiquitous.
The gradual liberalization of EU national policy
The Guidelines sit in stark contrast with European policy regulating research on human–animal chimeric embryos.
A discrete trend toward liberalization continues to slowly emerge in Europe.
Challenging the status quo: the role of regulation & oversight
European countries generally follow an ISSCR-style tiered approach to oversight, where SC research is subject to specialized or centralized ethics review depending on pre-determined criteria.
Governance and the role of criminal law: criminal law for misconduct related to embryo and hESC research is quite notable across European policies.
Legislative review in action
Some European countries have adopted statutorily mandated periodic reviews to assess the implementation and effects of their laws (e.g., France, Germany, The Netherlands), but they have not been widely used.
Future perspective
As old norms continue to govern the dynamic stem cell field, it is difficult but vital to assess whether governance mechanisms are still fulfilling their stewardship roles and how policy might evolve in the next decades. However, we do not expect a significant change in the status quo.
Financial & competing interests disclosure
This project has received funding from the European Union’s Horizon 2020 research and innovation program iPSpine under grant agreement No. 825925. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Additional information
Funding
References
- Landeweerd L , TownendD , MesmanJ , HoyweghenIV. Reflections on different governance styles in regulating science: a contribution to ‘Responsible Research and Innovation’. Life Sci. Soc. Policy11(8), (2015). https://lsspjournal.biomedcentral.com/articles/10.1186/s40504-015-0026-y
- International Society for Stem Cell Research (ISSCR) . Guidelines for Stem Cell Research and Clinical Translation (2021). www.isscr.org/docs/default-source/all-isscr-guidelines/2021-guidelines/isscr-guidelines-for-stem-cell-research-and-clinical-translation-2021.pdf?sfvrsn=979d58b1_4
- Baylis F . ISSCR guidelines fudge heritable human-genome editing. Nature594(7863), 333 (2021).
- Stein R . Controversial new guidelines would allow experiments on more mature human embryos. National Public Radio. (2021). www.npr.org/sections/health-shots/2021/05/26/1000126212/new-guidelines-would-allow-experiments-on-more-mature-human-embryos
- European Commission . “Horizon Europe: research and innovation funding programme until 2027. How to get funding, programme structure, missions, European partnerships, news and events”. https://ec.europa.eu/info/research-and-innovation/funding/funding-opportunities/funding-programmes-and-open-calls/horizon-europe_en (Accessed 13 Dec. 2021).
- Convention for the protection of human rights and dignity of the human being with regard to the application of biology and medicine: convention on human rights and biomedicine. Oviedo, 1997 Apr. 04. www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=164
- Estonia – artificial insemination and embryo protection act (1997). www.riigiteataja.ee/akt/1048155?leiaKehtiv
- Greece – law on application of medically assisted reproduction (2005). www.kodiko.gr/nomothesia/document/164338/nomos-3305-2005
- Additional protocol to the convention for the protection of human rights and dignity of the human being with regard to the application of biology and medicine, on the prohibition of cloning human beings (ETS No. 168). Paris, 1998 Dec. 1. https://rm.coe.int/168007f2ca
- Busardò FP , GulinoM , NapoletanoS , ZaamiS , FratiP. The evolution of legislation in the field of medically assisted reproduction and embryo stem cell research in European union members. Biomed. Res. Int.2014 (2014). www.hindawi.com/journals/bmri/2014/307160/
- Lithuania – Law on ethics of biomedical research (2000). https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/7aa28cc261bb11e5b316b7e07d98304b
- Netherlands – Embryo Law (2002). https://wetten.overheid.nl/BWBR0013797/2021-07-01
- Isasi RM , KnoppersBM. Beyond the permissibility of embryonic and stem cell research: substantive requirements and procedural safeguards. Hum. Reprod.21(10), 2474–2481 (2006).
- Isasi R , KnoppersBM. Toward commonality? Policy approaches to human embryonic stem cell research in Europe. In: Embryonic Stem Cell Patents: European Patent Law and Ethics.TorremansP, PlomerA ( Eds). Oxford University Press, Oxford, UK, 29–56 (2009).
- The European Group on Ethics in Science and New Technologies to the European Commission, ‘Recommendations on the Ethical Review of hESC FP7 Research Projects: Opinion No. 22’ (June 20, 2007).
- Additional Protocol to the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine, on the Prohibition of Cloning Human Beings (ETS No. 168). Paris, 1998 Dec. 1. https://rm.coe.int/168007f2ca
- Svoboda E . The next frontier for human embryo research. Nature597, S15–S17 (2021).
- Munsie M , HyunI , SugarmanJ. Ethical issues in human organoid and gastruloid research. Development144(6), 942–945 (2017).
- Tan T , WuJ , SiCet al. Chimeric contribution of human extended pluripotent stem cells to monkey embryos ex vivo. Cell184(8), 2020–2032.e14 (2021).
- Matthews KRW , IltisAS , MarquezNGet al. Rethinking human embryo research policies. Hastings Cent. Rep.51(1), 47–51 (2021).
- Shao Y , TaniguchiK , TownshendRF , MikiT , GumucioDL , FuJ. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat. Commun.8(1), (2017). www.nature.com/articles/s41467-017-00236-w
- Haremaki T , MetzgerJJ , RitoT , OzairMZ , EtocF , BrivanlouAH. Self-organizing neuruloids model developmental aspects of Huntington’s disease in the ectodermal compartment. Nat. Biotechnol.37(10), 1198–1208 (2019).
- van den Brink SC , van OudenaardenA. 3D gastruloids: a novel frontier in stem cell-based in vitro modeling of mammalian gastrulation. Trends Cell Biol.31(9), 747–759 (2021).
- Rossant J , TamPPL. Opportunities and challenges with stem cell-based embryo models. Stem Cell Reports16(5), 1031–1038 (2021).
- Bulgaria – Health law (2004). www.lex.bg/laws/ldoc/2135489147
- Iceland – Act on artificial insemination and the use of human gametes and embryos for stem cell research (1996). www.althingi.is/lagas/nuna/1996055.html
- Slovenia – Law on the treatment of infertility and biomedical assistance procedures (2000). http://pisrs.si/Pis.web/pregledPredpisa?id=ZAKO2518
- Spain – Law on biomedical research (2007). www.boe.es/buscar/doc.php?id=BOE-A-2007-12945#:~:text=Esta%20Ley%20tiene%20por%20objeto,humana%20que%20impliquen%20procedimientos%20invasivos
- Belgium – Law relating to research on embryos in vitro (2003). www.ejustice.just.fgov.be/eli/loi/2003/05/11/2003022592/justel
- Netherlands – Embryo law (2002). https://wetten.overheid.nl/BWBR0013797/2021-07-01
- Malta – Embryo protection act (2007). https://legislation.mt/eli/cap/524/20181001/eng
- United Kingdom – Human Fertilisation and Embryology Act 2008 explanatory notes (2008). www.legislation.gov.uk/ukpga/2008/22/notes
- United Kingdom – Human Fertilisation and Embryology Act (1990). www.legislation.gov.uk/ukpga/1990/37
- United Kingdom – Human Fertilisation and Embryology Act (2008). www.legislation.gov.uk/ukpga/2008/22
- McCracken J . Review of the human fertilization & embryology authority and the human tissue authority. 2013 Apr. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/216947/Justin_McCracken_report_of_review_of_HFEA_and_HTA.pdf (Accessed 2021Dec. 14).
- Additional protocol to the convention for the protection of human rights and dignity of the human being with regard to the application of biology and medicine, on the prohibition of cloning human beings (ETS No. 168). Paris, 1998 Dec. 1. https://rm.coe.int/168007f2ca
- United Kingdom – Human Fertilization and Embryology Act (1990). www.legislation.gov.uk/ukpga/1990/37
- Sweden – Genetic Integrity Act (2006). www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/lag-2006351-om-genetisk-integritet-mm_sfs-2006-351
- Belgium – Law relating to research on embryos in vitro (2003). www.ejustice.just.fgov.be/eli/loi/2003/05/11/2003022592/justel
- Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine. Oviedo, 1997 Apr. 04. www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=164
- Blackshaw BP , RodgerD. Why we should not extend the 14-day rule. Journal of Medical Ethics47, 712–714 (2021).
- Hyun I , WilkersonA , JohnstonJ. Embryology policy: Revisit the 14-day rule. Nature533, 169–171 (2016).
- Cyprus – Law on the Implementation of Medically Assisted Reproduction (2015). www.cylaw.org/nomoi/enop/non-ind/2015_1_69/full.html
- Portugal – Law on Medically Assisted Procreation (2006). https://dre.pt/dre/legislacao-consolidada/lei/2006-34529775
- Switzerland – Federal Act on Research on Embryonic Stem Cells (2011). www.fedlex.admin.ch/eli/cc/2005/104/de
- CHIMBRIDS – Chimeras and Hybrids in Comparative European and International Research – Scientific, Ethical, Philosophical and Legal Aspects. Taupitz J , WeschkaM. Springer-Verlag Berlin Heidelberg (2009).
- France – Law relating to the donation and use of elements and products of the human body, medically assisted procreation and prenatal diagnosis (1994). www.legifrance.gouv.fr/jorf/id/JORFTEXT000000549618
- France – Law relating to bioethics (2004). www.legifrance.gouv.fr/loda/id/JORFTEXT000000441469/
- France – Law on bioethics (2011). www.legifrance.gouv.fr/loda/id/JORFTEXT000024323102/
- French National Consultative Ethics Committee for Life and Health Sciences . An ethical reflection on research on cells of human embryonic origin, and research on the human embryo in vitro, Paris, 2010 Oct. www.cfef.org/archives/bricabrac/avis112.pdf
- France – Law n 2021-1017 of August 2, 2021 relating to bioethics (2021). www.legifrance.gouv.fr/jorf/id/JORFTEXT000043884384
- Denmark- Act amending the Act on artificial insemination in connection with medical treatment, diagnostics and research, etc. (Research on embryonic stem cells) (2003). www.retsinformation.dk/eli/lta/2003/427
- Norway – Act amending the Biotechnology Act (pre-implantation diagnostics and research on redundant fertilized eggs) (2007). https://lovdata.no/dokument/LTI/lov/2007-06-15-31
- Iceland – Act amending Act no. 55/1996, on artificial insemination, with subsequent amendments (2008). www.althingi.is/altext/stjt/2008.054.html
- Spain – Law on Biomedical Research (2007). www.boe.es/buscar/doc.php?id=BOE-A-2007-12945#:~:text=Esta%20Ley%20tiene%20por%20objeto,humana%20que%20impliquen%20procedimientos%20invasivos
- Iceland – Act amending Act no. 55/1996, on artificial insemination, with subsequent amendments (2008). www.althingi.is/altext/stjt/2008.054.html
- United Kingdom – Human Fertilisation and Embryology Act (2008). www.legislation.gov.uk/ukpga/2008/22
- German Reference Center for Ethics in the Biosciences. II. Selected national and international laws and regulations. www.drze.de/im-blickpunkt/stammzellen/gesetze-und-regelungen (Accessed 2021).
- Nations under God: How Churches Use Moral Authority to Influence Policy A1 – Grzymala-Busse, Anna M. PY – 2015 PB – Princeton University Press CY – Princeton SN – 9781400866458 UR – https://muse.jhu.edu/book/62410ER
- Lyons B . The Irish Council for Bioethics: An Unaffordable Luxury?Camb. Q Healthc Ethics21(3), 375–383 (2012).
- Gough F . Deliberating or dithering? Ireland and human embryonic stem cell research. Eur. J. Health Law20(2), 145–165 (2013).
- M.R. v T.R. [2009] IESC 82 www.courts.ie/Judgments.nsf/597645521f07ac9a80256ef30048ca52/0973cbd1fd5204028025768d003d60f7?
- Sivaraman MAF . Using Surplus Embryos and Research Embryos in Stem Cell Research: Ethical Viewpoints of Buddhist, Hindu and Catholic Leaders in Malaysia on the Permissibility of Research. Sci. Eng. Ethics24, 129–149 (2018).
- Beltrame L . The Italian Way to Stem Cell Research: Rethinking the Role of Catholic Religion in Shaping Italian Stem Cell Research Regulations. Dev. World Bioeth.17(3), 157–166 (2017).
- Pasotti J , StaffordN. It’s legal: Italian researchers defend their work with embryonic stem cells. Nature442, 229 (2006).
- Butler D . Vatican toughens stance on embryo research. Nature (2008). www.nature.com/articles/news.2008.1306
- https://timesofmalta.com/articles/view/campaign-against-stem-cell-research-in-spain.94784
- International Society for Stem Cell Research (ISSCR) . Guidelines for Stem Cell Research and Clinical Translation (2021). www.isscr.org/docs/default-source/all-isscr-guidelines/2021-guidelines/isscr-guidelines-for-stem-cell-research-and-clinical-translation-2021.pdf?sfvrsn=979d58b1_4
- Kimmelman J , HyunI , BenvenistyNet al. Policy: Global standards for stem-cell research. Nature533(7603), 311–313 (2016).
- Lomax GP , HullSC , IsasiR. The DISCUSS Project: Revised Points to Consider for the Derivation of Induced Pluripotent Stem Cell Lines From Previously Collected Research Specimens. Stem. Cells Transl Med.4(2), 123–129 (2015).
- Isasi R , KnoppersBM. Mind the gap: policy approaches to embryonic stem cell and cloning research in 50 countries. Eur. J. Health Law13(1), 9–25 (2006).
- Isasi R , NamoradoJ , MahN , BultjerN , KurtzA. A pathway for attesting ethical provenance of cell lines: Lessons from the European human pluripotent stem cell registry (hPSCreg). Stem. Cell Res.40 (2019). www.sciencedirect.com/science/article/pii/S1873506119301692?via=ihub
- Belgium – In Vitro Embryo Research Act (2003). www.ejustice.just.fgov.be/eli/loi/2003/05/11/2003022592/justel
- Greece – Law on Application of Medically Assisted Reproduction (2005). www.kodiko.gr/nomothesia/document/164338/nomos-3305-2005
- Hungary – Law on Health (1997). https://net.jogtar.hu/jogszabaly?docid=99700154.tv
- Montenegro – Law on Treatment of Infertility with Assisted Reproductive Technologies (2009). www.gov.me/dokumenta/621c6f39-0ae4-4656-9406-657c6d2ded31
- North Macedonia – Law on Biomedical Assistance in Fertilization (2008). http://zdravstvo.gov.mk/wp-content/uploads/2015/10/0-ZAKON-ZA-BIOMEDITSINSKO-POTPOMOGNATO-OPLODUVAN-E.pdf
- Portugal – Law on Medically Assisted Procreation (2006). https://dre.pt/dre/legislacao-consolidada/lei/2006-34529775
- Slovenia – Law on the Treatment of Infertility and Biomedical Assistance Procedures (2000). http://pisrs.si/Pis.web/pregledPredpisa?id=ZAKO2518
- United Kingdom – Human Fertilisation and Embryology Act (1990). www.legislation.gov.uk/ukpga/1990/37
- Cyprus – Law on the Implementation of Medically Assisted Reproduction (2015). www.cylaw.org/nomoi/enop/non-ind/2015_1_69/full.html
- Czechia – Act on Research on Human Embryonic Stem Cells and Related Activities (2006). www.zakonyprolidi.cz/cs/2006-227
- France – Public Health Code (2021). www.legifrance.gouv.fr/codes/id/LEGITEXT000006072665/
- Spain – Law on Biomedical Research (2007). www.boe.es/buscar/doc.php?id=BOE-A-2007-12945#:~:text=Esta%20Ley%20tiene%20por%20objeto,humana%20que%20impliquen%20procedimientos%20invasivos
- Switzerland – Stem Cell Research Act (2003). www.fedlex.admin.ch/eli/cc/2005/104/de
- International Society for Stem Cell Research (ISSCR) . Guidelines for Stem Cell Research and Clinical Translation (2016). www.isscr.org/docs/default-source/all-isscr-guidelines/guidelines-2016/isscr-guidelines-for-stem-cell-research-and-clinical-translationd67119731dff6ddbb37cff0000940c19.pdf
- International Society for Stem Cell Research (ISSCR) . Guidelines for Stem Cell Research and Clinical Translation (2021). www.isscr.org/docs/default-source/all-isscr-guidelines/2021-guidelines/isscr-guidelines-for-stem-cell-research-and-clinical-translation-2021.pdf?sfvrsn=979d58b1_4
- Dyer O . Swedish stem cell “pioneers” are found guilty of research misconduct. BMJ357, j1808 (2017).
- Editorial retraction [retraction of: Cell Cycle. 2012 Jan 1;11(1):65–78]. Cell Cycle16(3), 296 (2017).
- Kuhlmann S , StegmaierP , KonradK. The tentative governance of emerging science and technology – a conceptual introduction. Research Policy48(5), 1091–1097 (2019).
- France – Law n 2021–1017 of August 2, 2021 relating to bioethics (2021). www.legifrance.gouv.fr/jorf/id/JORFTEXT000043884384
- Germany – Stem Cell Act (2002). www.gesetze-im-internet.de/stzg/BJNR227700002.html
- Netherlands – Embryo Law (2002). https://wetten.overheid.nl/BWBR0013797/2021-07-01
- French Parliamentary Office for the Evaluation of Scientific and Technological Choices . Impact Study: Project of Law Relating to Bioethics. 2019 Jul. 23. https://www.assemblee-nationale.fr/dyn/15/textes/l15b2187_etude-impact#_Toc14771040
- French Parliamentary Office for the Evaluation of Scientific and Technological Choices . Impact Study: Project of Law Relating to Bioethics. 2019 Jul. 23. https://www.assemblee-nationale.fr/dyn/15/textes/l15b2187_etude-impact#_Toc14771040
- French National Consultative Ethics Committee (CCNE) . Opinion no. 129: “Contribution of the National Consultative Committee of ethics to the revision of the law of bioethics”, p. 52. https://www.ccne-ethique.fr/sites/default/files/2021-02/avis_129_vf.pdf
- France – Law on Bioethics (2011). www.legifrance.gouv.fr/loda/id/JORFTEXT000024323102/
- Netherlands – Embryo Law (2002). https://wetten.overheid.nl/BWBR0013797/2021-07-01
- Netherlands – Medical Ethics Note, no. 34990. 2018. Parliamentary paper, House of Representatives of the States General (2018). https://zoek.officielebekendmakingen.nl/kst-34990-1
- Germany – Stem Cell Act (2002). www.gesetze-im-internet.de/stzg/BJNR227700002.html
- Germany – Ordinance on the Central Ethics Commission for Stem Cell Research and on the competent authority under the Stem Cell Act (2002). www.gesetze-im-internet.de/zesv/BJNR266300002.html
- Central Ethics Committee for Stem Cell Research. Report of the Central Ethics Committee for Stem Cell Research (ZES): 16th Report after the Enactment of the Stem Cell Act (StZG) for the Reporting Period 1 January to 31 December 2018 (2019). www.rki.de/EN/Content/Institute/Committees/StemCell/16th-ReportZES.pdf?__blob=publicationFile
- Germany – Ninth report by the federal government on the implementation of the Stem Cell Act (2021). https://dserver.bundestag.de/btd/19/325/1932595.pdf
- Germany – Ethics Council Act (2007). www.gesetze-im-internet.de/ethrg/BJNR138500007.html
- German Ethics Council. Stem cell research – new challenges for the ban on cloning and treatment of artificially created germ cells? 2014 Sep. 15. www.domradio.de/sites/default/files/pdf/empfehlung-stammzellforschung.pdf
- The Queen on the application of (1) Quintavalle (2) CLC Claimant v Human fertilization and Embryology Authority (HFEA). England and Wales High Court. [2008] EWHC 3395 (Admin). www.bailii.org/ew/cases/EWHC/Admin/2008/3395.html
- Mummery C , AnthonyE. New guidelines for embryo and stem cell research. Nat. Rev. Mol. Cell Biol.22(12), 773–774 (2021).
- Adashi EY , CohenIG. Who will oversee the ethical limits of embryo research?Nat. Biotechnol.40, 463–464 (2022).