Abstract
Accumulating evidence suggests that vitamin D may play a role for cardiovascular health. Expression of the vitamin D receptor (VDR) and enzymes for vitamin D metabolism have been identified in the vasculature as well as in the heart. VDR knock-out mice suffer from cardiovascular disease (CVD) and even selective VDR deletion in cardiomyocytes causes myocardial hypertrophy. Many, but not all observational studies showed that vitamin D deficiency is associated with CVD and its risk factors. Low concentrations of 25-hydroxyvitamin D (25(OH)D) are an independent risk factor for cardiovascular events, in particular for strokes and sudden cardiac deaths. Only few randomized controlled trials (RCTs) are available on this topic. These RCTs are frequently limited by the additional supplementation of calcium which may increase the risk of CVD events. RCTs with pure vitamin D supplementation have partially but not consistently shown beneficial effects on cardiovascular risk factors such as arterial hypertension. A number of large RCTs on the impact of vitamin D supplementation on cardiovascular events and mortality have already started but limitations of the study designs such as inclusion of individuals with relatively high 25(OH)D concentrations have to be considered. At present, the evidence is not sufficient for general recommendations to supplement vitamin D in order to prevent and treat CVD. It should, however, be noted that justification for the prevention and treatment of vitamin D deficiency comes from evidence based benefits of vitamin D supplementation on musculoskeletal health.
Introduction
Vitamin D is classically known as a substance that prevents rickets in children and it is therefore recommended to supplement vitamin D in the first year(s) of life [Citation1]. It is established that vitamin D plays a crucial role in the regulation of mineral and bone metabolism [Citation1]. Meta-analyses of randomized controlled trials (RCT) documented that fractures and falls are significantly reduced by vitamin D supplementation [Citation2,Citation3]. As a consequence, vitamin D supplementation became a routine treatment for osteoporosis.
Large epidemiological studies have documented a high prevalence of vitamin D deficiency in general populations [Citation4]. This is most likely attributable to reduced sunlight exposure because UV-B induced vitamin D synthesis in the skin is the main determinant of circulating 25-hydroxyvitamin D (25[OH]D) concentrations that are measured to assess vitamin D status [Citation5]. Hence, vitamin D deficiency can be regarded as a lifestyle related public health problem. This is of growing interest because, beyond musculoskeletal diseases, vitamin D deficiency has also been associated with various extra-skeletal diseases including cancer, infections, autoimmune and cardiovascular diseases (CVD) [6– 8]. This makes sense when considering that the vitamin D receptor (VDR) is expressed in almost all human cells and that VDR activation regulates approximately three percent of the human genome [Citation9].
In 1981, Robert Scragg [Citation10] hypothesized that the seasonal decrease of CVD in summer might be a consequence of cardiovascular-protective actions of vitamin D. Accumulating evidence over the last three decades indeed indicates a beneficial role of vitamin D for cardiovascular health [Citation8]. Previous in-depth reviews have already summarized the data on the role of vitamin D for CVD and its risk factors, for strokes, heart and renal diseases [Citation8,Citation11–13]. In the present review, we aim to provide a brief overview on experimental, observational and interventional studies on these latter topics with a focus on the most recent data. Given that CVD is the major cause of death in Western countries we also summarize the current evidence relating vitamin D and total mortality. In addition, we want to provide an outlook on the future of vitamin D and CVD by discussing the currently ongoing large RCTs in this field with particular attention to their strengths and limitations.
Experimental studies
Expression of VDR and 1-α-hydroxylase, the enzyme that converts 25(OH)D to the most active vitamin D metabolite 1,25-dihydroxyvitamin D (1,25(OH)2D), has been found in the vessels and the heart [Citation8,Citation9,Citation12,Citation14,Citation15]. This suggests a role of vitamin D in cardiovascular pathophysiology, a notion that is supported by studies of knock-out mice for VDR or 1-α-hydroxylase. These mice suffer from cardiovascular pathologies including arterial hypertension, myocardial hypertrophy and increased thrombogenicity [Citation9,Citation16,Citation17]. Renin overexpression occurs in these models and the molecular pathways mediating renin suppression by VDR activation have already been characterized in detail [Citation9,Citation16,Citation17].
VDR activation has also been shown to prevent myocardial hypertrophy, an effect that may reflect direct vitamin D effects on the heart because even selective deletion of the VDR in cardiac myocytes leads to left ventricular hypertrophy despite normal calcium homeostasis and normal parathyroid hormone (PTH) concentrations [Citation12,Citation18]. Increased expression of various pro-hypertrophic genes such as modulatory calcineurin inhibitory protein 1 (MCIP1) is observed in these cardiomyocyte-specific knock-out mice [Citation12,Citation18]. Myocardial calcium homeostasis, which is crucial for myocardial electrophysiology and contractility, is also partially regulated by vitamin D [Citation12]. Apart from this it has been demonstrated that VDR activation inhibits the development of atherosclerotic lesions by e.g. inhibition of macrophage to foam cell formation or by suppression of vascular smooth muscle cell proliferation [Citation19,Citation20].
In rat models, it was shown that early life vitamin D deficiency is associated with impaired vascular endothelial and smooth muscle cell function [Citation21]. This is in line with a study on umbilical vein endothelial cells that showed significantly increased endothelial nitric oxide (NO) production after stimulation with 1,25 (OH)2D [Citation22]. In addition, there exists evidence from experimental studies suggesting that VDR activation may exert anti-oxidative properties [Citation23,Citation24].
Beyond direct effects on the cardiovascular system there is compelling experimental evidence that vitamin D may exert beneficial effects on common cardiovascular risk factors such as arterial hypertension, kidney dysfunction, inflammation, dyslipidemia, obesity or diabetes mellitus [Citation25–28]. Beyond suppressive effects on the renin angiotensin aldosterone system (RAAS), vitamin D has been shown to exert various other antihypertensive effects like modulation of vascular function including improved endothelial function [Citation21,Citation22,Citation25].
VDR activation has also been shown to be nephroprotective by e.g. antiproteinuric effects that may be partially mediated by protective effects on podocytes [Citation13]. VDR activation induces renal megalin expression which is required for tubular protein reabsorption [Citation29]. Inhibition of Tumor Necrosis Factor-α (TNF- α)/Epidermal Growth Factor Receptor (EGFR) signalling pathways including inhibition of the TNF-α converting enzyme (TACE) may also reduce renal parenchymal lesions and proteinuria [Citation29].
Moreover, vitamin D plays a crucial role in immune regulation. Vitamin D has several anti-inflammatory properties that are mediated by e.g. suppression of nuclear factor-κB (NF-κB) as well as by inhibition of pro-inflammatory and stimulation of anti-inflammatory cytokines [Citation30,Citation31]. It has been concluded that vitamin D causes a switch from a Th1/Th17 response to a Th2/Treg profile that may in turn protect against overwhelming inflammation and autoimmunity [Citation28].
Vitamin D, which is itself formed from 7-dehydrocholesterol in the human skin, is also involved in lipid metabolism and may e.g. lower triglyceride concentrations by decreasing hepatic triglyceride formation or by modulating hepatic calcium homeostasis [Citation32]. Mechanistic studies suggest that VDR activation may increase HDL-cholesterol and its main protein component apolipoprotein A-1 [Citation27]. Apart from this, it has been documented that vitamin D sequestration occurs in the adipose tissue so that obesity may cause vitamin D deficiency [Citation9].
Vitamin D may also be important for glucose homeostasis by directly modulating beta cell functions including calcium dependent processes that are involved in insulin secretion or by enhancing insulin signalling via up-regulation of the insulin-receptor [Citation9].
Observational studies on vitamin D, CVD and its risk factors
Numerous epidemiological studies have evaluated the associations of 25(OH)D with CVD events and its risk factors [Citation8,Citation11–13,Citation25–28]. Regarding CVD risk factors it has been shown in a meta-analysis that low concentrations of 25(OH)D are associated with an increased risk of prevalent and incident arterial hypertension [Citation33]. In this context, it has also been reported that low 25(OH)D concentrations are associated with increased activity of the RAAS [Citation34].
Prospective studies showed that individuals with 25(OH)D concentrations above 62.5 nmol/L (divide by 2.496 to convert nmol/L to ng/mL) had a 43 % lower risk of developing type 2 diabetes mellitus compared to individuals with 25(OH)D concentrations below 35 nmol/L [Citation35]. Inverse correlations of 25(OH)D and HbA1c have been found in some but not all studies addressing this issue [Citation36]. Observational data suggest that the association of vitamin D deficiency and increased risk of type 2 diabetes mellitus may be partially mediated by subclinical inflammation [Citation37].
An association of low 25(OH)D and inflammation (i.e. C-reactive protein) has been observed in the continuous National Health and Nutrition Examination Survey (NHANES), but not all studies found such associations [Citation7,Citation8,Citation28,Citation38].
Mixed results are available on the association of vitamin D status and dyslipidemia but most studies in this field reported an inverse association of 25(OH)D and serum triglycerides and positive correlations of 25(OH)D with HDL-cholesterol and apolipoprotein A-1 [Citation26,Citation27,Citation32]. It should, however, be considered that associations of vitamin D status with metabolic and inflammatory parameters may be confounded by the link of vitamin D and obesity [Citation9]. This is of particular importance because it is becoming increasingly clear that vitamin D deficiency is not the cause but the consequence of obesity [Citation39].
Given that low testosterone concentrations have been associated with increased cardiovascular risk it should be acknowledged that low 25(OH)D concentrations have been associated with low testosterone concentrations in epidemiological studies [Citation40,Citation41].
A poor vitamin D status has also been associated with lower glomerular filtration rate (GFR) and has been identified as a risk factor for rapid GFR loss in prospective analyses of the Cardiovascular Health Study [Citation13,Citation29,Citation42].
Regarding cross-sectional associations of vitamin D status with manifest vascular diseases there exist conflicting data with either an inverse or no association of 25(OH)D concentrations with coronary artery disease, coronary artery calcification and carotid intima-media thickness [Citation8,Citation43,Citation44]. Concerning vascular calcification it should be acknowledged that there might be an U-shaped association with increased risk of vascular calcification at both low 25(OH)D concentrations and vitamin D toxicity [Citation44]. Some but not all studies found an association of low 25(OH)D with endothelial dysfunction and arterial stiffness [Citation8,Citation45,Citation46], and numerous studies have almost consistently shown that low 25(OH)D concentrations are a risk factor for prevalent and incident heart failure [Citation12]. Myocardial infarction has also been linked to vitamin D deficiency in some but not all studies and epidemiological data showed an inverse association of 25(OH)D concentration and matrix metalloproteinase-9 (MMP-9), a marker of myocardial remodelling and inflammation [Citation47–49]. Most but not all studies showed that low 25(OH)D concentrations are associated with cerebrovascular events including strokes and a particular strong association has repeatedly been reported for low 25(OH)D and increased risk of sudden cardiac death [Citation50–56]. In general, it can be concluded that low 25(OH)D concentrations are a risk factor for cardiovascular events, a notion that is supported by meta-analyses of observational studies [Citation55–57]. Apart from this, it is also important to note that PTH, which increases as a consequence of vitamin D deficiency, can also be regarded as a cardiovascular risk factor and has been shown to predict cardiovascular events and mortality [Citation58–60].
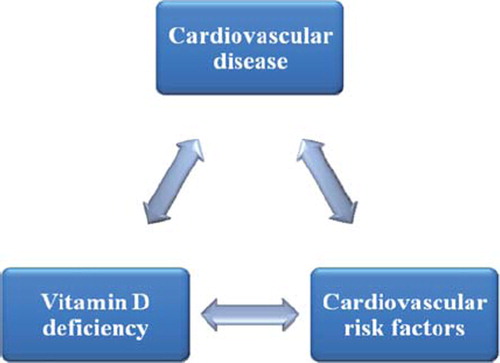
When interpreting the above mentioned observational studies it is difficult to differentiate whether the observed association between 25(OH)D concentrations and CVD reflects a “true” pathophysiologic relationship or whether it is confounded by the fact that patients suffering from CVD or risk conditions for CVD have reduced sunlight exposure due to their physical impairments or their risk factor related social behaviour. The situation is even more complex when considering the reciprocal influences of vitamin D deficiency, CVD and cardiovascular risk factors (see ). For example, low 25(OH)D concentrations may hypothetically contribute to myocardial dysfunction but heart failure associated limitations in mobility may reduce outdoor activities and sunlight exposure thus contributing to low 25(OH)D concentrations. On the other hand, heart failure may also cause wasting with weight loss and subsequently beneficial effects on metabolic risk factors (e.g. improved glucose homeostasis) but also higher 25(OH)D concentrations due to lower BMI.
Studies on vitamin D supplementation, cardiovascular risk factors and CVD events
In contrast to many observational studies on vitamin D status and CVD there are only few data on vitamin D supplementation and CVD and its risk factors. Meta-analyses on vitamin D treatment and blood pressure suggest that natural vitamin D may reduce systolic blood pressure in a range of approximately 2 to 6 mm Hg, but this result was not significant in all meta-analyses [Citation56,Citation61,Citation62]. UV-B exposure has previously been shown to reduce systolic and diastolic blood pressure compared to UV-A exposure, but this could not be replicated in a recent study by Scragg et al. [Citation63,Citation64]. By contrast, a study in 20 postmenopausal women showed that 25(OH)D supplementation is associated with a significant reduction in systolic blood pressure [Citation65]. Regarding diabetes mellitus, a meta-analysis by Mitri et al. concluded that vitamin D supplementation had no significant effect on glycemic outcomes in patients with normal glucose tolerance as well as in type 2 diabetics [Citation35]. Studies among patients with glucose intolerance, however, showed that vitamin D supplementation improved insulin resistance [Citation35]. Furthermore, a recent original study among adults at high risk of diabetes showed that vitamin D supplementation improved beta-cell function and there was a strong non-significant trend for an attenuated rise in HbA1c concentrations in individuals on vitamin D treatment [Citation66]. In line with this, another study showed that a vitamin D-fortified yogurt drink improved glycemic status in patients with type 2 diabetes mellitus [Citation67].
Interventional studies on vitamin D and blood lipids produced mixed results. Zittermann et al. showed that vitamin D supplementation significantly decreased serum triglycerides but also caused an increase in LDL-cholesterol [Citation26,Citation27,Citation32,Citation68]. RCTs also produced inconsistent results regarding the effects of vitamin D supplementation on inflammation [Citation7,Citation28,Citation69–72]. Some studies showed that vitamin D supplementation increases the anti-inflammatory cytokine interleukin-10 (IL-10) and decreases TNF-α [Citation70–72]. Circulating regulatory T cells (Tregs), that are considered to protect against autoimmunity including type 1 diabetes mellitus, can be increased by vitamin D supplementation [Citation73]. This is in line with observations by Hyppönen et al., who found a significantly decreased risk of type 1 diabetes mellitus in children receiving recommended vitamin D supplementation during their first years of life [Citation74]. In addition, genetic variants affecting 25(OH)D concentrations have also been linked to type 1 diabetes mellitus [Citation75,Citation76].
Limited and inconclusive data exist on the effect of vitamin D supplementation on markers of heart failure and some, but not all RCTs have shown that vitamin D supplementation improves endothelial function [Citation77–81]. Interestingly, paricalcitol, an active vitamin D analogue, was shown to reduce albuminuria in patients with diabetic nephropathy [Citation82].
RCTs on vitamin D supplementation and CVD events are sparse and a major problem with the interpretation of these studies is that vitamin D supplementation has often been combined with calcium supplementation [Citation83]. Considering that calcium supplementation may be associated with increased risk of cardiovascular events, as reported by a meta-analysis, we cannot draw adequate conclusions regarding the effect of vitamin D on CVD events when looking at studies with combined calcium plus vitamin D supplementation [Citation83]. When including studies with pure vitamin D supplementation, Wang et al. found a non-significant trend for reduced risk of CVD events (pooled relative risk, 0.90 [95 % CI, 0.77 to 1.05]) in patients randomized to vitamin D supplementation [Citation84]. Similar results i.e. a non-significant reduction of CVD events (hazard ratio = 0.91; 95 % CI = 0.79–1.05) were also reported by Avenell et al., who analyzed 5,292 older patients from the RECORD trial [Citation85]. In addition, it has been observed that chronic kidney disease (CKD) patients who were sufficiently treated with vitamin D experienced significantly less CVD events compared to untreated patients [Citation86]. Hence, limited evidence suggests that vitamin D supplementation might reduce CVD events in specific subgroups but this is not proven and has to be evaluated in further RCTs.
Vitamin D and total mortality
Given that CVDs are the major cause of death in Western countries it is of interest to evaluate whether vitamin D status or vitamin D supplementation is associated with total mortality. Prospective observational studies have largely, but not consistently shown, that low 25(OH)D concentrations are associated with increased risk of total mortality [Citation87,Citation88]. A meta-analysis of studies among the general population confirmed this notion and found a non-linear association of 25(OH)D and mortality with the lowest risk at 25(OH)D concentrations ranging from 75 to 87.5 nmol/L [Citation88]. Highest mortality risk was found in individuals with the lowest 25(OH)D concentrations [Citation88]. Among specific patient groups it has been observed that in CKD patients low 25(OH)D concentrations are also a risk factor for mortality [Citation89–91]. Importantly, recent observational data showed that vitamin D supplementation was associated with improved survival [Citation92]. This is in line with meta-analyses of RCTs that found significantly reduced mortality in patients randomized to vitamin D supplementation [Citation93]. In detail, a Cochrane review by Bjelakovic et al. reported a statistically significant 6 % reduction of mortality by vitamin D3 supplementation compared to placebo [Citation94]. It was calculated that when treating 161 individuals with vitamin D3, one additional death can be prevented [Citation94].
Future outlook
Although there exist accumulating data that vitamin D may be beneficial for CVD and its risk factors, evidence is still not sufficient to establish general recommendations to supplement vitamin D for the prevention and treatment of CVD. Therefore, well-designed large-scale RCTs are urgently needed. Such RCTs designed to evaluate vitamin D effects on CVD events and mortality have recently been started and include e.g. a study among 1,000 heart failure patients in Germany by Zittermann et al. (EVITA study) and a study among 5,100 older individuals in New Zealand by Scragg et al. (Vitamin D Assessment Study, ViDA). The largest study in this field is the VITAL Study (VITamin D and OmegA-3 TriaL) among 20,000 study participants in the US by Manson et al. [Citation95]. Strengths of these studies are e.g. the large number of study participants and the relatively long follow-up periods. However, it will take a few years from now until we can expect the published results of these trials between ∼2015 to 2017 [Citation95]. We should critically discuss whether the results of e.g. the VITAL Study can definitely answer the question whether vitamin D supplementation is effective for the prevention and treatment of CVD. In general, the VITAL Study includes study participants representing the older population in the US. Study participants are included regardless of their 25(OH)D concentrations at baseline and apart from the study medication (= 2,000 IU vitamin D per day or placebo) an intake of up to 800 IU vitamin D per day is allowed [Citation95]. Results of the VITAL study might therefore be limited by relatively high 25(OH)D concentrations in the placebo group. Although the results of the VITAL Study are of great importance because they allow drawing conclusions regarding general vitamin D supplementation in the older population the study is not mainly designed to address vitamin D benefits in individuals suffering from vitamin D deficiency. In this context, it should be considered that many observational studies showed a significantly increased risk of CVD events only in patients with 25(OH)D concentrations below ∼37.5 nmol/L [Citation8,Citation55–57]. Furthermore, beneficial outcomes are mostly observed in individuals with 25(OH)D concentrations ranging from ∼75 to 100 nmol/L and some study results indicate a slightly increasing risk for adverse events at very high 25(OH)D concentrations [Citation88,Citation95]. In our opinion, important vitamin D RCTs should therefore aim to study high-risk participants who are expected to be very sensitive to vitamin D supplementation (e.g. severely vitamin D deficient patients with high PTH concentrations and at high CVD risk) and should include an optimal vitamin D dosage regime (e.g. with the aim to reach the 25(OH)D target concentrations of ∼75 to 100 nmol/L). The time window for funding of such trials will likewise close within the next few years after the above mentioned RCTs have been published and we can only hope that the vitamin D story will not be similar to vitamin E [Citation96]. Vitamin E exerts anti-oxidative actions but large RCTs including relatively unselected study participants with vitamin E concentrations mainly in the sufficiency interval failed to prove significant benefits with suboptimal dosages of vitamin E [Citation96]. Therefore, the open question still remains whether there are relevant effects in vitamin E sensitive individuals with e.g. low vitamin E concentrations and/or high oxidative stress [Citation96,Citation97].
Conclusions
Experimental studies suggest that vitamin D plays a crucial role for the maintenance of cardiovascular health. This notion is supported by large epidemiological studies which highlight vitamin D deficiency as an independent cardiovascular risk factor. Data from interventional studies on vitamin D treatment and cardiovascular risk are, however, sparse and inconclusive and are often limited by the concomitant supplementation of vitamin D plus calcium. Large-scale RCTs on vitamin D and CVD risk have already started but their limitations such as inclusion of study participants regardless of their 25(OH)D concentrations may reduce the ability to detect beneficial vitamin D effects on cardiovascular morbidity and mortality in high-risk vitamin D deficient individuals. At present, the evidence is not sufficient for general recommendations to supplement vitamin D in order to prevent and treat CVD. It should, however, be noted that justification for the prevention and treatment of vitamin D deficiency needs only one proven benefit of vitamin D supplementation and this are (at least) the beneficial effects on musculoskeletal health [Citation1–3,Citation98].
Questions and Answers
M Kaelin, Switzerland
Are you confident that there will be randomised controlled trials that will give better results than those for vitamin E? When you mention that there are so many factors influencing CVD or strokes, there are always multifunctional cases and diseases. Will it therefore ever be possible to have comparable groups taking into account all these factors?
S Pilz
I hope that on-going trials will provide definite answers, but have severe limitations. These include that study participants are included irrespective of their serum 25(OH)D concentration. Quite a significant vitamin D intake is permitted so that may be a problem.
M Fukagawa, Japan
You did not mention FGF23. Did vitamin D supplementation increase the serum concentration of FGF23?
S Pilz
I have not looked at this.
M Fukagawa
I ask because, at least in CKD patients, treatment with active vitamin D sterols increases serum FGF23 concentrations and it is known that this therapy improves survival and reduces cardiac events. However, the increased FGF23 is another risk factor for cardiac function.
S Pilz
Yes, but for CKD I agree with what has been said by R Vieth; that we should consider the 25(OH)D concentration. If it is normal, we should think of prescribing active vitamin D. Sometimes there are difficulties in vitamin D deficient patients who are given additional 1,25(OH)2D3. Another point in CKD patients is that when you give calcitrol or other active vitamin D analogues, they may increase the degradation of 25(OH)D because of a reduction in the 24 hydroxylases.
M Fukagawa
So if I give a supplement that doesn't increase FGF23 in serum it may be good, because it may only be metabolised at the local level.
S Pilz
Yes.
JC Souberbielle
We have measured FGF23 after vitamin D supplementation in osteoporotic patients with low and moderate 25(OH)D concentrations at baseline and there were no changes in FGF23 in our study.
P Rozentryt, Poland
My question relates to consistency of data linking cardiovascular events and outcomes with vitamin D and those linking phosphates with cardiac events. They seem of similar quality. What is the primary event? Is it possible that increasing phosphate burden through inhibition of the FGF23 pathway might be a link.
S Pilz
There may be a link, I agree. However, I must also say that in some of the trials we were able to adjust for phosphate concentrations and it didn't change the relationship between vitamin D deficiency and cardiac events.
P Rozentryt
How can you explain the failure of paracalcitrol and regression of hypertrophy in renal patients?
S Pilz
You are referring to the paracalcitrol trial which studied renal patients and failed to show an effect on cardiac function. The issue is, was this the right therapy? It may be that the cardiomyocytes need more then 25(OH)D. If just a small amount of 25(OH)D is intracellularly converted to the active form then a much higher local concentration can be achieved than with systemic therapy with 1,25(OH)2D3 which may be harmful or have a very narrow therapeutic window.
R Jorde, Norway
I think we focus too much on what is the ‘sufficient’ level of intake. We should also consider what is too much. We have done several intervention studies and have given up to 6,000 U/day. We found that there is a small but significant increase in systolic blood pressure and a slight significant decrease in glucose tolerance, so we should be careful. The serum concentrations were 120–150 nmol/L.
Disclosure summary
Nothing to disclose.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Holick MF. McCollum Award Lecture, 1994: vitamin D—new horizons for the 21st century. Am J Clin Nutr 1994;60:619–30.
- Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 2009;169:551–61.
- Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 2009;339:b3692.
- Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J; IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20:1807–20.
- Macdonald HM, Mavroeidi A, Fraser WD, Darling AL, Black AJ, Aucott L, O'Neill F, Hart K, Berry JL, Lanham-New SA, Reid DM. Sunlight and dietary contributions to the seasonal vitamin D status of cohorts of healthy postmenopausal women living at northerly latitudes: a major cause for concern? Osteoporos Int 2011;22:2461–72.
- Pilz S, Tomaschitz A, Obermayer-Pietsch B, Dobnig H, Pieber TR. Epidemiology of vitamin D insufficiency and cancer mortality. Anticancer Res 2009;29:3699–704.
- Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 2011 Oct 13. doi: 10.1111/j.1365-2265. 2011.04261.x.
- Pilz S, Tomaschitz A, März W, Drechsler C, Ritz E, Zittermann A, Cavalier E, Pieber TR, Lappe JM, Grant WB, Holick MF, Dekker JM. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf) 2011;75:575–84.
- Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726–76.
- Scragg R. Seasonality of cardiovascular disease mortality and the possible protective effect of ultra-violet radiation. Int J Epidemiol 1981;10:337–41.
- Pilz S, Tomaschitz A, Drechsler C, Zittermann A, Dekker JM, März W. Vitamin D supplementation: a promising approach for the prevention and treatment of strokes. Curr Drug Targets 2011;12:88–96.
- Pilz S, Tomaschitz A, Drechsler C, Dekker JM, März W. Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res 2010;54:1103–13.
- Doorenbos CR, van den Born J, Navis G, de Borst MH. Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nat Rev Nephrol 2009;5:691–700.
- Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 25-hydroxyvitamin D3-1 alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation 2005;111:1666–71.
- Chen S, Glenn DJ, Ni W, Grigsby CL, Olsen K, Nishimoto M, Law CS, Gardner DG. Expression of the vitamin d receptor is increased in the hypertrophic heart. Hypertension 2008; 52:1106–12.
- Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab 2005;288:E125–32.
- Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1 alpha-hydroxylase knockout mice. Kidney Int 2008;74:170–9.
- Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner DG. Cardiomyocyte-specific deletion of the vitamin D receptor gene results in cardiac hypertrophy. Circulation 2011;124:1838–47.
- Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, Bernal-Mizrachi L, Bernal-Mizrachi C. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 2009;120:687–98.
- Chen S, Law CS, Gardner DG. Vitamin D-dependent suppression of endothelin-induced vascular smooth muscle cell proliferation through inhibition of CDK2 activity. J Steroid Biochem Mol Biol 2010;118:135–41.
- Tare M, Emmett SJ, Coleman HA, Skordilis C, Eyles DW, Morley R, Parkington HC. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol 2011;589: 4777–86.
- Molinari C, Uberti F, Grossini E, Vacca G, Carda S, Invernizzi M, Cisari C. 1α,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol Biochem 2011;27:661–8.
- Argacha JF, Egrise D, Pochet S, Fontaine D, Lefort A, Libert F, Goldman S, van de Borne P, Berkenboom G, Moreno-Reyes R. Vitamin D deficiency-induced hypertension is associated with vascular oxidative stress and altered heart gene expression. J Cardiovasc Pharmacol 2011;58: 65–71.
- Finch JL, Suarez EB, Husain K, Ferder L, Cardema MC, Glenn DJ, Gardner DG, Liapis H, Slatopolsky E. Effect of combining an ACE inhibitor and a VDR activator on glomerulosclerosis, proteinuria, and renal oxidative stress in uremic rats. Am J Physiol Renal Physiol. 2012;302:F141–9.
- Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009;6:621–30.
- Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res 2011;50:303–12.
- Jaimungal S, Wehmeier K, Mooradian AD, Haas MJ. The emerging evidence for vitamin D-mediated regulation of apolipoprotein A-I synthesis. Nutr Res 2011;31:805–12.
- Guillot X, Semerano L, Saidenberg-Kermanac'h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint Bone Spine 2010;77:552–7.
- Dusso AS, Tokumoto M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney Int 2011; 79:715–29.
- Geldmeyer-Hilt K, Heine G, Hartmann B, Baumgrass R, Radbruch A, Worm M. 1,25-dihydroxyvitamin D3 impairs NF-κB activation in human naïve B cells. Biochem Biophys Res Commun 2011; 407:699–702.
- Chen Y, Kong J, Sun T, Li G, Szeto FL, Liu W, Deb DK, Wang Y, Zhao Q, Thadhani R, Li YC. 1,25-Dihydroxyvitamin D3 suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-κB activation. Arch Biochem Biophys 2011;507:241–7.
- Zittermann A, Gummert JF, Börgermann J. The role of vitamin D in dyslipidemia and cardiovascular disease. Curr Pharm Des 2011;17:933–42.
- Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens 2011;29:636–45.
- Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, März W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta 2010;411:1354–60.
- Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr 2011;65: 1005–15.
- Hutchinson MS, Figenschau Y, Njølstad I, Schirmer H, Jorde R. Serum 25-hydroxyvitamin D concentrations are inversely associated with glycated haemoglobin (HbA(1c)). The Tromsø Study. Scand J Clin Lab Invest 2011;71: 399–406.
- Thorand B, Zierer A, Huth C, Linseisen J, Meisinger C, Roden M, Peters A, Koenig W, Herder C. Effect of serum 25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: results from the MONICA/KORA Augsburg study. Diabetes Care 2011;34:2320–2.
- Amer M, Qayyum R. Relation Between Serum 25-Hydroxyvitamin D and C-Reactive Protein in Asymptomatic Adults (From the Continuous National Health and Nutrition Examination Survey 2001 to 2006). Am J Cardiol 2012;109: 226–30.
- Dorjgochoo T, Shi J, Gao YT, Long J, Delahanty R, Xiang YB, Cai Q, Shu XO. Genetic variants in vitamin D metabolism-related genes and body mass index: analysis of genome-wide scan data of approximately 7000 Chinese women. Int J Obes (Lond) 2011 Dec 13. doi: 10.1038/ijo. 2011.246.
- Wehr E, Pilz S, Boehm BO, März W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen concentrations in men. Clin Endocrinol (Oxf) 2010;73: 243–8.
- Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, Wehr E, Zittermann A. Effect of vitamin D supplementation on testosterone concentrations in men. Horm Metab Res 2011;43:223–5.
- de Boer IH, Katz R, Chonchol M, Ix JH, Sarnak MJ, Shlipak MG, Siscovick DS, Kestenbaum B. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol 2011;6:2141–9.
- Joergensen C, Reinhard H, Schmedes A, Hansen PR, Wiinberg N, Petersen CL, Winther K, Parving HH, Jacobsen PK, Rossing P. Vitamin D levels and asymptomatic coronary artery disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Diabetes Care 2012;35: 168–72.
- Zittermann A, Schleithoff SS, Koerfer R. Vitamin D and vascular calcification. Curr Opin Lipidol 2007;18:41–6.
- Mayer O Jr, Filipovský J, Seidlerová J, Vaněk J, Dolejšová M, Vrzalová J, Cífková R. The association between low 25-hydroxyvitamin D and increased aortic stiffness. J Hum Hypertens 2011 Oct 20. doi: 10.1038/jhh.2011.94.
- Chitalia N, Recio-Mayoral A, Kaski JC, Banerjee D. Vitamin D deficiency and endothelial dysfunction in non-dialysis chronic kidney disease patients. Atherosclerosis. 2012;220: 265–8.
- Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 2008;168:1174–80.
- Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–96.
- Khalili H, Talasaz AH, Salarifar M. Serum vitamin D concentration status and its correlation with early biomarkers of remodeling following acute myocardial infarction. Clin Res Cardiol. 2011 Dec 11. [Epub ahead of print].
- Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, Lappé DL, Muhlestein JB; Intermountain Heart Collaborative (IHC) Study Group. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol 2010;106:963–8.
- Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, März W. Low vitamin D levels predict stroke in patients referred to coronary angiography. Stroke 2008; 39:2611–3.
- Deo R, Katz R, Shlipak MG, Sotoodehnia N, Psaty BM, Sarnak MJ, Fried LF, Chonchol M, de Boer IH, Enquobahrie D, Siscovick D, Kestenbaum B. Vitamin D, parathyroid hormone, and sudden cardiac death: results from the cardiovascular health study. Hypertension 2011;58:1021–8.
- Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, Espe K, Dekker F, Brandenburg V, März W, Ritz E, Wanner C. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J 2010;31:2253–61.
- Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, Boehm BO, Dobnig H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008;93:3927–35.
- Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med 2010;51:228–33.
- Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med 2010;152:307–14.
- Scragg R. Vitamin D and public health: an overview of recent research on common diseases and mortality in adulthood. Public Health Nutr 2011;14:1515–32.
- Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, Jenny NS, Siscovick DS. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol 2011;58:1433–41.
- Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, Melhus H, Held C, Lind L, Michaëlsson K, Arnlöv J. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 2009;119:2765–71.
- Pilz S, Tomaschitz A, Drechsler C, Ritz E, Boehm BO, Grammer TB, März W. Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur Heart J 2010;31: 1591–8.
- Witham MD, Nadir MA, Struthers AD. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertens 2009;27:1948–54.
- Wu SH, Ho SC, Zhong L. Effects of vitamin D supplementation on blood pressure. South Med J 2010;103:729–37.
- Krause R, Bühring M, Hopfenmüller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet 1998;352:709–10.
- Scragg R, Wishart J, Stewart A, Ofanoa M, Kerse N, Dyall L, Lawes CM. No effect of ultraviolet radiation on blood pressure and other cardiovascular risk factors. J Hypertens 2011;29:1749–56.
- Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, Sidelnikov E, Willett WC, Orav EJ, Stähelin HB, Wolfram S, Jetter A, Schwager J, Henschkowski J, von Eckardstein A, Egli A. Oral supplementation with 25(OH)D(3) versus vitamin D(3) : effects on 25(OH)D levels, lower extremity function, blood pressure and markers of innate immunity. Bone Miner Res 2011 Oct 25. doi: 10.1002/jbmr.551.
- Mitri J, Dawson-Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr 2011;94:486–94.
- Nikooyeh B, Neyestani TR, Farvid M, Alavi-Majd H, Houshiarrad A, Kalayi A, Shariatzadeh N, Gharavi A, Heravifard S, Tayebinejad N, Salekzamani S, Zahedirad M. Daily consumption of vitamin D- or vitamin D+calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr 2011;93:764–71.
- Zittermann A, Frisch S, Berthold HK, Götting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 2009;89:1321–7.
- Yusupov E, Li-Ng M, Pollack S, Yeh JK, Mikhail M, Aloia JF. Vitamin D and serum cytokines in a randomized clinical trial. Int J Endocrinol 2010;2010. pii: 305054.
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006;83:754–9.
- Smolders J, Peelen E, Thewissen M, Cohen Tervaert JW, Menheere P, Hupperts R, Damoiseaux J. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS One 2010;5:e15235.
- Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol 2010;21:353–61.
- Prietl B, Pilz S, Wolf M, Tomaschitz A, Obermayer-Pietsch B, Graninger W, Pieber TR. Vitamin D supplementation and regulatory T cells in apparently healthy subjects: vitamin D treatment for autoimmune diseases? Isr Med Assoc J 2010;12:136–9.
- Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001;358:1500–3.
- Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180–8.
- Cooper JD, Smyth DJ, Walker NM, Stevens H, Burren OS, Wallace C, Greissl C, Ramos-Lopez E, Hyppönen E, Dunger DB, Spector TD, Ouwehand WH, Wang TJ, Badenhoop K, Todd JA. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes 2011;60:1624–31.
- Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, Shah Y, Zhu H, Dong Y. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens. 2011 May;24(5):557–62.
- Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients - A randomised controlled trial. Nutr Metab Cardiovasc Dis 2010 Dec 29.
- Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian MR, Houshiarrad A, Gharavi A, Kalayi A, Shariatzadeh N, Zahedirad M, Khalaji N, Haidari H. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: a randomized double-blind clinical trial. BMC Med 2011; 9:125.
- Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, Li K, Bassali R, Guo DH, Thomas J, Pierce GL, White J, Holick MF, Zhu H. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab 2010;95:4584–91.
- Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail 2010;3:195–201.
- de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 2010;376(9752):1543–51.
- Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ 2011;342:d2040.
- Wang L, Manson JE, Song Y, Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–23.
- Avenell A, Maclennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, Grant AM, Campbell MK, Anderson FH, Cooper C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA; the RECORD Trial Group. Long-Term Follow-Up for Mortality and Cancer in a Randomized Placebo-Controlled Trial of Vitamin D3 and/or Calcium (RECORD Trial). J Clin Endocrinol Metab 2012;97:614–622.
- Lishmanov A, Dorairajan S, Pak Y, Chaudhary K, Chockalingam A. Treatment of 25-OH Vitamin D Deficiency in Older Men With Chronic Kidney Disease Stages 3 and 4 Is Associated With Reduction in Cardiovascular Events. Am J Ther 2011 Aug 17. [Epub ahead of print].
- Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, van Dam RM, Dekker JM. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf) 2009;71:666–72.
- Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr 2012;95:91–100.
- Pilz S, Tomaschitz A, Friedl C, Amrein K, Drechsler C, Ritz E, Boehm BO, Grammer TB, März W. Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant 2011;26:3603–9.
- Drechsler C, Verduijn M, Pilz S, Dekker FW, Krediet RT, Ritz E, Wanner C, Boeschoten EW, Brandenburg V; NECOSAD Study Group. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant 2011; 26:1024–32.
- Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis 2011;58:374–82.
- Vacek JL, Vanga SR, Good M, Lai SM, Lakkireddy D, Howard PA. Vitamin D Deficiency and Supplementation and Relation to Cardiovascular Health. Am J Cardiol 2012; 109:359–63.
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2011 Jul 6;(7):CD007470.
- Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials 2012;33:159–71.
- Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int 2010;21:1121–32.
- Robinson I, de Serna DG, Gutierrez A, Schade DS. Vitamin E in humans: an explanation of clinical trial failure. Endocr Pract 2006;12:576–82.
- Suksomboon N, Poolsup N, Sinprasert S. Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: systematic review of randomized controlled trials. J Clin Pharm Ther 2011;36:53–63.
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8.