Abstract
Background: From around the year 2000, Northern Europe experienced a rise in impetigo caused by Staphylococcus aureus resistant to fusidic acid. A single clone of S. aureus was found to be the bacterial pathogen involved in the impetigo outbreak in Norway, Sweden, the UK and Ireland, termed ‘the epidemic European fusidic acid-resistant impetigo clone’ (EEFIC). We have followed the incidence of impetigo during the years 2001–2012 based on all patients in general practice in the island community of Austevoll, Western Norway. We previously reported a marked decline of impetigo incidence in Austevoll, from 0.0260 cases per person-year in 2002 to 0.0038 in 2009. This article explores indications of an end to the impetigo epidemic caused by the EEFIC clone. Methods: All four general practitioners (GPs) in the community (mean population = 4400) were asked to diagnose impetigo in a uniform way and to take bacterial specimens from all impetigo cases. Phenotypic characteristics of specimen bacteria were determined for the whole period and molecular analyses were performed on isolates in the period 2008–2012. Results: We observed a further decline in incidence of impetigo in Austevoll in the study period. The proportion of fusidic acid-resistant S. aureus isolates decreased during the period 2002–2012, with a mean of 80% in the epidemic years of 2002–2004, 55% in 2005–2009, and 6% in 2010–2012. In total, 44 S. aureus isolates from impetigo were subject to molecular analyses in the period 2008–2012, and 11 were found to be related to the EEFIC. All EEFIC isolates were found in 2008–2009, with no new isolates in 2010–2012. Conclusion: There is an apparent end to the impetigo epidemic related to the EEFIC in this population in Western Norway.
Introduction
From around the year 2000, Northern Europe experienced a rise in superficial skin infections, most often diagnosed as impetigo. In many cases the infections were caused by Staphylococcus aureus resistant to fusidic acid [Citation1,Citation2]. In 2003, O’Neill and collaborators identified a single clone of S. aureus as being the bacterial pathogen involved in the impetigo outbreak in Norway, Sweden, the UK and Ireland [Citation3]. They termed this clone ‘the epidemic European fusidic acid-resistant impetigo clone’ (EEFIC), and later performed a comprehensive molecular characterization of the clone [Citation4].
Several studies documented the presence of the EEFIC being related to elevated numbers of impetigo cases in Norway [Citation1], Sweden [Citation2], the UK and Ireland [Citation3]. Later, the presence of the clone was also confirmed in France [Citation5] and the Netherlands [Citation6].
In 2011, we reported data on the incidence and bacteriology of impetigo in the Norwegian island community of Austevoll during the years 2001–2009 [Citation7]. Phenotypic characteristics of the bacteria were determined for the whole period, and in 2008 and 2009 molecular analyses were performed on selected isolates. The prevalence of the EEFIC was much less in 2009 than in 2008, indicating an attenuation of the impetigo epidemic caused by the EEFIC. Further suggestions of a decrease of the EEFIC came from studies from Sweden [Citation8] and Denmark [Citation9], showing falling rates of fusidic acid resistance in impetigo-related S. aureus isolates in the latter half of the first decade after the year 2000.
In the current study, we wanted to explore indications of an end to the impetigo epidemic caused by the EEFIC in the community of Austevoll, Norway, based on continued data for the years 2010–2012.
Materials and methods
The study was performed during the years 2001–2012 in the island municipality of Austevoll, Western Norway. The municipality had a mean population of 4400 inhabitants during the study period. The connection to the mainland is by boats and ferries only. During the study period the mean number of general practitioners (GPs) in the municipality was four. The GPs share a common Electronic Patient Journal (EPJ) system, and they agreed to record all cases of impetigo in a uniform way. Impetigo was defined as a superficial skin infection with spontaneous eruption of erosions being covered by honey-coloured crusts. The GPs were encouraged to take bacterial specimens from all impetigo cases. The bacterial specimens were sent to the Department of Microbiology, Haukeland University Hospital, Bergen, Norway. Annual incidence of impetigo was calculated and epidemic season was defined according to a standard procedure [Citation10].
In addition, the project leader (S.R.), who was one of the community GPs, collected bacterial specimens from cases of other bacterial skin infections, for comparison with the impetigo cases. These non-impetigo specimens were not collected according to specific criteria. Bacteria in all specimens were detected and identified by standard methods. The susceptibility of S. aureus to penicillin G, oxacillin, erythromycin, fusidic acid and clindamycin was determined using disc diffusion tests. From December 2012 antimicrobial susceptibility testing (AST) was done according to the EUCAST disc diffusion method. Before this, AST was done according to the Norwegian Working Group on Antibiotics (NWGA). Before the laboratory changed the method for susceptibility testing, the two methods were compared. Regarding susceptibility categorization (sensitive/intermediate/resistant), the two methods were found to be comparable.
Molecular epidemiological typing was performed on available S. aureus isolates in the period 2008–2012. Both pulsed-field gel electrophoresis (PFGE) and staphylococcal protein A typing (spa-typing) were used. Although different, the methods are thought to be comparable when it comes to classifying an isolate as belonging to the EEFIC clone or not. In this period, 83 impetigo cases were registered, 77 swabs were taken and S. aureus was isolated in 52 cases.
In the years 2008–2009, all available impetigo S. aureus isolates (n = 28) were investigated by PFGE. Band patterns were compared visually and differences were evaluated as described by Tenover et al. [Citation11]. Isolates indicated by PFGE to belong to the EEFIC (n = 11), and fusidic acid-resistant (FAR) isolates not found by PFGE to be related to the EEFIC (n = 2), were further analysed by spa typing, which was performed at St Olavs Hospital, Trondheim, Norway, as described previously [Citation12].
In the years 2010–2012, the PFGE method was no longer an available laboratory method. In this period, all available impetigo S. aureus isolates (n = 16) were investigated by spa typing instead. A few impetigo S. aureus isolates (6 of 34 isolates from 2008–2009 and 2 of 18 from 2010–2012) were lost to further molecular analyses.
By PFGE analysis, all 11 non-impetigo S. aureus isolates in the years 2008–2009 were found not to be related to the EEFIC, and were not subjected to further spa typing. Nineteen non-impetigo S. aureus isolates from 2010–2012 were all sent for spa typing. To be defined as EEFIC in the present study, isolates had to show spa typing at least closely related to the EEFIC reference strain (spa type t171) [Citation1,Citation3,Citation4].
Continuous surveillance of the impetigo incidence and FAR S. aureus prevalence has been the main focus of the Austevoll impetigo study. We have previously documented falling rates of these variables since the start of the epidemic in 2002–2003 and forward up to 2009 [Citation10]. We retrospectively collected data on prevalence and bacteriology of impetigo cases for the last half of 2001.
To investigate a possible end to the EEFIC- related impetigo epidemic in Austevoll, we here present the data for the years 2010–2012 as an extension of the data from the previous years. For overview, we also present some previously published data.
Ethics
Ethics approval was obtained from the Regional Committee for Medical and Health Research Ethics of Western Norway, and the study was also approved by the Ombudsman for Privacy in Research, Norwegian Social Science Data Services.
Results
In total, 484 impetigo cases were registered, and bacterial specimens were collected from 388 of these cases. shows the annual incidence of impetigo for the period 2001–2012. After a maximum in 2002, there was a marked decline in incidence, and there was no epidemic season after 2004 [Citation10]. Altogether, bacterial swabs were taken from 80% of the patients, and S. aureus was grown in 76% of the cases where swabs were taken (). The proportion of FAR S. aureus isolates decreased during the period 2002–2009, with a mean of 80% in the epidemic years 2002–2004, 55% in 2005–2009 [Citation7]. The decline continued and reached 6% in 2010–2012. These declining trends are depicted in .
Figure 1. Number of impetigo cases, S. aureus isolates in impetigo and fusidic acid-resistance in S. aureus in impetigo, in Austevoll 2002–2012.
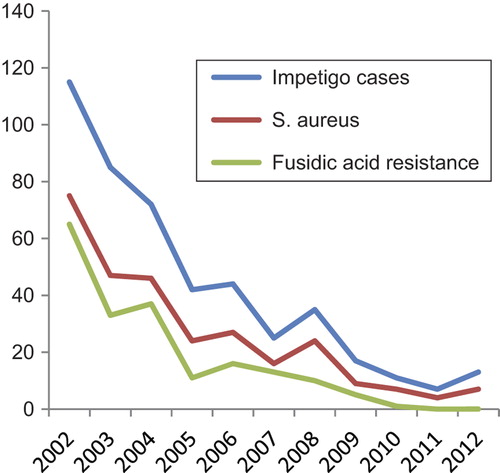
Table I. Yearly incidence rates and microbial characteristics of impetigo in the total population of Austevoll, Western Norway (n = 4400)a.
In total, 44 S. aureus isolates from impetigo were subject to molecular analyses, and 11 were found to be related to the EEFIC (). Of the 11 EEFIC isolates from 2008–2009, none showed PFGE identity with the reference strain collected in 2001, but all had closely related PFGE patterns. Eight had identical spa type (t171), while three strains had the closely related spa types of t659 (two isolates) and t645 [Citation7].
Table II. Susceptibility testing, spa typing and relatedness to EEFIC in 44 impetigo and 33 non-impetigo Staphylococcus aureus isolates from the years 2008–2012.
Of the 33 non-EEFIC S. aureus isolates from impetigo, 30 (91%) were susceptible to fusidic acid. The EEFIC clone was responsible for 77% (10 of 13) of impetigo S. aureus isolates with resistance to fusidic acid, and 91% (10 of 11) of EEFIC isolates were FAR [Citation7].
In 2010–2012, no new EEFIC isolates were found. The detailed listing of spa types from both impetigo and non-impetigo specimens from this period is shown in . Two spa types were involved in both impetigo and non-impetigo skin infections; t084 were found in seven impetigo infections and in three non-impetigo infections, and t127 were found in one impetigo infection and in four non-impetigo infections. spa type t127 was responsible for all cases of FAR during the years 2010–2012, both the one impetigo case and three non-impetigo cases. One isolate with spa type t127 was susceptible to fusidic acid.
Of 11 S. aureus isolates from skin infections other than impetigo during the years 2008–2009 analysed by susceptibility testing and PFGE, all turned out to be fusidic acid susceptible, and none were related to the EEFIC [Citation7]. In the years 2010–2012, 3 of the 19 S. aureus isolates from non-impetigo skin infections were FAR, but none were related to the EEFIC.
Discussion
In the present study there was a parallel decline in impetigo rate and the proportion of FAR S. aureus isolated from impetigo during the years 2002–2012. No impetigo isolates were FAR in 2011 and 2012. For the years 2008–2012 the vast majority of fusidic acid resistance in S. aureus in impetigo was caused by the EEFIC, making it reasonable to argue that the great predominance of fusidic acid resistance during the years 2002–2007 was also driven by the EEFIC.
The data from the present study suggest that, as far as the community of Austevoll is concerned, the epidemic related to the EEFIC has come to an end.
Whether this conclusion is generalizable to all of Norway, and to all of Northern Europe, is an important question. The EPISA study of antimicrobial susceptibility of S. aureus-related skin and soft tissue infections in general practice in France, the UK and Ireland during 2003–2004 found a high proportion of EEFIC identified in impetigo [Citation5]. In 2012, Dutch researchers investigated the prevalence of the EEFIC in general practice patients with skin or soft tissue infections during 2007 and 2008 [Citation6], and they identified EEFIC in a little over 30% of S. aureus isolates from skin lesions. However, these two studies do not give data about time trends of the EEFIC prevalence.
A Swedish follow-up study from the years 1993–2004 investigating the prevalence of S. aureus isolates being resistant to fusidic acid (FAR) in selected nationwide microbiological laboratories, found the proportion of FAR for superficial skin and soft tissue infections in children to decline from a maximum of about 47% in 2002 to 35% in 2004 [Citation13]. Another Swedish study of patients attending a dermatological outpatient clinic for impetigo and infected atopic dermatitis in the years 2004–2008 showed that the proportion of FAR in impetigo and infected atopic dermatitis caused by S. aureus was diminishing simultaneously, with the proportion of FAR in impetigo decreasing from 33% to 24% over the years [Citation8].
In 2011, a population-based study from Northern Denmark recorded bacterial data from skin and mucosal membranes of patients with skin infections for the time period of 1997–2008 [Citation9]. They found that the prevalence of S. aureus isolated in impetigo had a marked rise to a maximum in 2002, with a gradual decline thereafter. The proportion of the impetigo S. aureus isolates that were FAR was almost 50% during the years 2003–2006, and diminished after that. In the 2012 report of the Norwegian Antibiotic Resistance Surveillance system [Citation14], national data suggested the fusidic acid resistance to S. aureus in wound isolates to be reduced from a maximum of 25% in 2004 to a stable level of about 10% from the year 2008 on. A study from the UK for the years 1995–2010, which was based on a large primary care diagnosis database, showed national impetigo incidence to double from 1995 to 2001, and to regress to a little below the baseline of 1995 by 2010 [Citation15]. However, neither of these studies provided molecular data.
It is likely that these identifiable trends of diminishing prevalence of S. aureus fusidic acid resistance in Sweden, Denmark and Norway, and of impetigo in the UK, indicate a significant attenuation of the presence of EEFIC in these countries.
More epidemiological studies of superficial skin infections from different parts of Northern and Central Europe, involving application of molecular analyses of the bacteria, are needed to confirm this conclusion. However, we find the results from the current study sufficient to suggest that the impetigo epidemic related to the EEFIC is most likely near its end.
Another question is to what degree the EEFIC has specifically been the cause of impetigo and not of other skin infections like skin abscesses, paronychiae and other infections of fingers and toes, superinfection of atopic dermatitis, folliculitis, furunculosis and cellulitis.
When O’Neill et al first described the EEFIC [Citation4], they found that the clone possesses genes previously known to be linked to impetigo, like exfoliative toxins A and B and EDIN-C. In a previous article analysing our material from the years 2001–2005, we showed an inverse relationship between the proportion of FAR in impetigo and in other superficial skin infections, as the proportion of FAR in impetigo at the time was 76% and for other superficial skin infections it was 18% [Citation10].
Our recent data from 2010–2012 show that a diversity of spa types cause both impetigo and non-impetigo infections. The most common spa type found in impetigo is also frequently identified in non-impetigo skin infections, indicating that the strains of S. aureus that are most prevalent at present do not possess the same specificity for impetigo as the EEFIC did.
Based on our material, it seems reasonable to maintain that the EEFIC has been specifically inclined to be associated with impetigo, but not with other superficial skin infections. On the contrary, other studies such as the EPISA study [Citation5] and the study by Rijnders et al. from the Netherlands [Citation6], found the EEFIC in other types of skin infections as well as in impetigo. However, these studies do not seem to be based on accurate clinical data. Possibly, their results may have been subject to mixing of cases of impetigo and other skin infections, thus not being able to identify the high degree of impetigo specificity in EEFIC. The present study is based on cases with strict clinical diagnosis as an inclusion criterion.
The strengths of our study are the exact number of the total population under study, the clinical definition of the cases to be included as impetigo patients, the reliable identification of all impetigo patients and the uniform registration over a long timespan. Weaknesses of the study are that we do not have molecular analyses for the first 6 years, that the molecular methods were not uniformly performed in the periods 2008–2009 and 2010–2012, and that the number of patients in recent years has been small, thus reducing the generalizability of the conclusions.
The steady decline over the years of S. aureus fusidic acid resistance in impetigo and the consistent absence of EEFIC during the last 3 years are strong indications of the extinction of this epidemic in our community.
Acknowledgments
We thank Lillian Marstein, Department of Medical Microbiology, St Olav's University Hospital, who performed the spa typing analyses, and the GPs of Austevoll who diagnosed and treated the patients in this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the Foundation for Research in General Practice (grant no. 07/698), and the Norwegian Surveillance System for Antimicrobial Resistance (grant no. 22.12.2008).
References
- Tveten Y, Jenkins A, Kristiansen BE. A fusidic acid-resistant clone of Staphylococcus aureus associated with impetigo bullosa is spreading in Norway. J Antimicrob Chemother 2002;50:873–6.
- Osterlund A, Eden T, Olsson-Liljequist B, Haeggman S, Kahlmeter G. Swedish Study Group on Fusidic Acid- resistant Staphylococcus aureus. Clonal spread among Swedish children of a Staphylococcus aureus strain resistant to fusidic acid. Scand J Infect Dis 2002;34:729–34.
- O’Neill AJ, Larsen AR, Henriksen AS, Chopra I. A fusidic acid-resistant epidemic strain of Staphylococcus aureus carries the fusB determinant, whereas fusA mutations are prevalent in other resistant isolates. Antimicrob Agents Chemother 2004;48:3594–7.
- O’Neill AJ, Larsen AR, Skov R, Henriksen AS, Chopra I. Characterization of the epidemic European fusidic acid- resistant impetigo clone of Staphylococcus aureus. J Clin Microbiol 2007;45:1505–10.
- Larsen AR, Skov RL, Jarlier V, Henriksen AS. Epidemiological differences between the UK and Ireland versus France in Staphylococcus aureus isolates resistant to fusidic acid from community-acquired skin and soft tissue infections. J Antimicrob Chemother 2008;61:589–94.
- Rijnders MI, Wolffs PF, Hopstaken RM, den Heyer M, Bruggeman CA, Stobberingh EE. Spread of the epidemic European fusidic acid-resistant impetigo clone (EEFIC) in general practice patients in the south of The Netherlands. J Antimicrob Chemother 2012;67:1176–80.
- Rørtveit S, Skutlaberg DH, Langeland N, Rortveit G. Impetigo in a population over 8.5 years: incidence, fusidic acid resistance and molecular characteristics. J Antimicrob Chemother 2011;66:1360–4.
- Alsterholm M, Flytström I, Bergbrant IM, Faergemann J. Fusidic acid-resistant Staphylococcus aureus in impetigo contagiosa and secondarily infected atopic dermatitis. Acta Derm Venereol 2010;90:52–7.
- Dalager-Pedersen M, Søgaard M, Schønheyder HC. Staphylococcus aureus skin and soft tissue infections in primary healthcare in Denmark: a 12-year population-based study. Eur J Clin Microbiol Infect Dis 2011;30: 951–6.
- Rørtveit S, Rortveit G. Impetigo in epidemic and nonepidemic phases: an incidence study over 4(1/2) years in a general population. Br J Dermatol 2007;157:100–5.
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteriafor bacterial strain typing. J Clin Microbiol 1995;33:2233–9.
- Harmsen D, Claus H, Witte W, Rothgänger J, Claus H, Turnwald G, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 2003;41:5442–8.
- Osterlund A, Kahlmeter G, Haeggmann S, Olsson- Liljequist B. Staphylococcus aureus resistant to fusidic acid among Swedish children: a follow-up study. Scand J Infect Dis 2006;38:334–4.
- NORM/NORM-VET 2012. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø/Oslo 2013. ISSN:1502-2307 (print)/1890-9965. Available from: URL: http://www.vetinst.no/Publikasjoner/NORM-NORM-VET/NORM-NORM-VET-2012
- Shallcross LJ, Petersen I, Rosenthal J, Johnson AM, Freemantle M, Hayward AC. Use of primary care data for detecting impetigo trends, United kingdom, 1995-2010. Emerg Infect Dis 2013;19:1646–8.
