Abstract
Conclusion: As the periods of intratympanic injection of ovalbumin (OVA) to the middle ear became longer, marked eosinophil infiltration in the perilymphatic space was observed. Moreover severe morphological damage of the organ of Corti was observed in the 28-day antigen-stimulation side. These results indicate that eosinophilic inflammation occurred in the inner ear and caused profound hearing loss. Objective: The purpose of the present study was to elucidate the inner ear damage in a new animal model of eosinophilic otitis media (EOM) which we recently constructed. Methods: We constructed the animal model of EOM by intraperitoneal and intratympanic injection of OVA. Infiltrating cells and the inner ear damage were examined by histological study. Results: In the inner ear, a few eosinophils were seen in the scala tympani of the organ of Corti and the dilation of capillaries of the stria vascularis was observed in the 7-day stimulation side. In the 14-day antigen stimulation side, some eosinophils and macrophages were seen in not only the scala tympani but also the scala vestibule. In the 28-day antigen-stimulation side, severe morphological damage of the organ of Corti and many eosinophils, red blood cells, and plasma cells infiltrating the perilymph were observed.
Introduction
Eosinophils play an important role as effecter cells in the pathogenesis of various allergic diseases; therefore they are also used as diagnostic markers in bronchial asthma, allergic rhinitis, and so on. Intractable otitis media with eosinophil-enriched middle ear effusion was named as eosinophilic otitis media (EOM) by Tomioka et al. [Citation1]. It has been gradually recognized that EOM, previously called allergic otitis media [Citation2], is not necessarily a rare disease. The most common characteristic of EOM is the presence of highly viscous middle ear effusion enriched with eosinophils. ‘Eosinophil dominant middle ear effusion’ and ‘highly viscous middle ear effusion’ were included in the diagnostic criteria of EOM [Citation3], and other criteria were ‘resistance to conventional treatment,’ ‘association with bronchial asthma,’ and ‘association with nasal polyposis.’
EOM is not a fatal disease, but carries a high risk of hearing loss. In the first report of EOM, Tomioka et al. revealed that one of three cases of EOM resulted in deafness [Citation4]. Later, Iino et al. reported that deterioration of bone conductive hearing loss (BCHL) was found in 81 of 138 patients with EOM, and 8 cases had complete hearing loss in 1 or both ears [Citation3]. The hearing loss in EOM is progressive high-tone BCHL and is profound, if suitable treatments including systemic and/or topical steroids are not carried out [Citation5,6]. There are some cases that are refractory to such treatments and develop deafness. Cochlear implant surgery was shown to be successful in a case of profound deafness with EOM [Citation7].
Recent research has gradually revealed the pathological conditions of the middle ear in EOM. Significantly numerous activated eosinophils (EG2-positive cells) were observed in the middle ear mucosa and a high concentration of eosinophil cationic protein (ECP) was also detected in middle ear effusion [Citation5,8]. The presence of eosinophil chemoattractants, such as IL-5 and eotaxin, in the middle ear effusion has also been proved [Citation9,10]. On the other hand, the etiology of sensory hearing loss of EOM is still obscure, since it is impossible to take human specimens of this disease, although eosinophilic inflammation of the middle ear likely affects inner ear function. Recently, we constructed a new animal model of eosinophil infiltration in the middle ear mucosa, as a model of EOM [Citation11]. General and topical sensitization and intraperitoneal and intratympanic injection with ovalbumin (OVA) led to eosinophil infiltration in the middle ear mucosa, and the number of eosinophils was increased as the periods of topical sensitization became longer. In the present study, we performed a histological investigation to elucidate the inner ear damage in this animal model of EOM.
Material and methods
Fifteen healthy Hartley guinea pigs weighing 250–350 g were used in the present study. General and topical sensitization was performed as in our previous report [Citation11]. Briefly, the animals were intraperitoneally injected with OVA 2000 μg and aluminum hydroxide (alum) 100 mg on day 0, and with 100 μg OVA and 100 mg alum on days 7 and 14 for general sensitization. Then, they were topically boosted by daily application of 100 μg OVA solution by nasal drip and intratympanic injection of 0.1 ml OVA (1000 μg/ml) in the right ear, and 0.1 ml saline in the left ear for control from day 21 for 7 days (n = 5), 14 days (n = 5), and 28 days (n = 5). All procedures were carried out under anesthesia with sodium pentobarbital (20 mg/kg i.p.). After the final OVA injection, the animals were deeply anesthetized with sodium pentobarbital (50 mg/kg i.p.) and injected intratympanically with 10% formaldehyde. The temporal bones were dissected and post-fixed with 10% formaldehyde (4 h), and decalcified with EDTA 2Na in 0.1 M TRIS (pH 7.2). Then, paraffin-embedded sections (3 μm) were prepared and stained with hematoxylin and eosin (H&E).
The sections were observed using an Olympus microscope (BX51), and assessed using digital images via a digital camera (Olympus DP72) and imaging software (DP2-BSW). The number of eosinophils was counted and calculated per unit area in the middle ear mucosa in five areas, and the mean per unit area was calculated. In the inner ear, infiltrating cells and damage of the organ of Corti were examined in the three groups of animals with different boosted periods.
Statistical analysis was performed using an unpaired t test to compare the OVA-injected side and control side. A p value < 0.05 was considered statistically significant.
This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 1983, and all animal experiments in this paper followed the Guidelines for Animal Experimentation, Hirosaki University.
Results
Tympanic cavity and middle ear mucosa
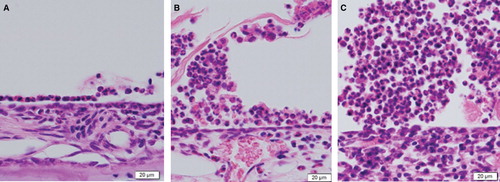
In the guinea pigs sensitized by nasal drip and intratympanic injection of OVA for 7 days, some eosinophils and a few plasmacytes were seen in the tympanic cavity of the antigen-stimulation side under microscopic observation (), although middle ear effusion was not noticed macroscopically. In the 14-day stimulation side, middle ear effusion containing even more eosinophils was observed in the tympanic cavity (). Furthermore, numerous eosinophils, some plasmacytes, and lymphocytes were seen in the tympanic cavity of the 28-day stimulation side (). Partial permeation of bacteria and a fair number of neutrophils were also seen.
Figure 1. Histological sections of the middle ear of an animal model for eosinophilic otitis media (EOM) (H&E staining): from the 7-day (A), 14-day (B), and 28-day (C) ovalbumin (OVA) stimulation sides.
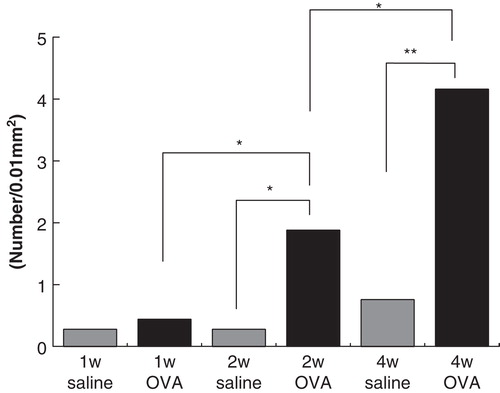
In the middle ear mucosa, eosinophil infiltration was increased as the period of OVA injection became longer in our previous study [Citation11]. Statistical analysis of middle ear mucosa showed that there was no significant difference between the antigen-stimulation side (mean ± SD: 0.44 ± 0.41) and the control side (mean ± SD: 0.28 ± 0.23) in the 7-day stimulation, but the number of eosinophils in the mucosal layer in the 14-day antigen-injected side (mean ± SD: 1.88 ± 0.78) was significantly larger than in the control side (mean ± SD: 0.28 ± 0.39). Moreover, the number of eosinophils of the 28-day stimulation side (mean ± SD: 4.16 ± 1.42) was significantly increased in the control side (mean ± SD: 0.76 ± 0.83) and the 14-day stimulation side ().
Inner ear
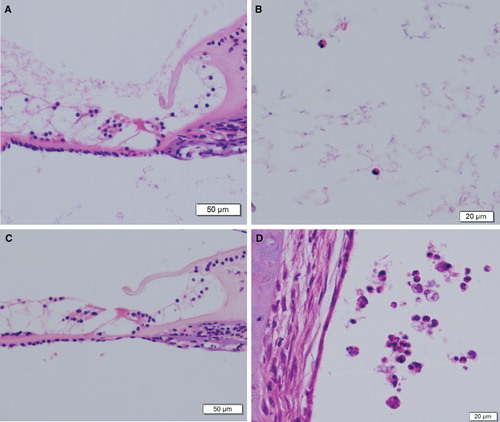
There was no obvious morphologic damage in the cochlea and the organ of Corti was well preserved at the light microscopic level in the 7-day stimulation (). However, a few eosinophils were seen in the scala tympani of the organ of Corti (). In addition, dilation of capillaries of the stria vascularis was observed, and no eosinophil infiltration was seen in the spiral ligament or the stria vascularis (data not shown). In the 14-day antigen-stimulation side, the morphology of the cochlea was almost preserved (). However, some eosinophils and macrophages were seen in not only the scala tympani but also the scala vestibuli ().
Figure 3. Histological sections of the inner ear from 7-day (A, B), and 14-day (C, D) antigen-stimulation sides (H&E staining). (A) The organ of Corti in the basal turn of the cochlea. (B) Eosinophils in the scala tympani. (C) The organ of Corti from a 14-day stimulation animal. (D) Eosinophils and macrophages in the scala vestibuli of the apical turn under high power field.
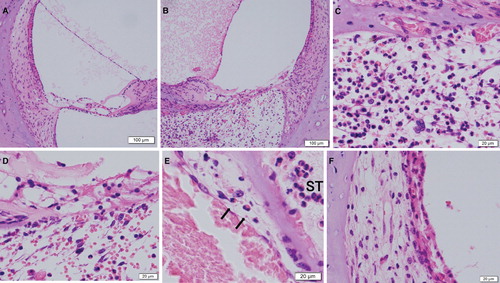
Although the morphology of the cochlea in the 28-day intratympanic injection of saline was also well preserved (), obvious morphological damage was seen in the 28-day antigen-stimulation side (). There were numerous eosinophils and red blood cells, some lymphocytes, and a few plasma cells, neutrophils, and macrophages in the scala tympani. In addition, capillaries of the basilar membrane were ruptured (), and severe morphological damage of the organ of Corti occurred (). Individual variation in the level of inner ear damage was seen. Migrating eosinophils from vessels were also seen in the venules neighboring the scala tympani (). Morphological damage of the spiral ligament and the stria vascularis was also severe in the 28-day antigen-stimulation side ().
Figure 4. Histological sections from the inner ear in 28-day stimulation sides (H&E staining). (A) The saline injection side of the basal turn of the cochlea under low power field. (B) The ovalbumin (OVA) injection side of the basal turn of the cochlea. (C) The basilar membrane and the scala tympani of (B) under high power field. (D) The organ of Corti under high power field. (E) Migrating eosinophils (arrows) from vessels in the venules neighboring the scala tympani (ST). (F) The spiral ligament and the stria vascularis of (E) under high power field.
A few animals in the 28-day antigen-stimulation group showed short duration post OVA injection nystagmus after 2 weeks or more. Although this may indicate the rupture of the round window membrane (RWM), we did not clarify this by histological examination. In the present study, hearing tests such as auditory brainstem response (ABR) were not performed, since the perforation of the tympanic membrane in our animal model was due to repetitive injections.
Discussion
EOM is known to be a high risk disease often involving sensory hearing loss, although its etiology is still obscure. Previous reports showed some cases of profound deafness [Citation4,5,7], and Iino et al. reported 5.8% of 138 patients developing unilateral or bilateral deafness [Citation3]. Nakagawa et al. reported that EOM damages high-tone sensory hearing in a time-dependent manner [Citation6], and Iino et al. also demonstrated that the incidence of deterioration of high-tone hearing loss in EOM is higher than in non-eosinophilic chronic otitis media [Citation12]. The sensory hearing loss will progress unless a correct diagnosis is made and anti-allergic treatment for EOM is started, and associated middle ear conditions are risk factors for high-tone sensory hearing loss. Therefore, inflammatory products of eosinophilic inflammation in the middle ear are likely to influence inner ear damage via the RWM [Citation6,12].
Animal models are useful to elucidate the pathologic conditions of the inner ear. Previous experimental studies of middle ear allergy already showed that a single topical challenge by antigen after general sensitization caused the infiltration of eosinophils in the middle ear mucosa [Citation13,14]. These previous studies were performed to clarify whether allergic reaction was involved in the etiology of the otitis media with effusion. Since the purpose of our investigation was to create a model animal in which many eosinophils infiltrated the middle ear mucosa, we used the general sensitization by OVA and prolonged intratympanic injection with OVA.
As a result, we succeeded in creating an animal model using guinea pigs in which the number of eosinophils infiltrating both middle ear mucosa and middle ear effusion increased as the periods of topical sensitization became longer [Citation11]. Although mice are generally used for animal models, we used guinea pigs, because the mouse temporal bone is too small to use in experiments involving inner ear damage.
In the early period (7-day stimulation) a few eosinophils infiltrated both the tympanic cavity and the scala tympani. As the periods of sensitization to the middle ear became longer, the number of eosinophils increased not only in the middle ear but also in the perilymphatic space. Moreover, morphological damage to the organ of Corti, the spiral ligament, and the stria vascularis were observed in the 28-day antigen-stimulation side. These results indicated that eosinophilic inflammation occurred in the inner ear as well as in the middle ear.
The middle ear and inner ear were separated only by the soft tissue RWM structure, and its permeability has already been investigated [Citation15]. Low molecular weight substances, such as steroids, can pass thorough the RWM, and high molecular weight substances, such as albumin (MW 67 000), can also pass through the RWM during inflammation. Previous reports demonstrated that eotaxin (8.4 kDa) and IL-5 (50–60 kDa), eosinophil chemoattractants, were contained in middle ear effusion of EOM patients at high concentration [Citation9,10]. In the 7-day stimulation side, these substances, especially eotaxin, may easily pass from the middle ear through the RWM into the perilymphatic space of the cochlea, and play an important role in eosinophilic inflammation in the inner ear. Permeability of Cys-leukotrienes (LTs), LTC4 and LTB4, through the RWM has also been demonstrated [Citation16], and they may have acted on capillary dilation of the stria vascularis in the 7-day stimulation side. Although we did not perform hearing tests in the present study, animals may show slight and reversible hearing loss.
In the prolonged topical stimulation model of 28-day intratympanic injection with OVA, numerous eosinophil infiltrations were seen in the scala tympani, and also severe morphological damage of the cochlea, the organ of Corti, and the stria vascularis, which was thought to be the cause of deafness. Eosinophils include cytotoxic granule proteins, cytokines, chemokines, and lipid mediators, and highly toxic granule proteins released from inappropriate accumulated eosinophils cause severe tissue damage. Moreover, it is likely that cytokines and chemokines released from eosinophils have significant paracrine and autocrine relevance at local sites [Citation17].
Infiltration by a few eosinophils in the early stage, such as 7-day stimulation, may be reversible. On the other hand, infiltration by a lot of eosinophils over a long term, such as 28-day stimulation, seems to cause irreversible inner ear damage. Moreover, it is still unclear whether all the numerous eosinophils in the perilymph migrate from venules or through the RWM. Further study is needed to clarify these issues regarding the cause of irreversible inner ear damage in detail.
Several kinds of anti-allergic drugs, Cys-LTs receptor antagonists, phosphodiesterase inhibitors, and so on, are used for the management of EOM. Especially for eosinophilic inflammation, administration of systemic or topical steroids is the most effective treatment. With regard to the permeability of the RWM, triamcinolone acetonide can pass through the RMW from the tympanic cavity into the inner ear [Citation15]. Iino et al. have reported that topical administration of triamcinolone acetonide is effective for EOM regarding hearing preservation [Citation18]. As a topical treatment for EOM, it is also important to remove middle ear effusion enriched with eosinophils and their inflammatory mediators as soon as possible. Heparin is used for easy removal of gelatinous middle ear effusion and its inhibitory effects on eosinophilic chemotaxis and neutralizing effects on eosinophil granule cationic proteins [Citation19]. A recent report indicates that long-term anti-IgE therapy could be effective for EOM [Citation20]. Our newly constructed animal model may be useful for establishing a new approach to treatment for EOM.
To our knowledge, this is the first report to indicate that prolonged topical stimulation with antigen in an animal model of EOM shows severe inner ear damage due to eosinophilic inflammation. Although this may cause severe sensory hearing loss, hearing tests were not performed in the present study. Further physiological study is needed to clarify the relationship between hearing level and morphological damage of the inner ear due to eosinophilic inflammation.
Acknowledgments
This study was supported by a Grant-in-aid for Scientific Research (C) from the Ministry of Education, Science, Sports, and Culture of Japan.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Tomioka S, Kobayashi T, Takasaka T. Intractable otitis media in patients with bronchial asthma (eosinophilic otitis media). In Sanna M, editor. Cholesteatoma and mastoid surgery. Rome: CIC Edizioni Internazionali. 1997. p 851–3.
- Derlacki EL. Aural manifestations of allergy. Ann Otol Rhinol Laryngol 1952;6:179–88.
- Iino Y, Tomioka-Matsutani S, Matsubara A, Nakagawa T, Nonaka M. Diagnostic criteria of eosinophilic otitis media, a newly recognized middle ear disease. Auris Nasus Larynx 2011;38:456–61.
- Tomioka S, Yuasa R, Iino Y. 1993. Intractable otitis media in cases with bronchial asthma. Recent advances in otitis media. In Mogi G, Honjo I, Ishii T, Takasaka T, editors. Proceedings of the second extraordinary international symposium on recent advances on otitis media. Amsterdam: Kugler Publications, Inc. p 183–6.
- Nagamine H, Iino Y, Kojima C, Miyazawa T, Iida T. Clinical characteristics of so called eosinophilic otitis media. Auris Nasus Larynx 2002;29:19–28.
- Nakagawa T, Matsubara A, Shiratsuchi H, Kakazu Y, Nakashima T, Koike K. Intractable otitis media with eosinophils: importance of diagnosis and validity of treatment for hearing preservation. ORL J Otorhinolaryngol Relat Spec 2006;68:118–22.
- Iwasaki S, Nagura M, Mizuta K. Cochlear implantation in a patient with eosinophilic otitis media. Eur Arch Otorhinolaryngol 2006;263:365–9.
- Iino Y, Nagamine H, Yabe T, Matsutani S. Eosinophils are activated in middle ear mucosa and middle ear effusion of patients with intractable otitis media associated with bronchial asthma. Clin Exp Allergy 2001;31:1135–43.
- Nonaka M, Fukumoto A, Ozu C, Mokuno E, Baba S, Pawankar R, et al. IL-5 and eotaxin levels in middle ear effusion and blood from asthmatics with otitis media with effusion. Acta Otolaryngol 2003;123:383–7.
- Iino Y, Kakizaki K, Katano H, Saigusa H, Kanegasaki S. Eosinophil chemoattractants in the middle ear of patients with eosinophilic otitis media. Clin Exp Allergy 2005;35:1370–6.
- Nishizawa H, Matsubara A, Nakagawa T, Ohta N, Izuhara K, Shirasaki T, et al. The role of periostin in eosinophilic otitis media. Acta Otolaryngol 2012;132:838–44.
- Iino Y, Usubuchi H, Kodama K, Takizawa K, Kanazawa T, Ohta Y. Bone conduction hearing level in patients with eosinophilic otitis media associated with bronchial asthma. Otol Neurotol 2008;29:949–52.
- Ryan AF, Catanzaro A, Wasserman SI, Harris JP. Secondary immune response in the middle ear: immunological, morphological, and physiological observations. Ann Otol Rhinol Laryngol 1986;95:242–9.
- Tomonaga K, Chaen T, Kurono Y, Mogi G. Type I allergic reactions of the middle ear and eustachian tube: an experimental study. Auris Nasus Larynx 1990;17:121–31.
- Juhn SK, Hamaguchi Y, Goycoolea M. Review of round window membrane permeability. Acta Otolaryngol Suppl 1988;457:43–8.
- Lee SH, Woo HW, Jung TTK, Lee C, Miller SK, Park YM, et al. Permeability of arachidonic acid metabolites through the round window membrane in chinchillas. Acta Otolaryngol Suppl 1992;493:165–9.
- Walsh GM. Eosinophil granule proteins and their role in disease. Curr Opin Hematol 2001;8:28–33.
- Iino Y, Nagamine H, Kakizaki K, Komiya T, Katano H, Saruya S, et al. Effectiveness of instillation of triamcinolone acetonide into the middle ear for eosinophilic otitis media associated with bronchial asthma. Ann Allergy Asthma Immunol 2006;97:761–6.
- Matsubara A, Kuroda R, Usami S, Takahata J, Shinkawa H. 2001. The effect of topical administration of heparin to the eosinophilic otitis media. In Takasaka T, Yuasa R, Hozawa K, editors. Recent advances in otitis media. Bologna: Monduzzi Editore. p 403–6.
- Iino Y, Hara M, Hasegawa M, Matsuzawa S, Shinnabe A, Kanazawa H, et al. Clinical efficacy of anti-IgE therapy for eosinophilic otitis media. Otol Neurotol 2012;33:1218–24.