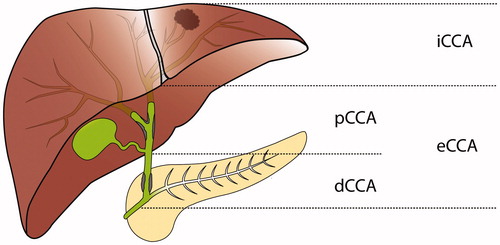
Cholangicarcinoma (CCA) is a rare malignancy that arises in connection to the bile ducts and show various degrees of cholangiocyte differentiation. Cholangiocarcinomas are classified based on anatomical location () as intrahepatic (iCCA) and extrahepatic (eCCA). Extrahepatic CCA has then been further divided into perihilar (pCCA), also known as Klatskin tumours, and distal (dCCA).[Citation1] In the current classification of CCA, iCCA arises in the liver parenchyma, pCCA arises between the second order bile ducts and the insertion of the cystic duct, and dCCA arises between the insertion of the cystic duct and to, but not including, the ampulla of Vater.[Citation2,Citation3] In the 7th edition of the American Joint Committee on Cancer staging manual published in 2010, a unique staging system was introduced for pCCA and dCCA. This represented an improvement from the 6th edition, in which eCCA was staged in the same way regardless of perihilar or distal location.[Citation3] Cholangiocarcinomas are sometimes investigated together with gallbladder cancer, as well as ampullary carcinomas and the term biliary tract cancer is applied.
Figure 1. Current classification of cholangiocarcinoma: dCCA: distal cholangiocarcinoma; eCCA: extrahepatic cholangiocarcinoma; iCCA: intrahepatic cholangiocarcinoma; pCCA: perihilar cholangiocarcinoma.
In the International classification of disease of oncology (ICD-O), third edition CCA is topographically classified in the category C22 “liver and intrahepatic bile ducts” or the category C24 “other and unspecified parts of the biliary tract”.[Citation4] Current epidemiological data can therefore only be used to evaluate iCCA and eCCA, and any unique epidemiological characteristics of pCCA and dCCA has not been recorded. The incidence of cholangiocarcinoma shows great variation between and within countries.[Citation5] In western countries, the age specified incidence rates are between 0.5 and 1.5 per 100,000 persons/year.[Citation6] Several authors have suggested that the misclassification of “Klatskin” tumours in the ICD-O second edition, as intrahepatic lesions, have influenced epidemiological trends.[Citation7,Citation8] However, other investigators have found this to have an overall marginal impact.[Citation9]
Cholangiocarcinoma has a dismal prognosis with an overall 5-year survival of <5%.[Citation6] The only treatment that offers a possibility of cure is surgical resection. However, this is currently only possible for about one-third of patients and the rate of recurrence after surgery is high.[Citation10] The differences in diagnostic and surgical approaches have been the rationale underlying the current classification into iCCA, pCCA and dCCA, and a significant number of studies has looked into the surgical treatment and outcome in the different subcategories.[Citation11] Furthermore, several authors have suggested that this subclassification is warranted not only due to differences in clinical management, but also due to differences in tumour biology.[Citation12–14] Differences between intrahepatic and extrahepatic cholangiocarcinomas have been well documented from different fields, as presented below:
Epidemiological research has shown different trends in incidence for iCCA and eCCA with the worldwide incidence of iCCA increasing substantially, and the incidence of eCCA remaining stable or even slightly decreasing.[Citation5,Citation15]
Studies elucidating the cellular origin of CCA have shown the possibility of iCCA arising from transdifferentiated hepatocytes or hepatic progenitors, whilst eCCA are believed to arise from the biliary epithelium or peribiliary glands.[Citation14,Citation16] In one study, iCCA could be divided based on expression of mucins into a group being clinicopathological similar to pCCA, and another similar to hepatocyte progenitors, possibly reflecting either large bile ducts or primary liver cells as cellular origin.[Citation17]
In a meta-analysis of immunohistochemical markers, 18 of 57 markers were found to be significantly differentially expressed between iCCA and eCCA. These included potential pharmacological targets like EGFR, c-erbB-2, and VEGF-A. In addition, only 16 markers had a similar prevalence with narrow confidence intervals.[Citation18]
Genomic profiling has identified different somatic mutations in iCCA and eCCA, respectively.[Citation19] In the currently largest genomic profiling of eCCA mutations in potential pharmacological targets, such as KRAS, ERBB2, PTEN and more, were identified. Notably, no mutations were found in IDH1/2 or FGFR2 gene fusions, which has been described as important potential targets in iCCA.[Citation20,Citation21]
A limited number of studies have investigated differences within the extrahepatic group and the conclusions that currently can be drawn are limited. One example is the differential expression of cell cycle related proteins between pCCA and dCCA, where some studies has shown that dCCA expressed a pattern more closely resembling pancreatic ductal adenocarcinoma (PDAC).[Citation22,Citation23]
Previous research in the field of cholangiocarcinoma, such as identifying genetic and epigenetic alterations, differentially expressed proteins and disease biomarkers in tissue or biofluids, has often grouped iCCA, pCCA and dCCA together. This sometimes also have included gallbladder or ampullary carcinomas, without taking into account the validity problem of the various causes, with various outcomes. The same goes for oncological treatment studies. One important first step towards a more personalised medicine strategy in the treatment of patients with cholangiocarcinoma is a proper characterisation of the unique subcategories of cholangiocarcinomas and the identification of unique biomarkers for diagnosis, prognosis and therapeutic outcome prediction. This development has been ongoing, primarily in iCCA, in which several studies have investigated the molecular pathogenesis and identified potential biomarkers, as well as goals for targeted therapy.[Citation20] The number of studies characterising eCCA is significantly lower and there is a need of elucidating the molecular pathogenesis, as well as identifying unique biomarkers of pCCA and dCCA, respectively. This division will be necessary, not only because there are underlying differences in tumour biology, but also to deal with unique clinical problems in pCCA and dCCA. One of the most promising improvements is the possibility of treating pCCA with neoadjuvant therapy followed by liver transplantation. In a highly selected group of patients, this regime has shown a 5-year survival as high as 68%.[Citation24,Citation25] Finding biomarkers for response to neoadjuvant treatment, and the identification of prognostic biomarkers in this setting could further improve patient selection and survival. In dCCA, a differential diagnostic problem exists versus other periampullary tumours, such as PDAC and ampullary carcinoma. Several studies are investigating the place of neoadjuvant regimes for PDAC [Citation26,Citation27] and the need of separation between dCCA and PDAC preoperatively will be warranted for optimal management.
For the forthcoming research, in patients with assumed cholangiocarcinomas, in order to improve understanding of the different types of malignancies, and provide novel possibilities of personalised treatment options and improved outcome, we propose the following:
First, studies investigating molecular pathogenesis, biomarkers and treatment should define patient cohorts as iCCA, pCCA and dCCA, and analyse them as separate entities. If tumour groups are mixed, the validity problem this causes has to be considered. Secondly, further research efforts has to be put into characterising the molecular pathogenesis and identifying diagnostic, prognostic and predictive biomarkers for pCCA, and dCCA, as the current knowledge is poor. Finally, in upcoming versions, the ICD system should incorporate separate topographical classification codes for pCCA and dCCA.
Acknowledgements
We would like to express our gratitude to Martin Berntsson for assistance in creating the illustration of the different subcategories of cholangiocarcinoma.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473.
- Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11:13–21.
- Edge S, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. New York (NY): Springer Verlag; 2010.
- International classification of diseases for oncology: ICD-O. Geneva: WHO; 2000.
- Bergquist A, von Seth E. Epidemiology of cholangio-carcinoma. Best Pract Res Clin Gastroenterol. 2015;29:221–232.
- Mosconi S, Beretta GD, Labianca R, et al. Cholangiocar-cinoma. Crit Rev Oncol Hematol. 2009;69:259–270.
- Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–875.
- Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854.
- Tyson GL, Ilyas JA, Duan Z, et al. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci. 2014;59:3103–3110.
- Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–1669.
- Akamatsu N, Sugawara Y, Hashimoto D. Surgical strategy for bile duct cancer: advances and current limitations. World J Clin Oncol. 2011;2:94–107.
- Ghouri YA, Mian I, Blechacz B. Cancer review: cholangiocarcinoma. J Carcinogen. 2015;14:1
- Cardinale V, Bragazzi MC, Carpino G, et al. Cholangiocarcinoma: increasing burden of classifications. Hepatobiliary Surg Nutr. 2013;2:272–280.
- Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229.
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125.
- Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–3918.
- Komuta M, Govaere O, Vandecaveye V, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–1888.
- Wiggers JK, Ruys AT, Groot Koerkamp B, et al. Differences in immunohistochemical biomarkers between intra- and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29: 1582–1594.
- Putra J, de Abreu FB, Peterson JD, et al. Molecular profiling of intrahepatic and extrahepatic cholangiocarcinoma using next generation sequencing. Exp Mol Pathol. 2015;99:240–244.
- Moeini A, Sia D, Bardeesy N, et al. Molecular pathogenesis and targeted therapies of intrahepatic cholangiocarcinoma. Clin Cancer Res. 2015. [Epub ahead of print].
- Lee H, Wang K, Johnson A, et al. Comprehensive genomic profiling of extrahepatic cholangiocarcinoma reveals a long tail of therapeutic targets. J Clin Pathol 2015; Oct 23. pii: jclinpath-2015-203394. [Epub ahead of print].
- Jarnagin WR, Klimstra DS, Hezel M, et al. Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol. 2006;24:1152–1160.
- Argani P, Shaukat A, Kaushal M, et al. Differing rates of loss of DPC4 expression and of p53 overexpression among carcinomas of the proximal and distal bile ducts. Cancer. 2001;91:1332–1341.
- Robles R, Sánchez-Bueno F, Ramírez P, et al. Liver transplantation for hilar cholangiocarcinoma. World J Gastroenterol. 2013;19:9209–9215.
- Darwish Murad S, Kim WR, Therneau T, et al. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56:972–981.
- Heinrich S, Pestalozzi B, Lesurtel M, et al. Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study). BMC Cancer. 2011;11:346
- Nitsche U, Wenzel P, Siveke JT, et al. Resectability after first-line FOLFIRINOX in initially unresectable locally advanced pancreatic cancer: a single-center experience. Ann Surg Oncol 2015; Sep 8. [Epub ahead of print].
