Abstract
Background. Various substances including uric acid, organic acids and drugs are transported by organic anion transporters (OATs) in the kidney. In addition, a member of the OAT family, urate transporter 1 (URAT1), is involved in the reabsorption of uric acid from the renal tubule. Benzbromarone inhibits URAT1 to block uric acid reabsorption. Methods. Our group previously observed higher salivary uric acid levels than serum levels in patients taking benzbromarone, and reported the possible existence of URAT1-like uric acid excretion mechanism in the salivary gland. The purpose of this study was to elucidate the uric acid excretion mechanism in salivary gland tissues using rabbit anti-human OAT1-4 and URAT1 polyclonal antibodies with EnVision™ system. Results. In the salivary gland, OAT1 was expressed in ductal cells. OAT2 was found in both ductal cells and serous acinar cells and weak expression was also observed in several nuclei. OAT3 expression was observed in serous acinar cells and nuclei and OAT4 was expressed only in ductal cells. URAT1 expression was observed in the cytoplasm of ductal cells and strong punctuate staining was seen in part of the supra-nuclear cytoplasm. The number of cells expressing URAT1 was smaller compared with OATs. In the kidney, however, OAT1-4 and URAT1 were strongly expressed on proximal renal tubules. Conclusions. The present study confirmed the existence of OAT1-4 and URAT1 in the salivary gland. These results may support the previous speculation that benzbromarone inhibits URAT1 to block uric acid reabsorption in the salivary gland, resulting in higher salivary uric acid levels than serum levels.
Introduction
Organic anions (organic acids) and organic cations (organic bases) are collective terms for substances that possess a carbon backbone and a portion with net negative or positive charge in their basic structure. Many of the compounds that are harmful to or unwanted by the body (such as metabolites, drugs and toxins) are included in this group of organic substances. Uric acid, which is the final product of purine derivatives, is one of the organic anions and exists in the ionized form as urate. Urate produced in the liver and gastrointestinal tract is dissolved in blood as sodium urate and deposits as urate crystals when reaching a supersaturation state. These deposits form gouty tophus or calculus in soft tissues, joint cavities and renal tubules, causing gouty arthritis and impairing renal function. Furthermore, hyperuricemia is a promoting arteriosclerosis factor and often co-exists with hypertension, hyperlipidemia and diabetes. In recent years, hyperuricemia has been regarded as a risk factor of ischemic heart diseases. On the other hand, since uric acid possesses an antioxidant effect, this compound plays a protective role against oxidative stress (radical scavenger) and is associated with homeostasis of the body [Citation1–5].
Based on the differences in substrate selectivity, organic ion transporters can be broadly divided into organic anion, organic cation and amphoteric ion transporters. Organic ions metabolized in the liver are excreted from the kidney. Since many of the drug metabolites become organic anions, organic anion transporters (OATs) are indispensable for drug disposal. OATs are expressed in renal tubule, liver and various types of endothelial cells and OAT1-7 as well as OAT10 have been cloned [Citation6–8].
One member of the OAT family, urate transporter 1 (URAT1), is expressed in the brush border of the proximal renal tubule (brush border membrane) and mediates reabsorption of uric acid to regulate blood uric acid concentration. Benzbromarone, which is a drug widely used for the treatment of hyperuricemia, inhibits URAT1-mediated uric acid reabsorption to enhance urinary excretion and lower blood uric acid level [Citation9–11]. Moreover, salicylate and benzyl penicillin have been reported to interact with URAT1 and exert an effect on urinary uric acid excretion [Citation12–14]. Various studies have demonstrated the expression of OAT1-3 in the basement membrane of proximal renal tubules (basolateral membrane) and OAT4 and URAT1 in the luminal brush border of proximal renal tubules [Citation4,10,15].
Shibasaki et al. [Citation16] reported that salivary uric acid levels were higher than blood levels in patients on benzbromarone and proposed the possible existence of URAT1, OATs or other mechanisms related to uric acid excretion and absorption in salivary gland tissues. However, despite a large number of reports on OATs and URAT1, an extensive search of literature identified no report on the expression of these transporters in the human salivary gland.
In the present study, we performed an immunohistochemical investigation on the expression of OAT1-4 and URAT1 in salivary gland tissues including the submandibular gland and parotid gland. Kidney tissue was used as a positive control of OATs and URAT1 expression.
Materials and methods
Tissue samples
The tissue samples used in this study were paraffin-embedded blocks of major salivary gland and kidney tissues archived at the Department of Pathology, The Nippon Dental University, School of Life Dentistry at Niigata. The salivary gland tissue specimens were histopathologically normal in general without inflammation or tumor. Tissues from patients who had undergone radiotherapy and tissues containing calcified substances such as salivary calculus were excluded, because of the possibility of strong degeneration. For kidney samples also, generally normal tissues were used. Sixteen salivary gland samples (15 submandibular glands and one parotid gland) were studied. This study was approved by the Ethics Committee of The Nippon Dental University, Life Dentistry at Niigata (NDU: 154) and the tissues were used only for the investigations described below.
Immunohistochemical staining
From the paraffin embedded tissues, serial sections of 3 μm thickness were prepared. After deparaffinization, the sections were incubated with Immunosaver (333; Nissin, Tokyo, Japan) at 98°C for 45 min to retrieve antigen and then treated with 0.3% hydrogen peroxide in methanol to block endogenous peroxidase. The samples were incubated with 5% normal goat serum (X0907; Dako, Glostrup, Denmark) for 10 min at room temperature and were incubated with the diluted primary antibodies overnight at 4°C. Among the primary antibodies used, OAT1 (KE038; Trans Genic Inc., Kumamoto, Japan), OAT2 (KE031; Trans Genic Inc.), OAT3 (KE032; Trans Genic Inc.) and OAT4 (KE033; Trans Genic Inc.) were diluted 1/1000 and URAT1 (KE094; Trans Genic Inc.) was diluted 1/400. Then the samples were treated with the EnVision™ System (K5027; Dako). DAB+(3-3'-Diaminobenzidine Tetrahydrochloride) Liquid (K3468; Dako) was used for color development. After nucleus staining with hematoxylin, the sections were examined under a light microscope. Positive or negative expression of each transporter, the site of expression and immunostaining property were recorded. For negative control, the primary antibody was replaced with normal rabbit serum (X0902; Dako).
A positive reaction was recorded only when the assessments of the investigators were positive.
Results
Localization of OAT1-4 expression in salivary gland
OAT1 expression was observed in ductal cells and vascular endothelial cells. In the duct cells, uneven immunostaining in the cytoplasm, with some areas showing strong staining, was observed (). In the vascular endothelial cells, OAT1 was expressed along the duct lumen side (data not shown).
Figure 1. Histopathological and immunohistochemical findings of OAT1-4 in human submandibular gland. (A, C, E and G) Hematoxylin-eosin staining of serial sections for the corresponding OAT1-4 immunostained sections (B, D, F and H). (B) OAT1 is strongly expressed in the cytoplasm of striated ductal cells. (D) OAT2 is expressed in the cytoplasm of striated ductal cells and serous acinar cells, as well as in several nuclei. (F) OAT3 is strongly expressed in the whole cytoplasm and several nuclei of serous acinar cells. (H) OAT4 is expressed in the cytoplasm of striated ductal cells.
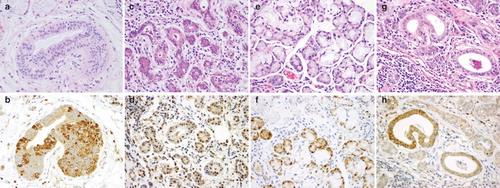
Diffuse cytoplasmic expression of OAT2 was found both in both ductal cells and serous acinar cells and weak expression was also observed in several nuclei ().
OAT3 expression was observed in serous acinar cells and nuclei, but definitive expression was not observed in the ducts or other tissues. In serous acinar cells, OAT3 was strongly expressed in the whole cytoplasm and several nuclei ().
OAT4 expression was observed only in ducts. Both diffuse strong expression in the whole cytoplasm and uneven expression (as observed for OAT1) in a number of cells in the duct were observed ().
Localization of URAT1 expression in salivary gland
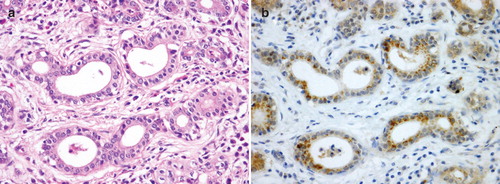
URAT1 expression was observed in the cytoplasm of duct cells and strong punctuate staining was seen in part of the supra-nuclear cytoplasm. The number of cells expressing URAT1 was fewer and the staining intensity was weaker compared with other OATs and some salivary glands were assessed as negative. Expression was not clearly found in acinar cells or other tissues ().
Figure 2. Histopathological and immunohistochemical findings of URAT1 in the human submandibular gland. (A) Hematoxylin-eosin staining of a serial section. (B) Immunohistochemical findings of URAT1 shows expression in the cytoplasm of striated ductal cells, with strong punctuate staining in part of the supra-nuclear cytoplasm. Compared with the staining results of OAT1-4, fewer cells are positive and staining intensity is weak.
Localization of OAT1-4 and URAT1 in the kidney
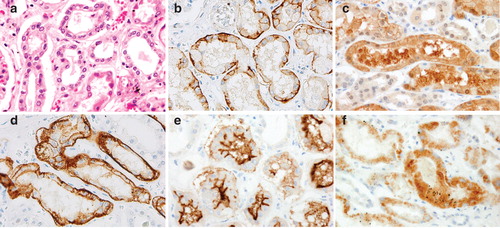
The expressions of OAT1-4 and URAT1 in the kidney are as follows. For all transporters, definitive expression was observed mainly in the proximal renal tubule of the renal cortex. OAT1 and OAT3 showed similar expression patterns: both were expressed strongly along the basement membrane (basolateral membrane) of proximal renal tubules (). The expression of OAT2 was weaker compared with OAT1 and OAT3 and diffuse cytoplasmic expression was observed in proximal renal tubules (). OAT4 and URAT1 were expressed along the luminal brush border (brush border membrane) of proximal renal tubules and the expression patterns were different from those of OAT1-3. OAT4 was strongly expressed along the cell membrane of the luminal brush border (). However, URAT1 expression was observed in the cytoplasm on the duct lumen side and also in luminal brush border, showing slight difference from the expression pattern of OAT4 (). OAT1-4 were distributed uniformly in the renal cortex, while URAT1 expression tended to be less compared with OAT1-4.
Figure 3. Histopathological and immunohistochemical findings of OAT1-4 and URAT1 in the human kidney. (A) Hematoxylin-eosin staining of a serial section. (B–F) Immunohistochemical findings of OAT1-4 and URAT1. Definitive expressions of all the transporters are observed mainly in proximal renal tubules of the renal cortex. (B and D) The expression patterns of OAT1 and OAT3 are similar, with strong expression along the basement membrane (basolateral membrane) of renal tubules. (C) Diffuse OAT2 expression is observed in the cytoplasm of proximal renal tubular cells. (E) OAT4 is expressed strongly along the luminal brush border (brush border membrane) of proximal renal tubules. (F) URAT1 expression is found in the cytoplasm on the lumen side and in luminal brush border, but the number of positive cells tends to be fewer compared to OAT1–4.
Discussion
OATs are expressed in the epithelium of various organs and mediate the absorption or excretion of endogenous and exogenous substances such as uric acid, organic acids, drugs and their metabolites. All the OATs are expressed in the kidney as well as in other organs and tissues such as the liver, brain, placenta and muscle cells [Citation10,17–19]. URAT1 was identified by Enomoto et al. [Citation10] in 2002 as a new urate transporter belonging to the OAT family. This transporter mediates reabsorption of uric acid in proximal renal tubules to regulate blood uric acid level. URAT1 is considered to be expressed specifically in the brush border of proximal renal tubules. However, because expression in vascular smooth muscle cells has also been reported [Citation20], the existence of URAT1 in organs other than the kidney has been suggested.
In the present study, the expressions of OAT1-4 and URAT1 in the salivary gland and kidney were investigated using an immunohistochemical method. While OAT1-4 and URAT1 were all positive in salivary gland and kidney tissues, their localizations were considerably different. OAT1-3 were expressed in the cell membrane and cytoplasm on the basement membrane side of renal tubules, whereas they were expressed in multiple sites including the cytoplasm and some nuclei of striated ductal cells and acinar cells in the salivary gland. On the other hand, OAT4 and URAT1 expressions were observed in the cytoplasm and cell membrane on the luminal side of renal tubules, while they were found mainly in the cytoplasm of striated ductal cells in the salivary gland. The finding that transporters are expressed in striated ductal cells as in renal tubule cells suggests that the ductal cells are involved in the transport of organic acids and drug metabolites. Especially, the observation of URAT1 expression in the salivary gland indicates the possible existence of a uric acid reabsorption mechanism also in these glands and supports the report of Shibasaki et al. [Citation16] that benzbromarone may act on URAT1 in the salivary gland to inhibit uric acid reabsorption, resulting in higher uric acid levels in saliva than in blood.
According to previous reports, the mechanisms of action of OAT1-4 and URAT1 on uric acid metabolism in renal tubules are proposed as follows. Uric acid reabsorbed in renal tubular cells via URAT1 is transported by exchange with dicarboxylic acid mediated by OAT1 and OAT3 and excreted to the blood vessel side or taken up into renal tubule cells from the blood vessel side [Citation21–24]. Compared with OAT1 and OAT3, OAT2 has a different mechanism of action: an H + and/or Cl− concentration gradient is involved in the uptake of uric acid from the blood vessel side into renal tubular cells [Citation25]. OAT4 and URAT1 are considered to mediate uptake of uric acid from the renal tubule into renal tubular cells by exchange with organic anions such as lactic acid and nicotinic acid [Citation21–24].
In the kidney, OAT1-4 and URAT1 are distributed along the renal tubular cell membrane and are involved in reabsorption and excretion of uric acid, organic acids, drugs and their metabolites. In the salivary gland, on the other hand, OAT1-4 and URAT1 are distributed in the whole tissues including ductal cells and acinar cells, suggesting that these transporters are associated with the transport of organic acids and uric acid in the whole salivary gland. However, unlike in the kidney where the transporters are expressed mainly along the cell membrane, whether the transporters on the luminal side or basement membrane side of the salivary gland carry out the transport function could not be clarified from the present study.
In the present study, cells expressing URAT1 in the salivary gland were fewer than those expressing OAT1-4 in the salivary gland and URAT1 in the kidney. This finding probably reflects less URAT1 protein present in the salivary gland ductal cells and weaker reaction with the secondary antibody for color development. In the kidney also, URAT1 expressing cells were fewer than OAT1-4 expressing cells. A possible explanation is that on the luminal side of renal tubules, URAT1 is involved specifically in uric acid reabsorption and consequently the amount of transporter protein is less than OAT1-4 that participates in the metabolites of diverse compounds.
While we found no clear correlation of salivary gland URAT1 and OAT1-4 expression levels with blood uric acid levels or gender of individual patients (data not shown), the factors associated with the quantity and localization of transporter expression should be examined in further studies.
In recent years, new uric acid excretion transporters such as glucose transporter 9 (GLUT9) [Citation26,27] and ATP-binding cassette sub-family member 2 (ABCG2) [Citation28] have been identified and their pathological association with hyperuricemia and gout is being investigated. Further studies are required to study the expression of these transporters in the salivary gland and the relationship with OATs and URAT1.
Acknowledgment
We are grateful to Mrs Michiko Moride and all members of the Department of Pathology, School of Life Dentistry at Niigata, The Nippon Dental University for advice and technical guidance. Transporter expression of all samples was evaluated by two investigators (R.I and K.S).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Richette P, Bardin T. Gout. Lancet 2010;375:318–28.
- So A. Developments in the scientific and clinical understanding of gout. Arthritis Res Ther 2008;10:221–6.
- Mount DB. Molecular physiology and the four-component model of renal urate transport. Curr Opin Nephrol Hypertens 2005;14:460–3.
- Nigam SK, Bush KT, Bhatnagar V. Drug and toxicant handling by the OAT organic anion transporters in the kidney and other tissues. Nat Clin Pract Nephrol 2007;3:443–8.
- Enomoto A, Endou H. Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clin Exp Nephrol 2005;9:195–205.
- Ahn SY, Nigam SK. Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol 2009;76:481–90.
- Bahn A, Hagous Y, Reuter S, Balen D, Brzica H, Kric W, Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem 2008;283:16332–41.
- Sekine T, Cha SH, Endou H. The multispecific organic anion transporter (OAT) family. Pfugers Arch 2000;440:337–50.
- Kunishima C, Inoue I, Oikawa T, Nakajima H, Komada T, Katayama S. Activating effect of Benzbromarone, a uricosuric drug, on peroxisome proliferators-activated resceptors. PPAR Res 2007:2007;1–5.
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Ho Cha S, Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002;17:447–52.
- Iwanaga T, Kobayashi D, Hirayama M, Tamai I. Involvement of uric acid transporter in increased renal clearance of the xanthine oxidase inhibitor oxypurinol induced by a uricosuric agent, benzbromarone. Drug Metab Dispos 2005;33:1791–5.
- El-Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. Effect of hypouricemic and hyperuricemic drugs on the renal urate efflux transporter, multidrug resistance protein 4. Br J Pharmacol 2008;155:1066–75.
- Imaoka T, Kusuhara H, Adachi-Akahane S, Hasegawa M, Morita N, Endou H, The renal-specific transporter mediates facilitative transport of organic anions at the brush border membrane of mouse renal tubules. J Am Soc Nephrol 2004;15:2012–22.
- Shin HJ, Takeda M, Enomoto A, Fujimura M, Miyazaki H, Anzai N, Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrology 2011;16:156–62.
- Hagos Y. Human renal organic anion transporters 4 operates as an asymmetric urate transporter. J Am Soc Nephrol 2007;18:430–9.
- Shibasaki K, Kimura M, Ikarashi R, Yamaguchi A, Watanabe T. Uric acid concentration in saliva and its changes with the patients receiving treatment for hyperuricemia. Metabolomics 2011; doi:10.1007/s11306-011-0334-z; online published.
- Enomoto A, Niwa T. Roles of organic anion transporters in the progression of chronic renal failure. Ther Apher Dial 2007;11:27–31.
- Saito H. Pathophysiological regulation of renal SLC22A organic ion transporters in acute kidney injury: pharmacological and toxicological implications. Pharmacol Ther 2010;125:79–91.
- Hwang JS, Park EY, Kim WY, Yang CW, Kim J. Expression of OAT1 and OAT3 in differentiating proximal tubules of the mouse kidney. Histol Histopathol 2010;25:33–44.
- Price KL, Sautin YY, Long DA, Zhang L, Miyazaki H, Mu W, human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol 2006;17:1791–5.
- Anzai N, Miyazaki H, Noshiro R, Khamdang S, Chairoungdua A, Shin HJ, The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J Biol Chem 2004;279:45942–50.
- Hagous Y, Bahn A, Vormefelde SV, Brockmöller J, Burckhardt G. Torasemide transport by organic anion transporters contributes to hyperuricemia. J Am Soc Nephrol 2007;18:3101–9.
- Hediger MA, Johnson RJ, Miyazaki H, Endou H. Molecular physiology of urate transport. Physiology (Bethesda) 2005;20:125–33.
- Zhang R, Yang X, Li J, Wu J, Peng WX, Dong XQ, Upregulation of rat renal cortical organic anion transporter (OAT1 and OAT3) expression in response to ischemia/reperfusion injury. Am J Nephrol 2008;8:772–83.
- Saito M, Mamada H, Anzai N, Shirasaka Y, Nakanishi T, Tamai I. Renal secretion of uric acid by Organic Anion Transporter 2. Biol Pharm Bull 2010;33:498–503.
- Matsuo H, Chiba T, Nagamori A, Domoto H, Phetdee K, Wiriyasermkul P, Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet 2008;83:744–51.
- Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in human. J Biol Chem 2008;238:26834–8.
- Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Ikebuchi Y, Common defects of ABCG2, a high-capacity urate exporter, cause gout. Sci Transl Med 2009;1:5ra11.